Abstract
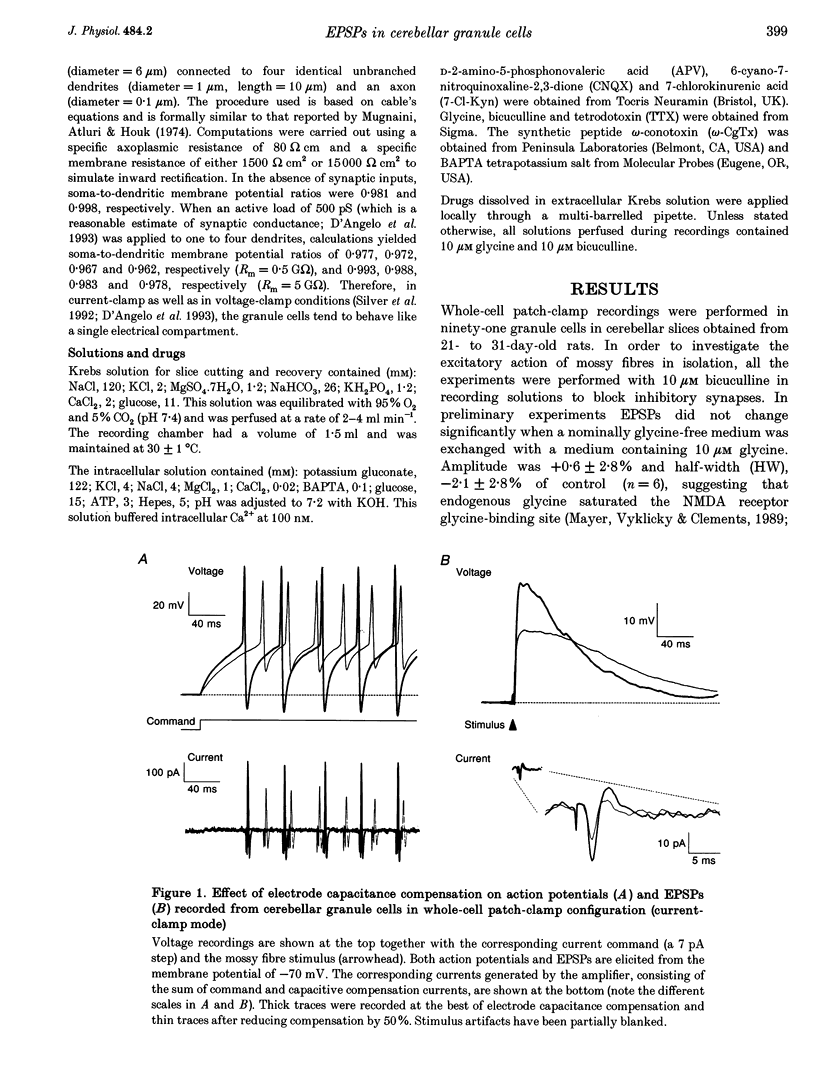
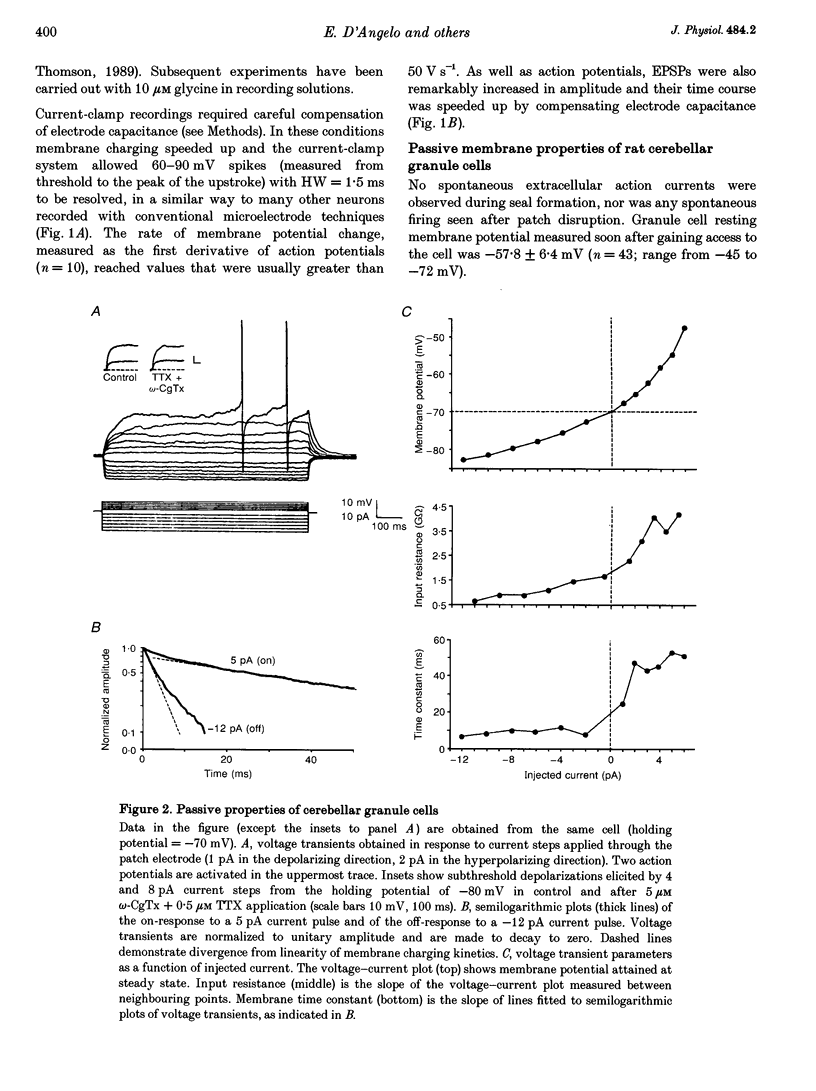
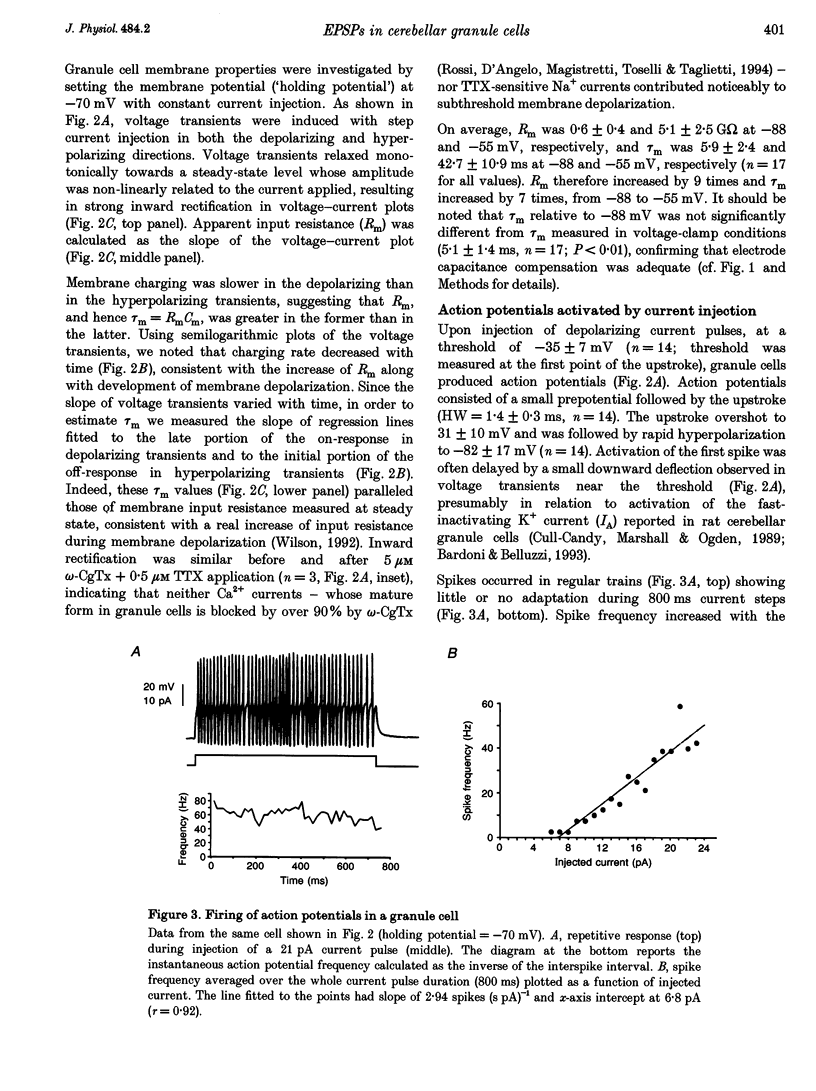
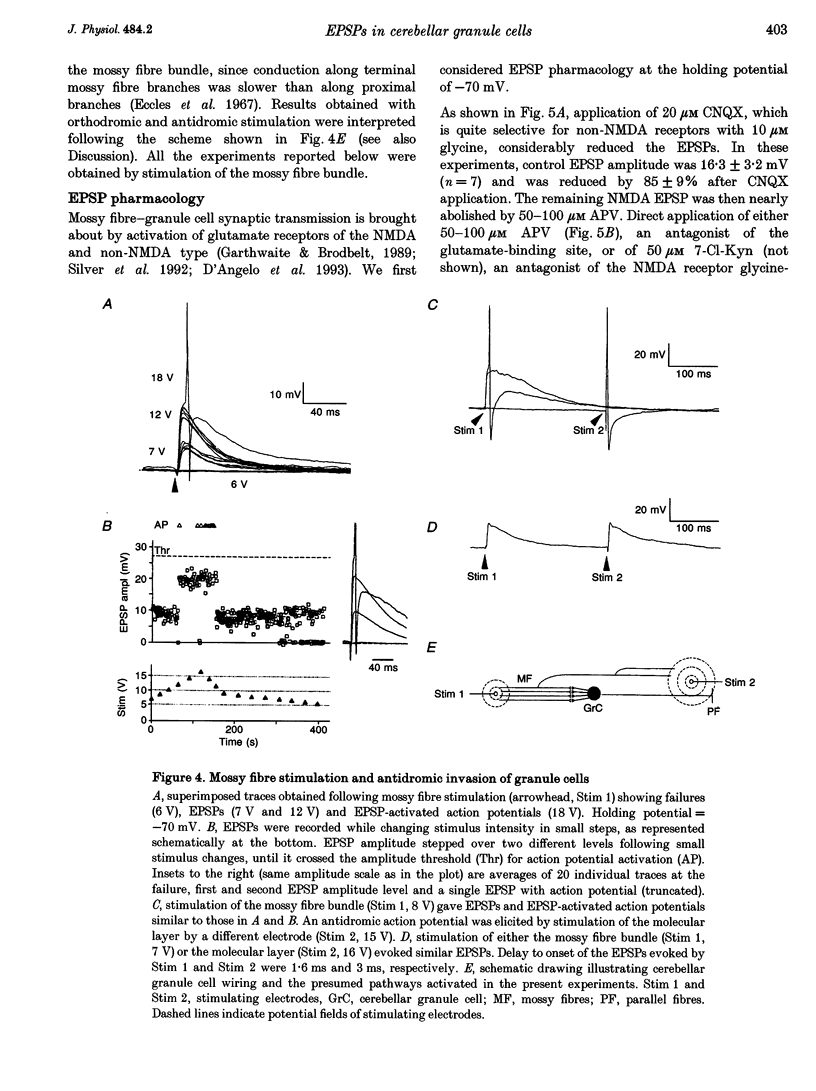
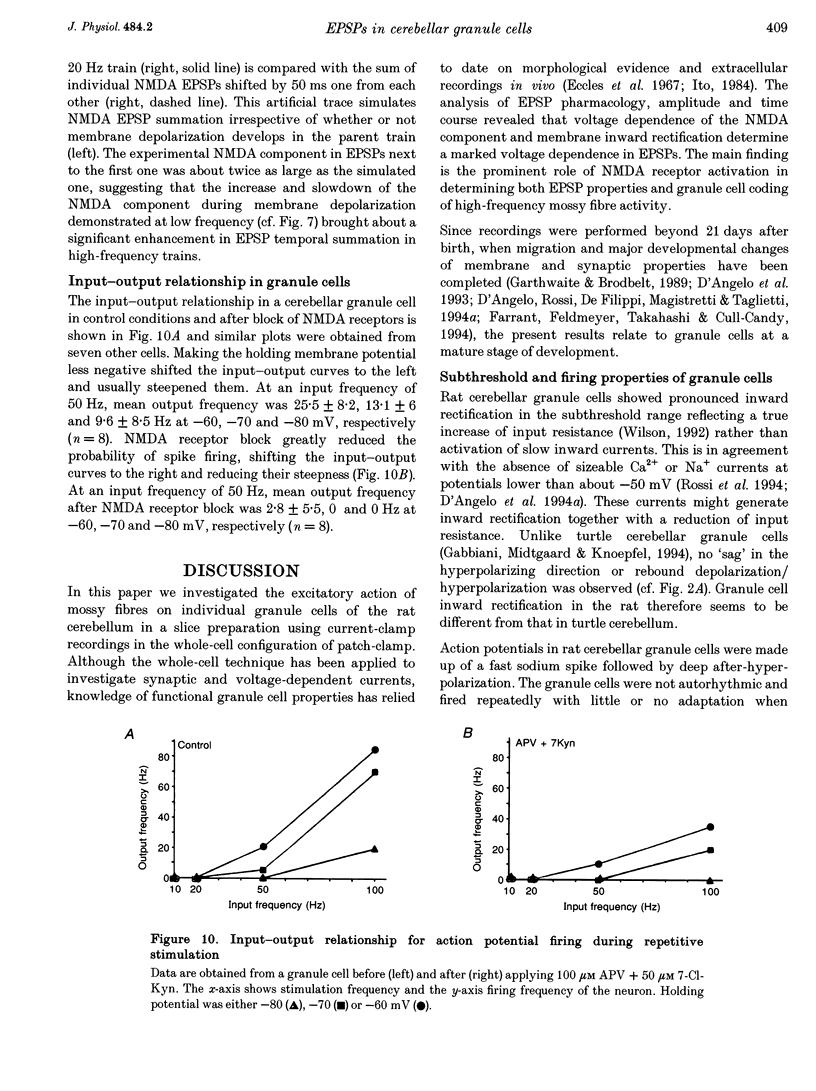
1. Current-clamp recordings were made in whole-cell patch-clamp configuration from ninety-one granule cells in parasagittal cerebellar slices obtained from 21- to 31-day-old rats. Recordings were performed at 30 degrees C. 2. Resting membrane potential was -58 +/- 6 mV (n = 43). The membrane voltage response to step current injection showed inward rectification consistent with increasing input resistance during membrane depolarization. Over -35 +/- 7 mV (n = 14) repetitive firing with little or no adaptation was activated. Spike frequency increased nearly linearly with injected current. 3. Unitary EPSPs obtained by stimulating the mossy fibre bundle had an amplitude of 11.4 +/- 2.1 mV (n = 22, holding potential = -75 mV). Synchronous activation of greater than one to two mossy fibres was needed to elicit action potentials. Antidromic stimulation elicited antidromic spikes and also EPSPs, presumably through a mossy fibre 'axon reflex'. 4. EPSPs were brought about by NMDA and non-NMDA receptor activation, accounting for about 70 and 30%, respectively, of peak amplitude at the holding potential of -70 mV. The EPSP decay conformed to passive membrane discharge after blocking the NMDA receptors. 