Abstract
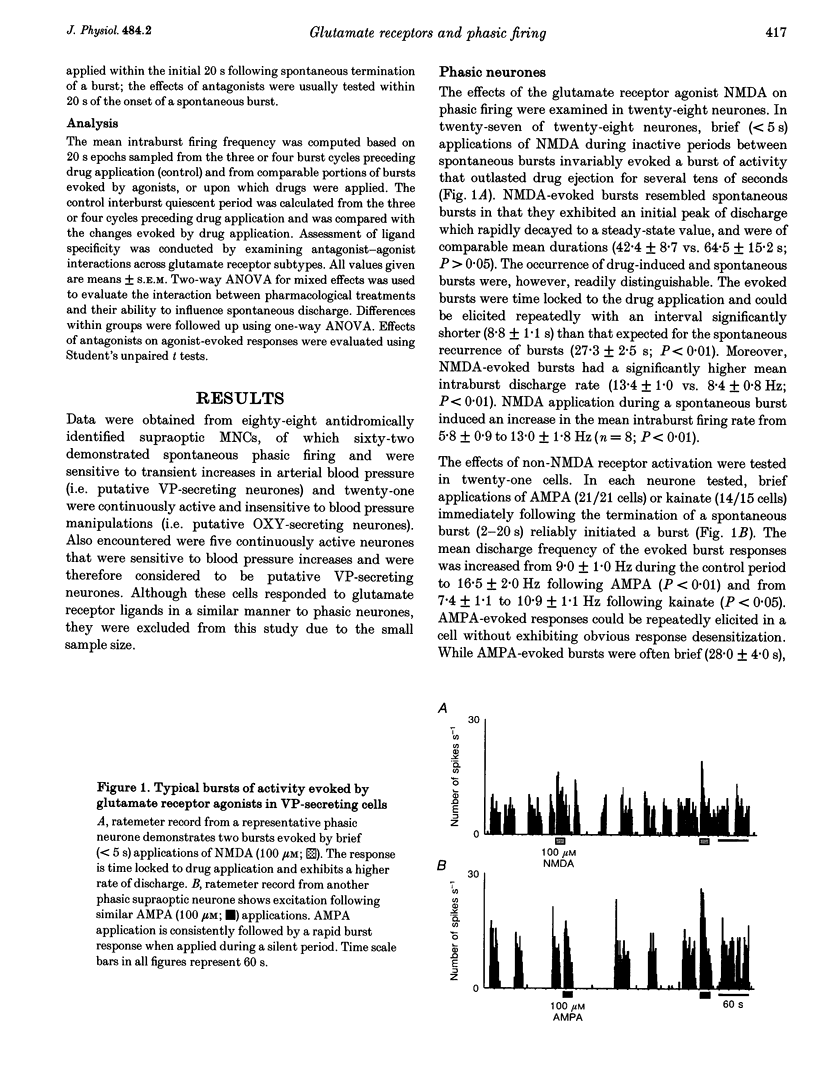
