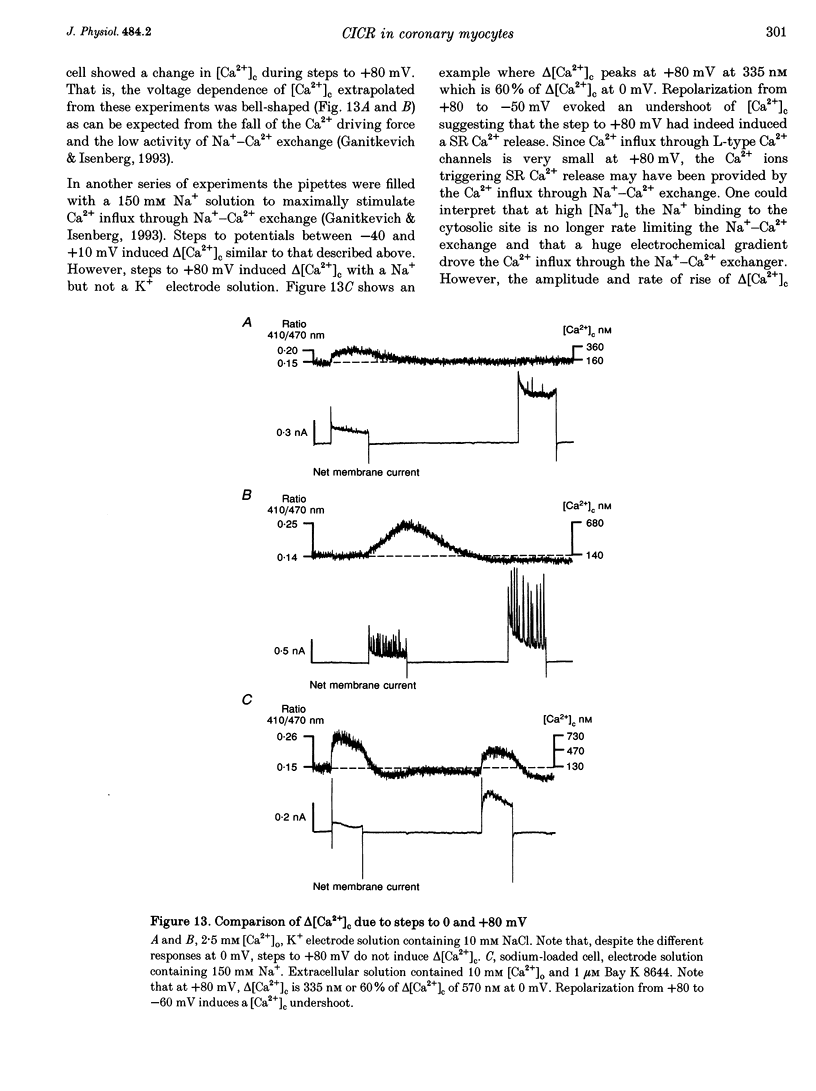
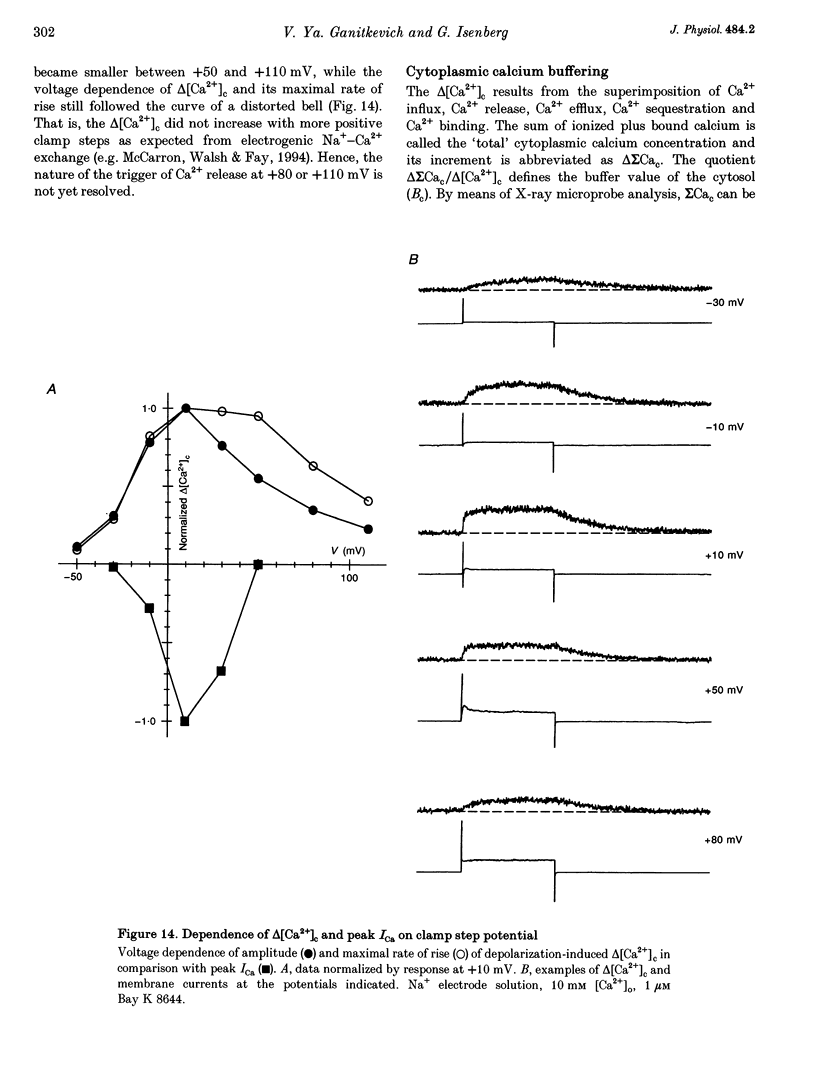
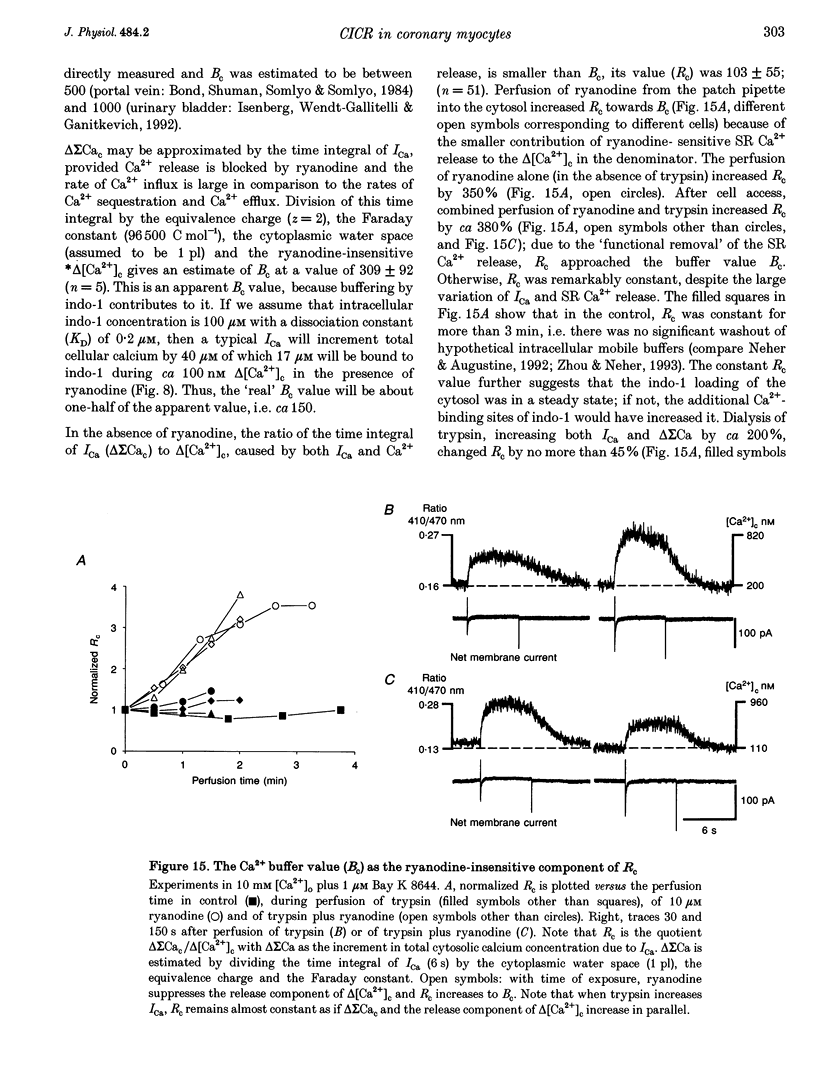
Abstract
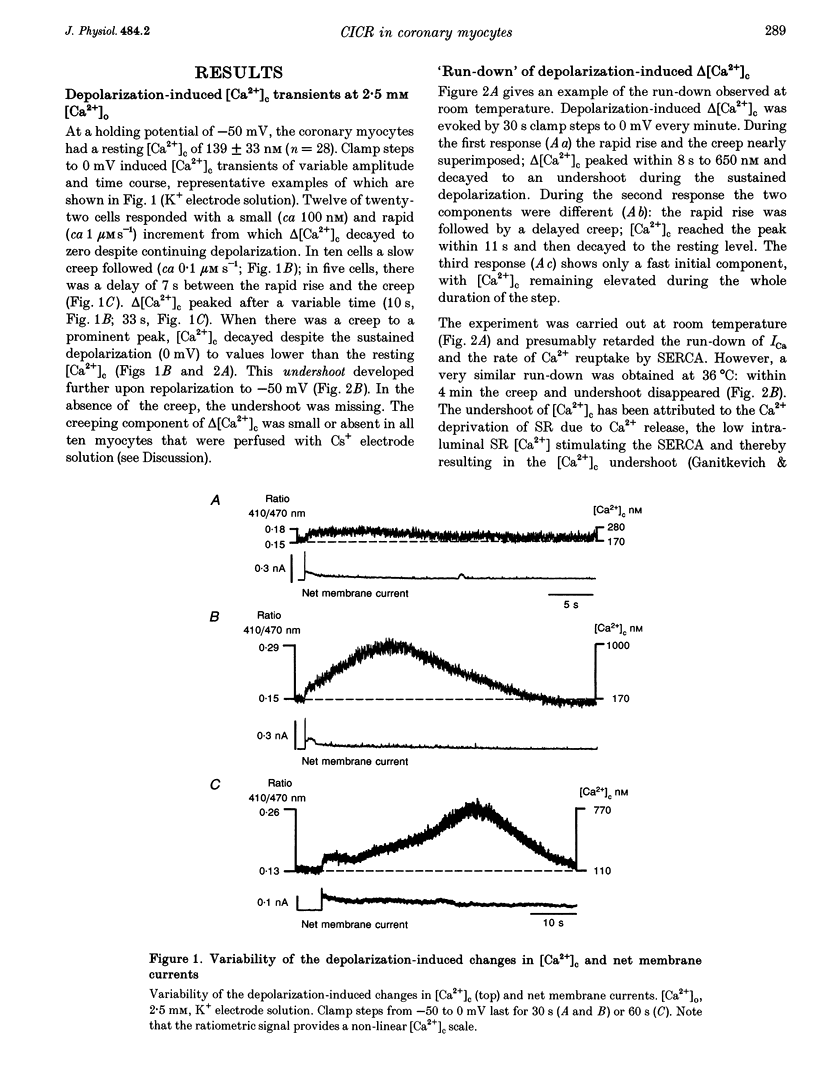
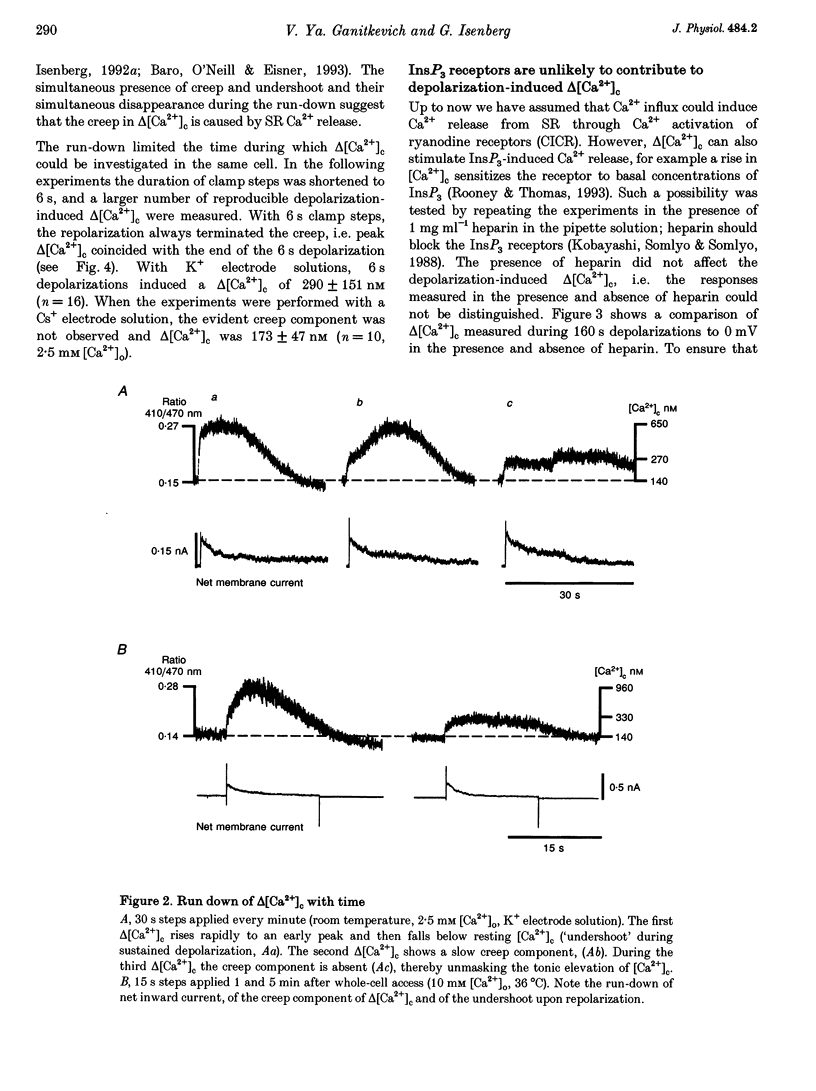
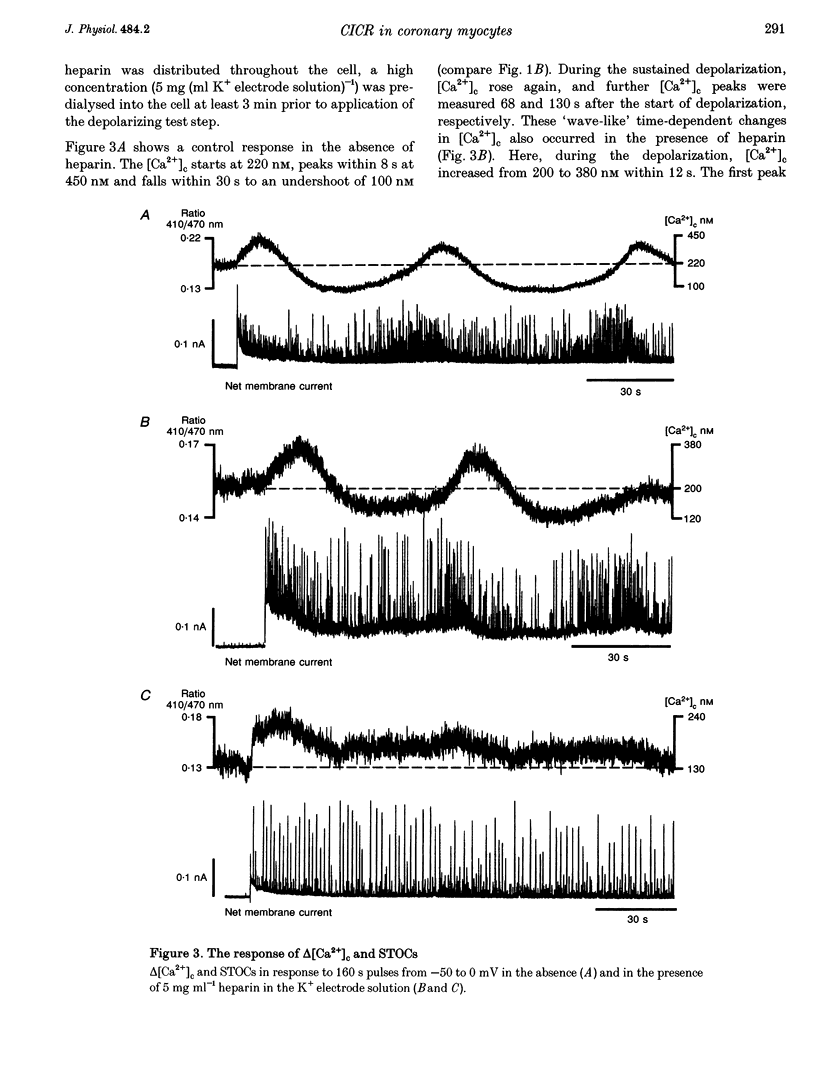
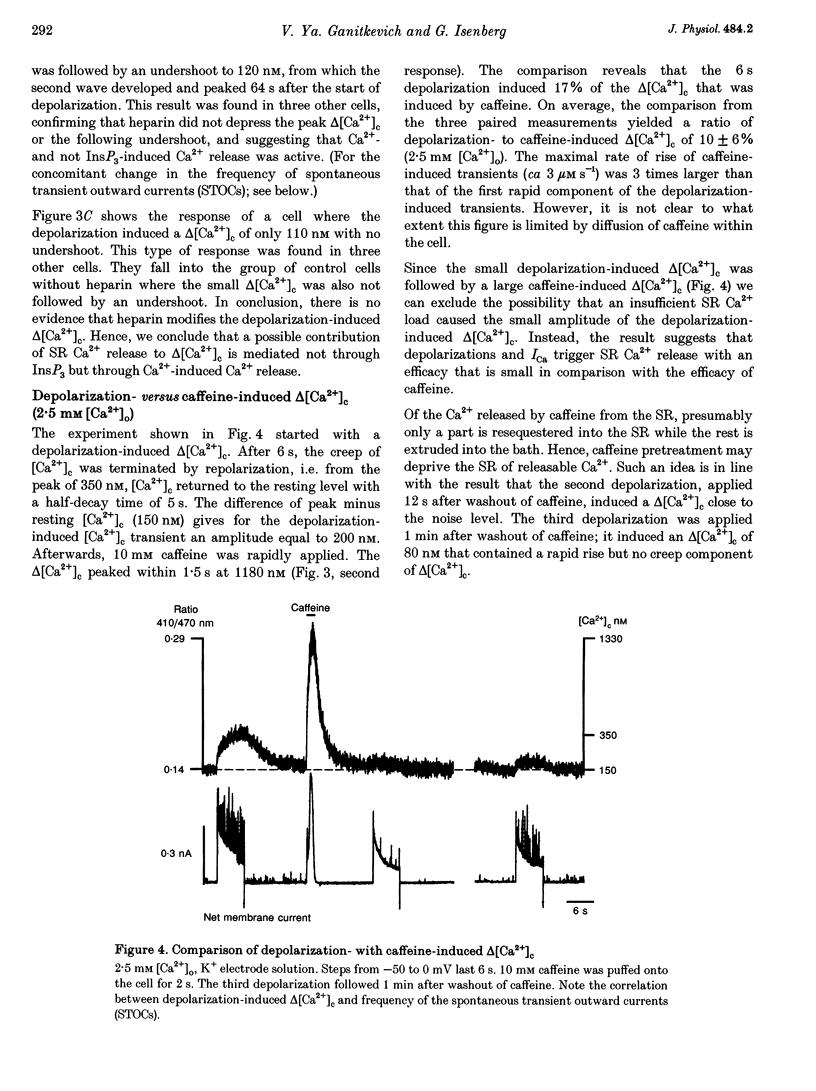
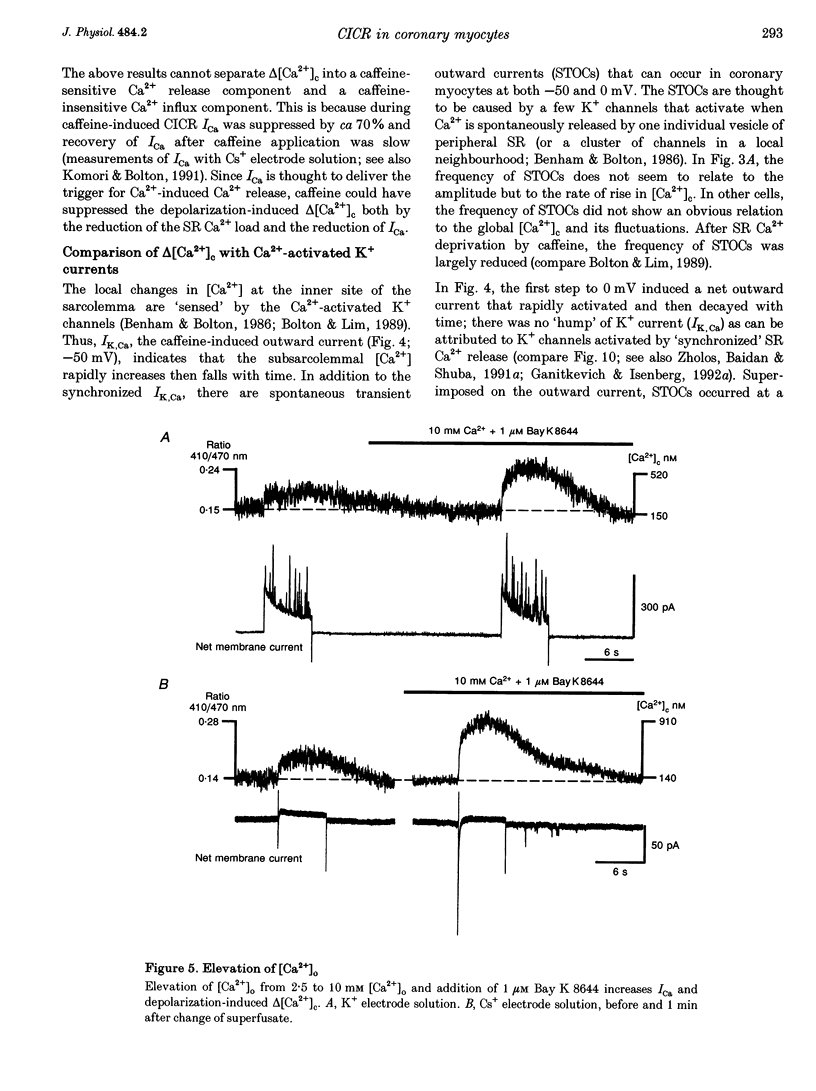
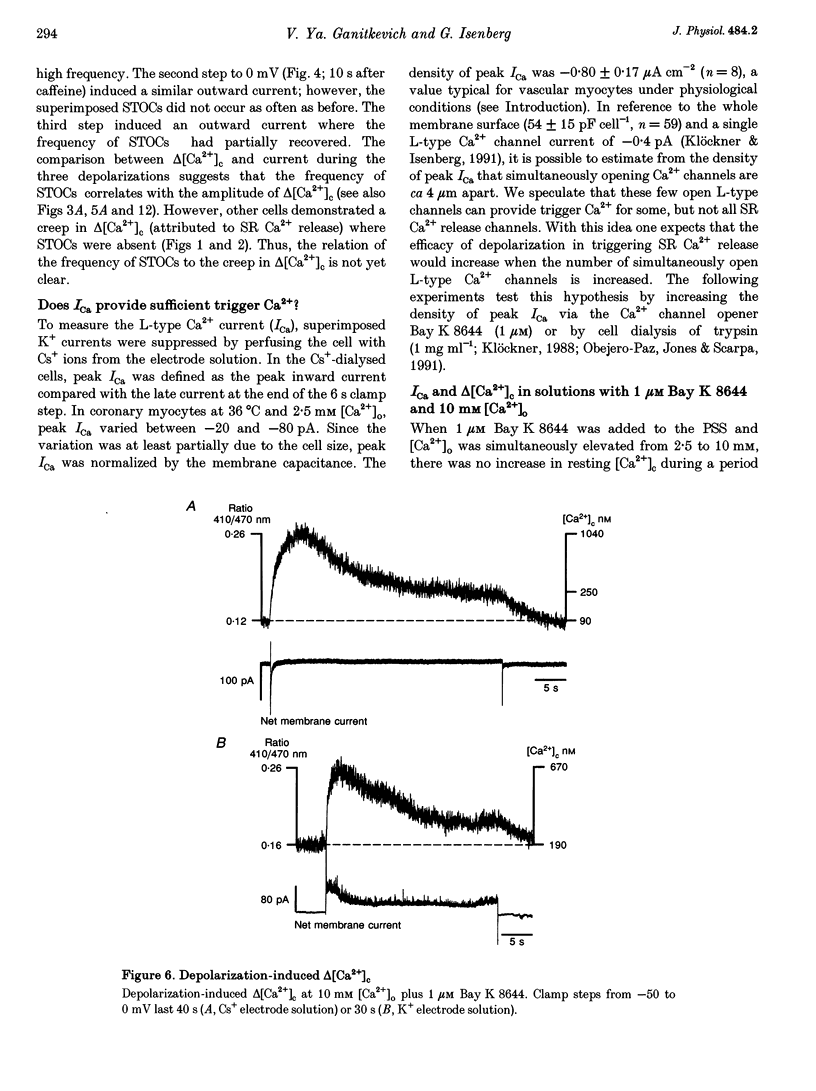
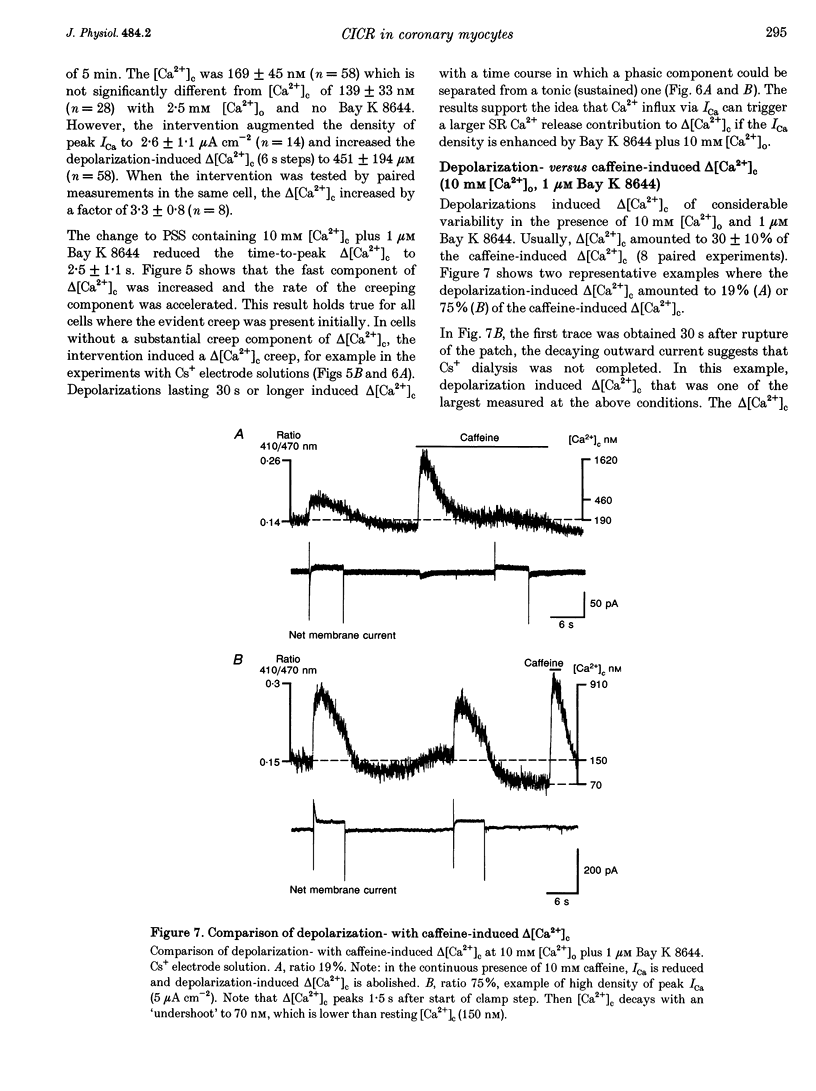
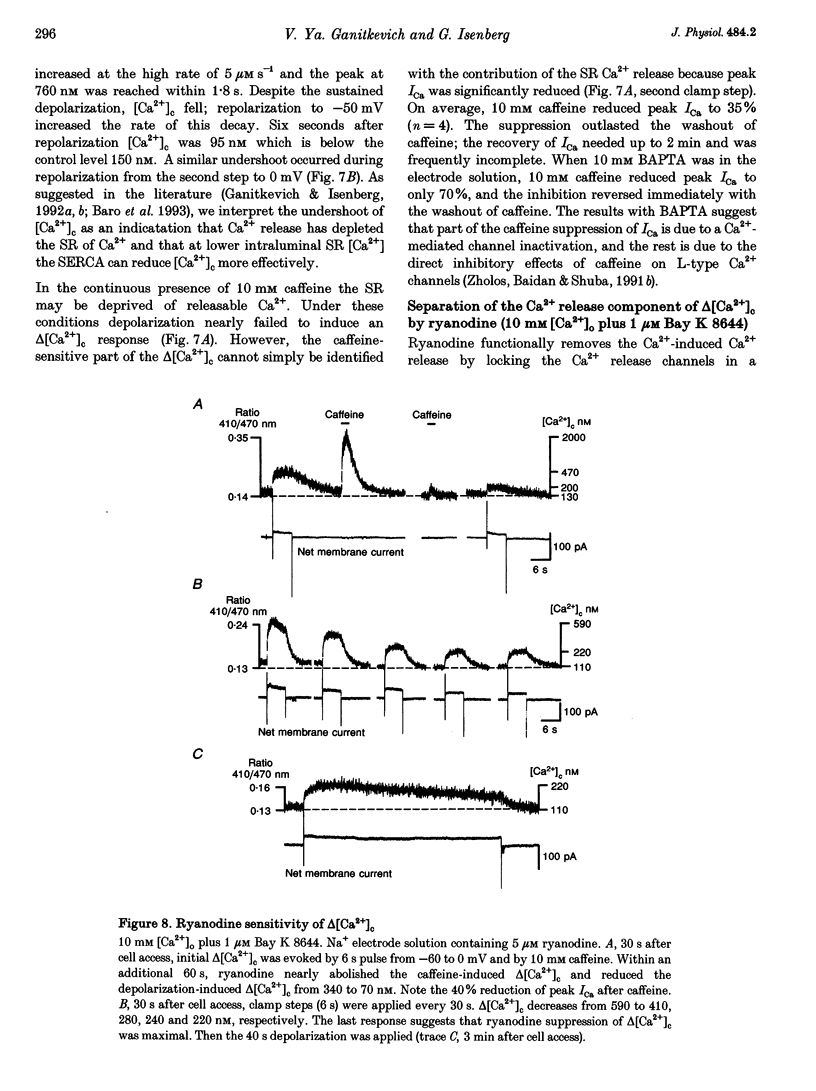
1. Increments in cytosolic Ca2+ concentration (delta[Ca2+]c) were measured in single smooth muscle cells from guinea-pig coronary artery together with the density of peak Ca2+ currents (ICa) in response to clamp steps from -50 to 0 mV. The comparison of depolarization- with caffeine-induced delta[Ca2+]c was used to define the efficacy by which ICa can trigger Ca2+ release from the sarcoplasmic reticulum (SR). 2. At 2.5 mM extracellular calcium concentration ([Ca2+]o), depolarization induced a rapid rise of delta[Ca2+]c followed by a slow creep. Peak [Ca2+]c occurred within ca 30 s and could be followed by an undershoot and a second rise in [Ca2+]c. The creep was blocked by ryanodine but was insensitive to block of InsP3 receptors with heparin. The creep was not observed in Cs(+)-filled cells. After disappearance of the creep, a tonic delta[Ca2+]c became unmasked. 