5. No appreciable correlation was found between the time-to-peak and decay time constant of the EPSPs, consistent with the compact electrotonic structure of these neurons. 6. During membrane depolarization EPSP amplitude increased transiently, due to both a voltage-dependent increase of the NMDA component and inward rectification. In addition, EPSPs slowed down due to a slowdown of the NMDA component. 7. Temporal summation during high-frequency stimulation was sustained by NMDA receptors, whose contribution to depolarization tended to prevail over that of non-NMDA receptors during the trains. A block of the NMDA receptors resulted in reduced depolarization and output spike frequency. 8. This study, as well as extending previous knowledge to the intracellular level in vivo, provides evidence for a primary role of NMDA receptors in determining mossy fibre excitation of granule cells. It is suggested that the marked voltage dependence of the EPSP time course, which was mainly caused by voltage dependence in NMDA conductance, promotes the NMDA receptor-dependent enhancement of granule cell coding observed during repetitive mossy fibre activity.
Full text
PDF


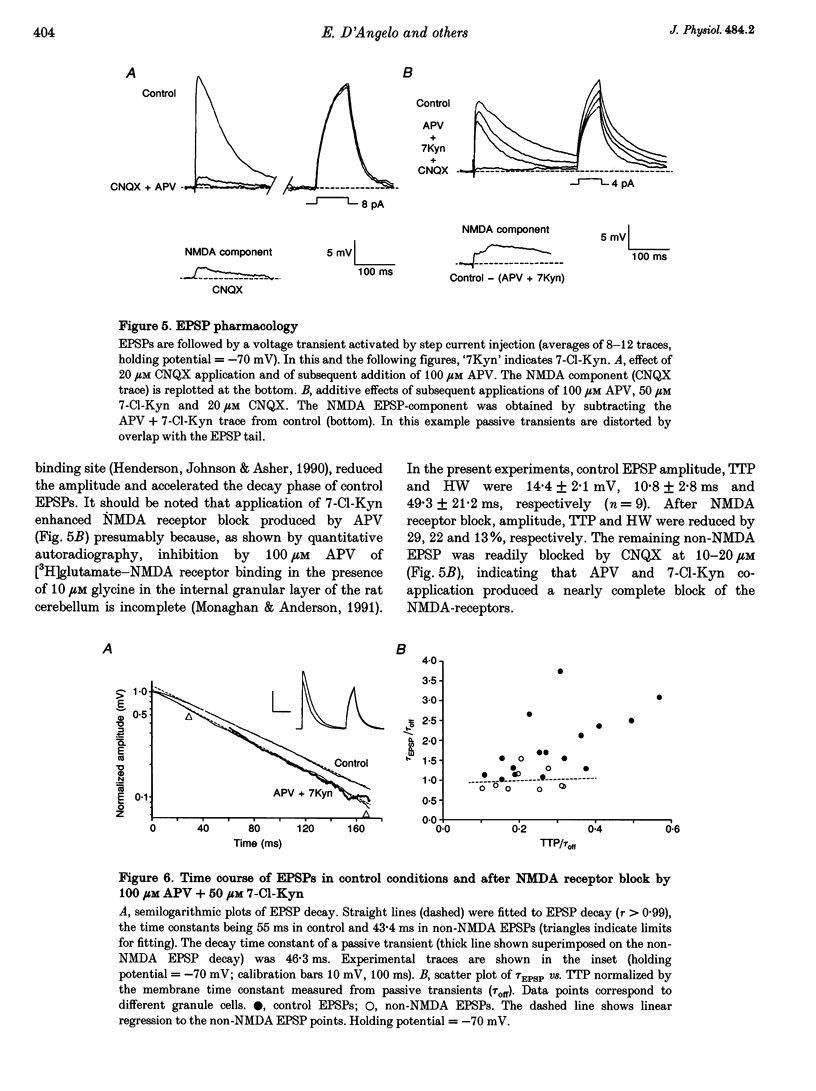
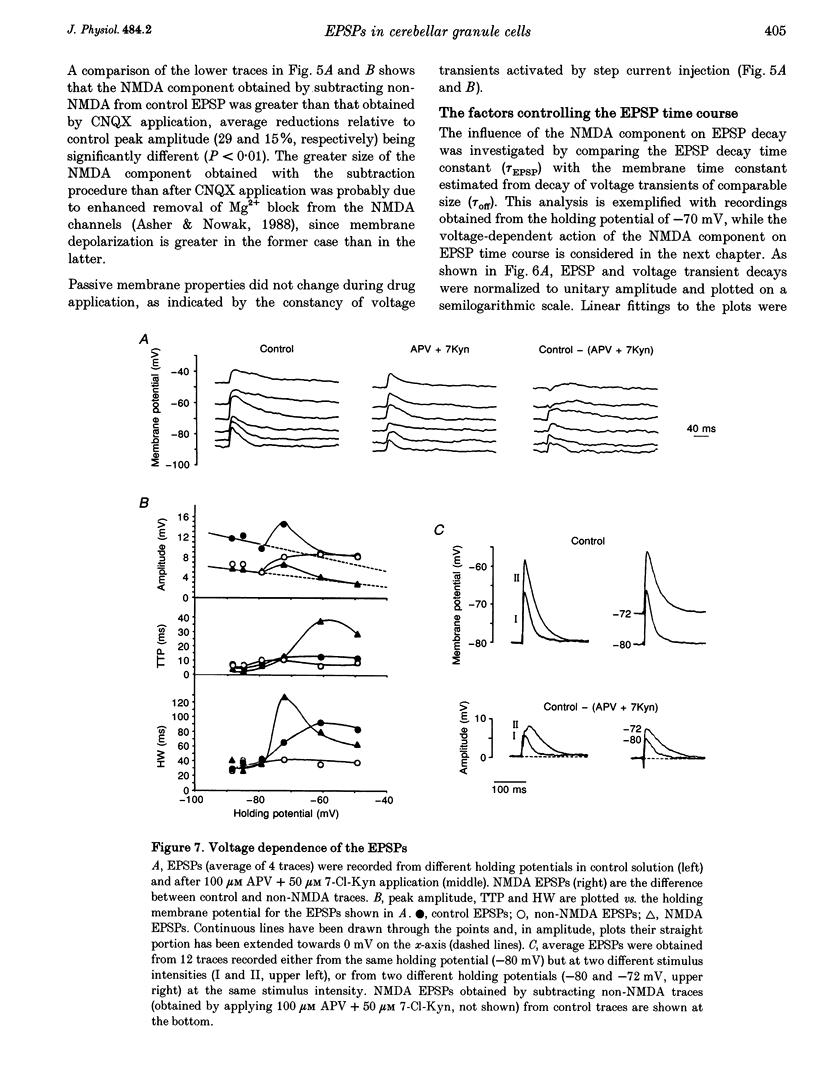
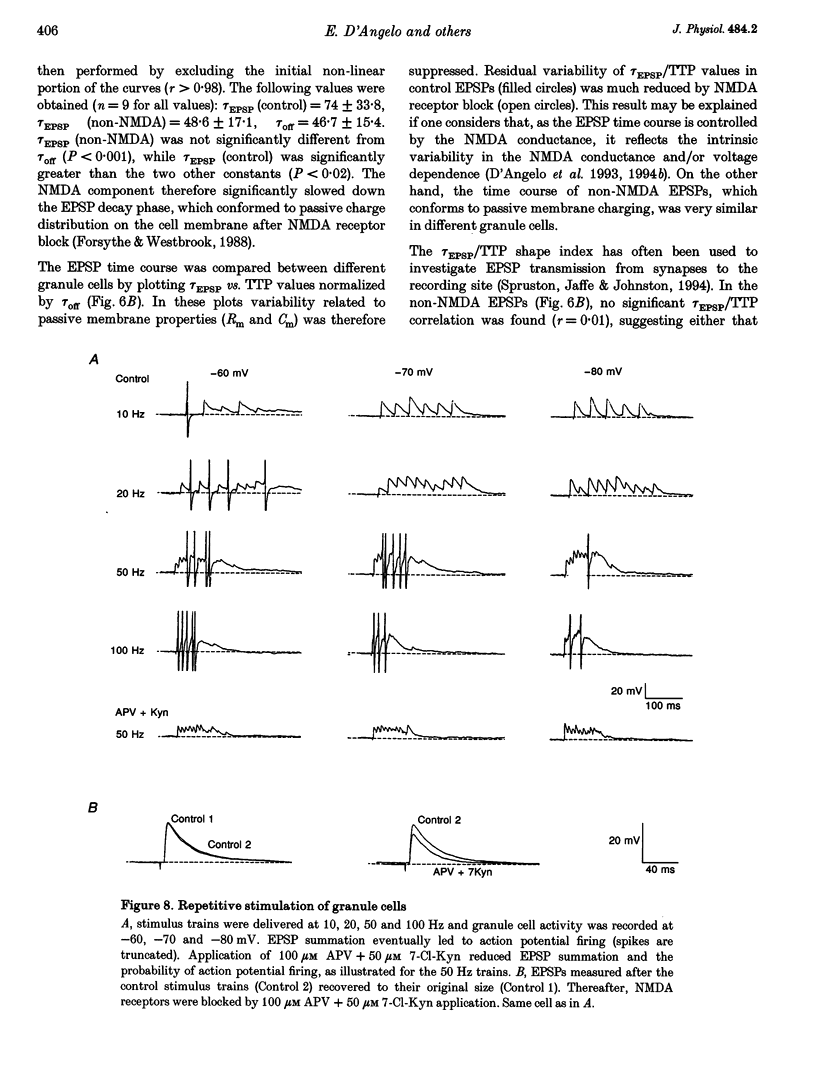

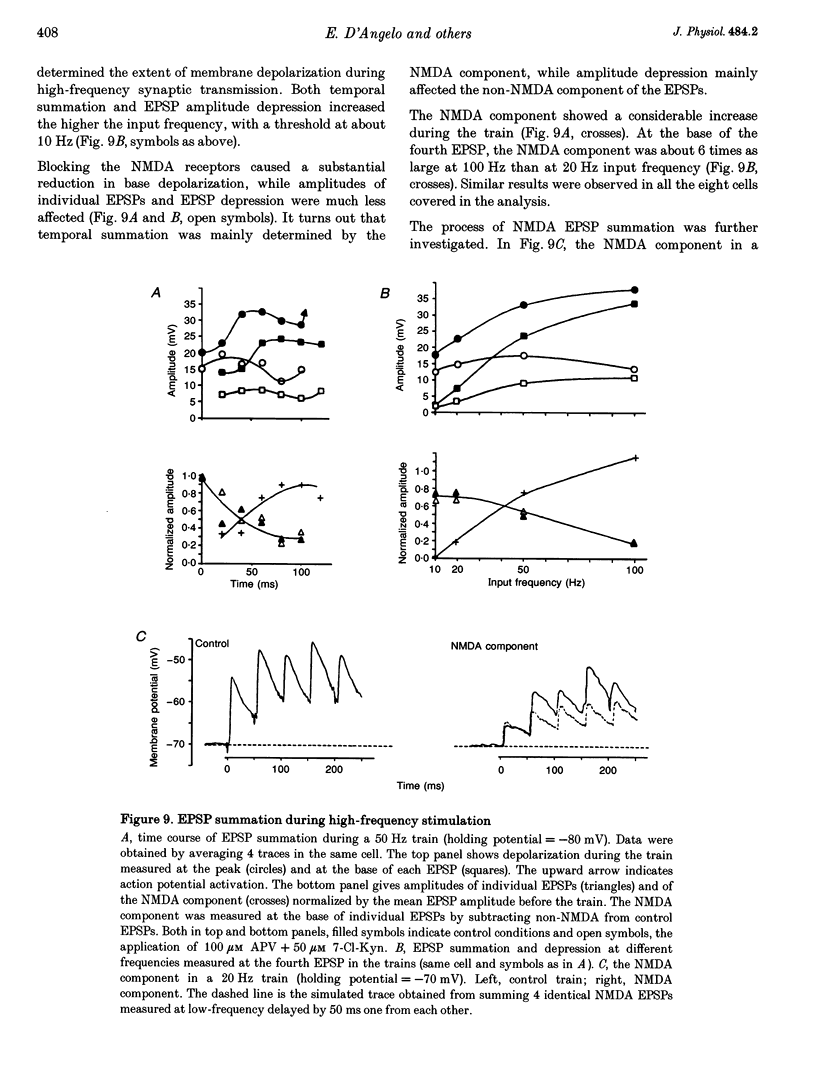




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascher P., Nowak L. The role of divalent cations in the N-methyl-D-aspartate responses of mouse central neurones in culture. J Physiol. 1988 May;399:247–266. doi: 10.1113/jphysiol.1988.sp017078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour B. Synaptic currents evoked in Purkinje cells by stimulating individual granule cells. Neuron. 1993 Oct;11(4):759–769. doi: 10.1016/0896-6273(93)90085-6. [DOI] [PubMed] [Google Scholar]
- Bardoni R., Belluzzi O. Kinetic study and numerical reconstruction of A-type current in granule cells of rat cerebellar slices. J Neurophysiol. 1993 Jun;69(6):2222–2231. doi: 10.1152/jn.1993.69.6.2222. [DOI] [PubMed] [Google Scholar]
- Collingridge G. L., Herron C. E., Lester R. A. Frequency-dependent N-methyl-D-aspartate receptor-mediated synaptic transmission in rat hippocampus. J Physiol. 1988 May;399:301–312. doi: 10.1113/jphysiol.1988.sp017081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Herron C. E., Lester R. A. Synaptic activation of N-methyl-D-aspartate receptors in the Schaffer collateral-commissural pathway of rat hippocampus. J Physiol. 1988 May;399:283–300. doi: 10.1113/jphysiol.1988.sp017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L. The Sharpey-Schafer Prize Lecture. The mechanism of induction of NMDA receptor-dependent long-term potentiation in the hippocampus. Exp Physiol. 1992 Nov;77(6):771–797. doi: 10.1113/expphysiol.1992.sp003645. [DOI] [PubMed] [Google Scholar]