3. At 2.5 mM [Ca2+]o, peak ICa was -0.80 +/- 0.17 microA cm-2. delta[Ca2+] peaked at the end of the 6 s pulse at 202 +/- 98 nM while caffeine-induced delta[Ca2+]c peaked at 1330 +/- 410 nM. The ratio of depolarization- to caffeine-induced delta[Ca2+]c was 10 +/- 6%. 4. In media containing 10 mM [Ca2+]o plus 1 microM Bay K 8644, peak ICa was -2.6 +/- 1.1 microA cm-2 and delta[Ca2+]c peaked within 2.5 s at 451 +/- 194 nM. Paired measurements yielded the ratio of depolarization- to caffeine induced delta[Ca2+]c as 30 +/- 10%. Depolarization-induced delta[Ca2+]c was nearly blocked by caffeine and reduced by ryanodine to 30%, suggesting the contribution of Ca2+ release from caffeine- and ryanodine-sensitive Ca2+ stores. 5. Trypsin (1 mg ml-1) in the electrode solution (10 mM [Ca2+]o plus 1 microM Bay K 8644) increased peak ICa up to 12.5 microA cm-2. ICa induced a delta[Ca2+]c of 990 +/- 210 nM and was accompanied by a 'hump' of IK,Ca. When applied briefly after peak delta[Ca2+]c, caffeine increased [Ca2+]c only moderately. The results suggest that a peak ICa can trigger a synchronized whole-cell Ca2+ release only if ICa is strongly augmented. 6. Amplitude and rate of rise of delta[Ca2+]c were graded by test step potentials along a bell-shaped voltage-dependent curve, similar to that of L-type ICa. Steps to +80 mV induced no delta[Ca2+]c when the electrode solution contained 10 mM Na+. However, with 150 mM intrapipette Na+, pulses to +80 mV induced delta[Ca2+]c.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF

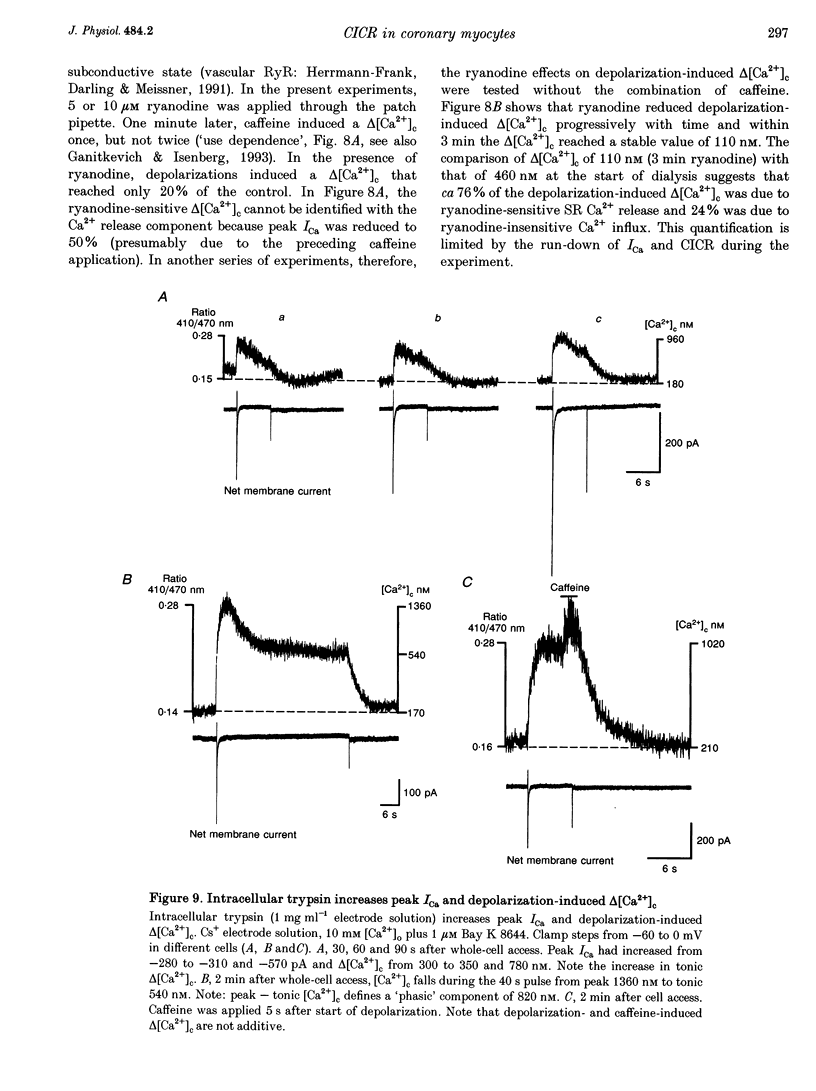
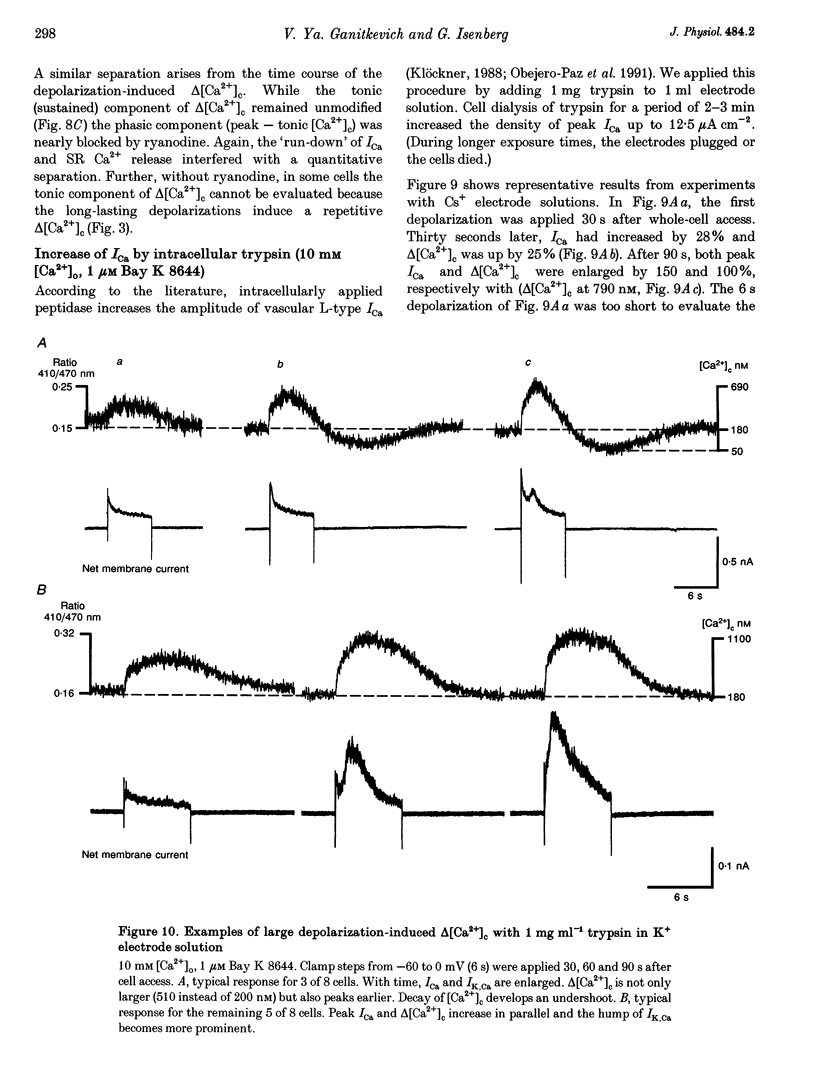
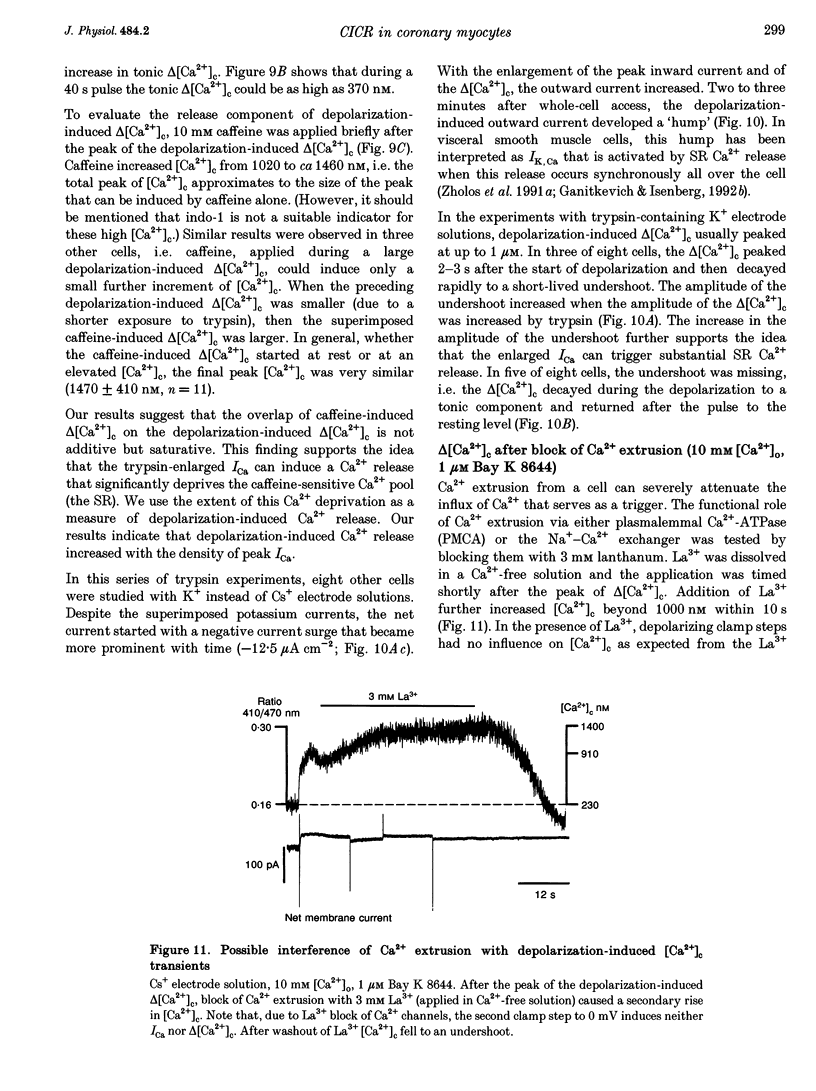
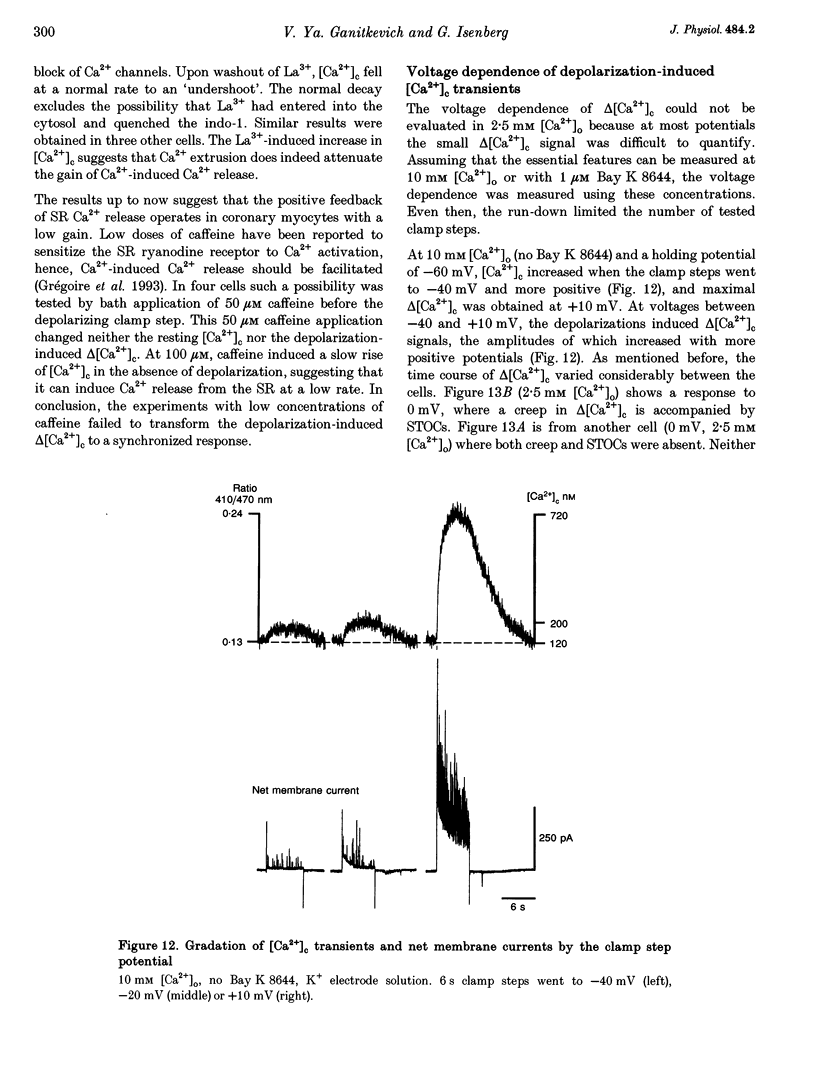



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson P. I., Bolton T. B., Lang R. J., MacKenzie I. Calcium currents in single isolated smooth muscle cells from the rabbit ear artery in normal-calcium and high-barium solutions. J Physiol. 1988 Nov;405:57–75. doi: 10.1113/jphysiol.1988.sp017321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allbritton N. L., Meyer T. Localized calcium spikes and propagating calcium waves. Cell Calcium. 1993 Nov;14(10):691–697. doi: 10.1016/0143-4160(93)90095-n. [DOI] [PubMed] [Google Scholar]
- Baró I., O'Neill S. C., Eisner D. A. Changes of intracellular [Ca2+] during refilling of sarcoplasmic reticulum in rat ventricular and vascular smooth muscle. J Physiol. 1993 Jun;465:21–41. doi: 10.1113/jphysiol.1993.sp019664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Bolton T. B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986 Dec;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bolton T. B., Lim S. P. Properties of calcium stores and transient outward currents in single smooth muscle cells of rabbit intestine. J Physiol. 