- Connors B. W., Gutnick M. J. Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci. 1990 Mar;13(3):99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Marshall C. G., Ogden D. Voltage-activated membrane currents in rat cerebellar granule neurones. J Physiol. 1989 Jul;414:179–199. doi: 10.1113/jphysiol.1989.sp017683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo E., Rossi P., De Filippi G., Magistretti J., Taglietti V. The relationship between synaptogenesis and expression of voltage-dependent currents in cerebellar granule cells in situ. J Physiol Paris. 1994;88(3):197–207. doi: 10.1016/0928-4257(94)90006-x. [DOI] [PubMed] [Google Scholar]
- D'Angelo E., Rossi P., Garthwaite J. Dual-component NMDA receptor currents at a single central synapse. Nature. 1990 Aug 2;346(6283):467–470. doi: 10.1038/346467a0. [DOI] [PubMed] [Google Scholar]
- D'Angelo E., Rossi P., Taglietti V. Different proportions of N-methyl-D-aspartate and non-N-methyl-D-aspartate receptor currents at the mossy fibre-granule cell synapse of developing rat cerebellum. Neuroscience. 1993 Mar;53(1):121–130. doi: 10.1016/0306-4522(93)90290-v. [DOI] [PubMed] [Google Scholar]
- D'Angelo E., Rossi P., Taglietti V. Voltage-dependent kinetics of N-methyl-D-aspartate synaptic currents in rat cerebellar granule cells. Eur J Neurosci. 1994 Apr 1;6(4):640–645. doi: 10.1111/j.1460-9568.1994.tb00309.x. [DOI] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Farrant M., Feldmeyer D., Takahashi T., Cull-Candy S. G. NMDA-receptor channel diversity in the developing cerebellum. Nature. 1994 Mar 24;368(6469):335–339. doi: 10.1038/368335a0. [DOI] [PubMed] [Google Scholar]
- Forsythe I. D., Westbrook G. L. Slow excitatory postsynaptic currents mediated by N-methyl-D-aspartate receptors on cultured mouse central neurones. J Physiol. 1988 Feb;396:515–533. doi: 10.1113/jphysiol.1988.sp016975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani F., Midtgaard J., Knöpfel T. Synaptic integration in a model of cerebellar granule cells. J Neurophysiol. 1994 Aug;72(2):999–1009. doi: 10.1152/jn.1994.72.2.999. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Brodbelt A. R. Synaptic activation of N-methyl-D-aspartate and non-N-methyl-D-aspartate receptors in the mossy fibre pathway in adult and immature rat cerebellar slices. Neuroscience. 1989;29(2):401–412. doi: 10.1016/0306-4522(89)90067-5. [DOI] [PubMed] [Google Scholar]
- Henderson G., Johnson J. W., Ascher P. Competitive antagonists and partial agonists at the glycine modulatory site of the mouse N-methyl-D-aspartate receptor. J Physiol. 1990 Nov;430:189–212. doi: 10.1113/jphysiol.1990.sp018288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S., Nicoll R. A., Perkel D. J., Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol. 1990 Mar;422:203–225. doi: 10.1113/jphysiol.1990.sp017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kase M., Miller D. C., Noda H. Discharges of Purkinje cells and mossy fibres in the cerebellar vermis of the monkey during saccadic eye movements and fixation. J Physiol. 1980 Mar;300:539–555. doi: 10.1113/jphysiol.1980.sp013178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller B. U., Konnerth A., Yaari Y. Patch clamp analysis of excitatory synaptic currents in granule cells of rat hippocampus. J Physiol. 1991 Apr;435:275–293. doi: 10.1113/jphysiol.1991.sp018510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J. D., Jones R. S. A reevaluation of excitatory amino acid-mediated synaptic transmission in rat dentate gyrus. J Neurophysiol. 1990 Jul;64(1):119–132. doi: 10.1152/jn.1990.64.1.119. [DOI] [PubMed] [Google Scholar]
- Llano I., Gerschenfeld H. M. Inhibitory synaptic currents in stellate cells of rat cerebellar slices. J Physiol. 1993 Aug;468:177–200. doi: 10.1113/jphysiol.1993.sp019766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Vyklicky L., Jr, Clements J. Regulation of NMDA receptor desensitization in mouse hippocampal neurons by glycine. Nature. 1989 Mar 30;338(6214):425–427. doi: 10.1038/338425a0. [DOI] [PubMed] [Google Scholar]