1989 Feb;409:385–401. doi: 10.1113/jphysiol.1989.sp017504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond M., Kitazawa T., Somlyo A. P., Somlyo A. V. Release and recycling of calcium by the sarcoplasmic reticulum in guinea-pig portal vein smooth muscle. J Physiol. 1984 Oct;355:677–695. doi: 10.1113/jphysiol.1984.sp015445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond M., Shuman H., Somlyo A. P., Somlyo A. V. Total cytoplasmic calcium in relaxed and maximally contracted rabbit portal vein smooth muscle. J Physiol. 1984 Dec;357:185–201. doi: 10.1113/jphysiol.1984.sp015496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Devine C. E., Somlyo A. V., Somlyo A. P. Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol. 1972 Mar;52(3):690–718. doi: 10.1083/jcb.52.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Ca2+ entry through Na(+)-Ca2+ exchange can trigger Ca2+ release from Ca2+ stores in Na(+)-loaded guinea-pig coronary myocytes. J Physiol. 1993 Aug;468:225–243. doi: 10.1113/jphysiol.1993.sp019768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Caffeine-induced release and reuptake of Ca2+ by Ca2+ stores in myocytes from guinea-pig urinary bladder. J Physiol. 1992 Dec;458:99–117. doi: 10.1113/jphysiol.1992.sp019408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Contribution of two types of calcium channels to membrane conductance of single myocytes from guinea-pig coronary artery. J Physiol. 