- Midtgaard J. Membrane properties and synaptic responses of Golgi cells and stellate cells in the turtle cerebellum in vitro. J Physiol. 1992 Nov;457:329–354. doi: 10.1113/jphysiol.1992.sp019381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H., Sprengel R., Schoepfer R., Herb A., Higuchi M., Lomeli H., Burnashev N., Sakmann B., Seeburg P. H. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992 May 22;256(5060):1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Mugnaini E., Atluri R. L., Houk J. C. Fine structure of granular layer in turtle cerebellum with emphasis on large glomeruli. J Neurophysiol. 1974 Jan;37(1):1–29. doi: 10.1152/jn.1974.37.1.1. [DOI] [PubMed] [Google Scholar]
- Redman S. Quantal analysis of synaptic potentials in neurons of the central nervous system. Physiol Rev. 1990 Jan;70(1):165–198. doi: 10.1152/physrev.1990.70.1.165. [DOI] [PubMed] [Google Scholar]
- Rossi P., D'Angelo E., Magistretti J., Toselli M., Taglietti V. Age-dependent expression of high-voltage activated calcium currents during cerebellar granule cell development in situ. Pflugers Arch. 1994 Nov;429(1):107–116. doi: 10.1007/BF02584036. [DOI] [PubMed] [Google Scholar]
- Silver R. A., Traynelis S. F., Cull-Candy S. G. Rapid-time-course miniature and evoked excitatory currents at cerebellar synapses in situ. Nature. 1992 Jan 9;355(6356):163–166. doi: 10.1038/355163a0. [DOI] [PubMed] [Google Scholar]
- Spruston N., Jaffe D. B., Johnston D. Dendritic attenuation of synaptic potentials and currents: the role of passive membrane properties. Trends Neurosci. 1994 Apr;17(4):161–166. doi: 10.1016/0166-2236(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Stern P., Edwards F. A., Sakmann B. Fast and slow components of unitary EPSCs on stellate cells elicited by focal stimulation in slices of rat visual cortex. J Physiol. 1992 Apr;449:247–278. doi: 10.1113/jphysiol.1992.sp019085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutor B., Hablitz J. J. EPSPs in rat neocortical neurons in vitro. II. Involvement of N-methyl-D-aspartate receptors in the generation of EPSPs. J Neurophysiol. 1989 Mar;61(3):621–634. doi: 10.1152/jn.1989.61.3.621. [DOI] [PubMed] [Google Scholar]
- Thomson A. M. Glycine modulation of the NMDA receptor/channel complex. Trends Neurosci. 1989 Sep;12(9):349–353. doi: 10.1016/0166-2236(89)90042-8. [DOI] [PubMed] [Google Scholar]
- Trussell L. O., Zhang S., Raman I. M. Desensitization of AMPA receptors upon multiquantal neurotransmitter release. Neuron. 1993 Jun;10(6):1185–1196. doi: 10.1016/0896-6273(93)90066-z. [DOI] [PubMed] [Google Scholar]