1990 Jul;426:19–42. doi: 10.1113/jphysiol.1990.sp018125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich V. Y., Isenberg G. Contribution of Ca(2+)-induced Ca2+ release to the [Ca2+]i transients in myocytes from guinea-pig urinary bladder. J Physiol. 1992 Dec;458:119–137. doi: 10.1113/jphysiol.1992.sp019409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grégoire G., Loirand G., Pacaud P. Ca2+ and Sr2+ entry induced Ca2+ release from the intracellular Ca2+ store in smooth muscle cells of rat portal vein. J Physiol. 1993 Dec;472:483–500. doi: 10.1113/jphysiol.1993.sp019957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Schiefer A., Isenberg G. Ca2+ load of guinea-pig ventricular myocytes determines efficacy of brief Ca2+ currents as trigger for Ca2+ release. J Physiol. 1994 Nov 1;480(Pt 3):411–421. doi: 10.1113/jphysiol.1994.sp020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann-Frank A., Darling E., Meissner G. Functional characterization of the Ca(2+)-gated Ca2+ release channel of vascular smooth muscle sarcoplasmic reticulum. Pflugers Arch. 1991 May;418(4):353–359. doi: 10.1007/BF00550873. [DOI] [PubMed] [Google Scholar]
- Iino M. Calcium release mechanisms in smooth muscle. Jpn J Pharmacol. 1990 Dec;54(4):345–354. doi: 10.1254/jjp.54.345. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Han S. Gradation of Ca(2+)-induced Ca2+ release by voltage-clamp pulse duration in potentiated guinea-pig ventricular myocytes. J Physiol. 1994 Nov 1;480(Pt 3):423–438. doi: 10.1113/jphysiol.1994.sp020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargacin G., Fay F. S. Ca2+ movement in smooth muscle cells studied with one- and two-dimensional diffusion models. Biophys J. 1991 Nov;60(5):1088–1100. doi: 10.1016/S0006-3495(91)82145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S., Somlyo A. V., Somlyo A. P. Heparin inhibits the inositol 1,4,5-trisphosphate-dependent, but not the independent, calcium release induced by guanine nucleotide in vascular smooth muscle. Biochem Biophys Res Commun. 1988 Jun 16;153(2):625–631. doi: 10.1016/s0006-291x(88)81141-0. [DOI] [PubMed] [Google Scholar]
- Komori S., Bolton T. B. Inositol trisphosphate releases stored calcium to block voltage-dependent calcium channels in single smooth muscle cells. Pflugers Arch. 1991 Jun;418(5):437–441. doi: 10.1007/BF00497770. [DOI] [PubMed] [Google Scholar]
- Langton P. D., Standen N. B. Calcium currents elicited by voltage steps and steady voltages in myocytes isolated from the rat basilar artery. J Physiol. 1993 Sep;469:535–548. doi: 10.1113/jphysiol.1993.sp019828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarron J. G., Walsh J. V., Jr, Fay F. S. Sodium/calcium exchange regulates cytoplasmic calcium in smooth muscle. Pflugers Arch. 1994 Feb;426(3-4):199–205. doi: 10.1007/BF00374772. [DOI] [PubMed] [Google Scholar]
- Neher E., Augustine G. J. Calcium gradients and buffers in bovine chromaffin cells. J Physiol. 1992 May;450:273–301. doi: 10.1113/jphysiol.1992.sp019127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowycky M. C., Pinter M. J. Time courses of calcium and calcium-bound buffers following calcium influx in a model cell. Biophys J. 1993 Jan;64(1):77–91. doi: 10.1016/S0006-3495(93)81342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obejero-Paz C. A., Jones S. W., Scarpa A. Calcium currents in the A7r5 smooth muscle-derived cell line. Increase in current and selective removal of voltage-dependent inactivation by intracellular trypsin. J Gen Physiol. 1991 Dec;98(6):1127–1140. doi: 10.1085/jgp.98.6.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney T. A., Thomas A. P. Intracellular calcium waves generated by Ins(1,4,5)P3-dependent mechanisms. Cell Calcium. 1993 Nov;14(10):674–690. doi: 10.1016/0143-4160(93)90094-m. [DOI] [PubMed] [Google Scholar]
- Smirnov S. V., Aaronson P. I. Ca2+ currents in single myocytes from human mesenteric arteries: evidence for a physiological role of L-type channels. J Physiol. 1992 Nov;457:455–475. doi: 10.1113/jphysiol.1992.sp019387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trafford A. W., O'Neill S. C., Eisner D. A. Factors affecting the propagation of locally activated systolic Ca transients in rat ventricular myocytes. Pflugers Arch. 1993 Oct;425(1-2):181–183. doi: 10.1007/BF00374521. [DOI] [PubMed] [Google Scholar]
- Zholos A. V., Baidan L. V., Shuba M. F. Properties of the late transient outward current in isolated intestinal smooth muscle cells of the guinea-pig. J Physiol. 1991 Nov;443:555–574. doi: 10.1113/jphysiol.1991.sp018851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zholos A. V., Baidan L. V., Shuba M. F. Some properties of Ca(2+)-induced Ca2+ release mechanism in single visceral smooth muscle cell of the guinea-pig. J Physiol. 1992 Nov;457:1–25. doi: 10.1113/jphysiol.1992.sp019362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zholos A. V., Baidan L. V., Shuba M. F. The inhibitory action of caffeine on calcium currents in isolated intestinal smooth muscle cells. Pflugers Arch. 1991 Oct;419(3-4):267–273. doi: 10.1007/BF00371106. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Neher E. Mobile and immobile calcium buffers in bovine adrenal chromaffin cells. J Physiol. 1993 Sep;469:245–273. doi: 10.1113/jphysiol.1993.sp019813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Breemen C., Saida K. Cellular mechanisms regulating [Ca2+]i smooth muscle. Annu Rev Physiol. 1989;51:315–329. doi: 10.1146/annurev.ph.51.030189.001531. [DOI] [PubMed] [Google Scholar]