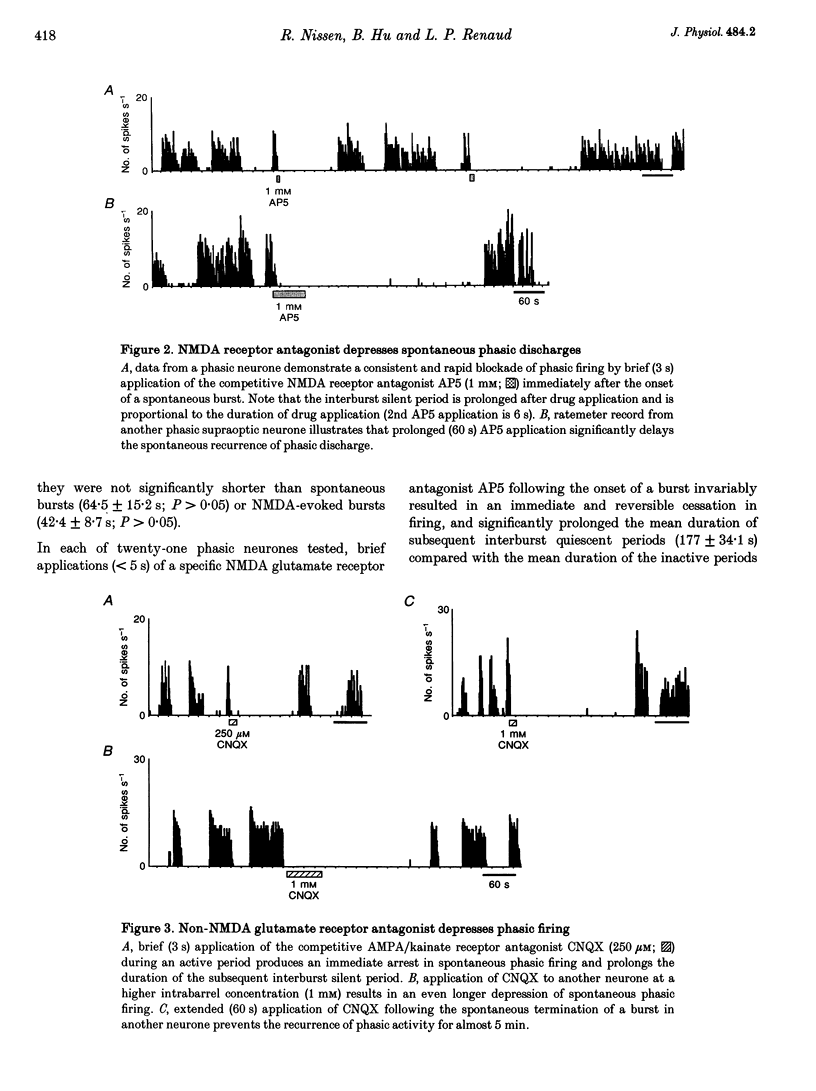
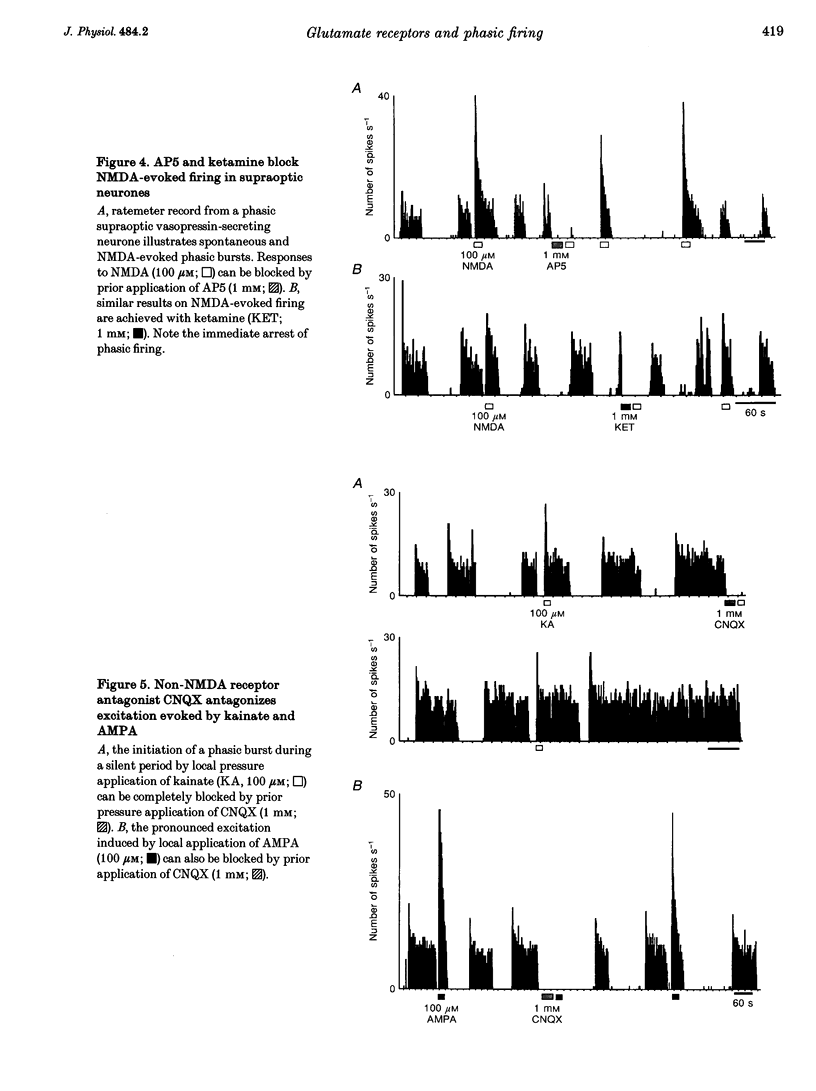
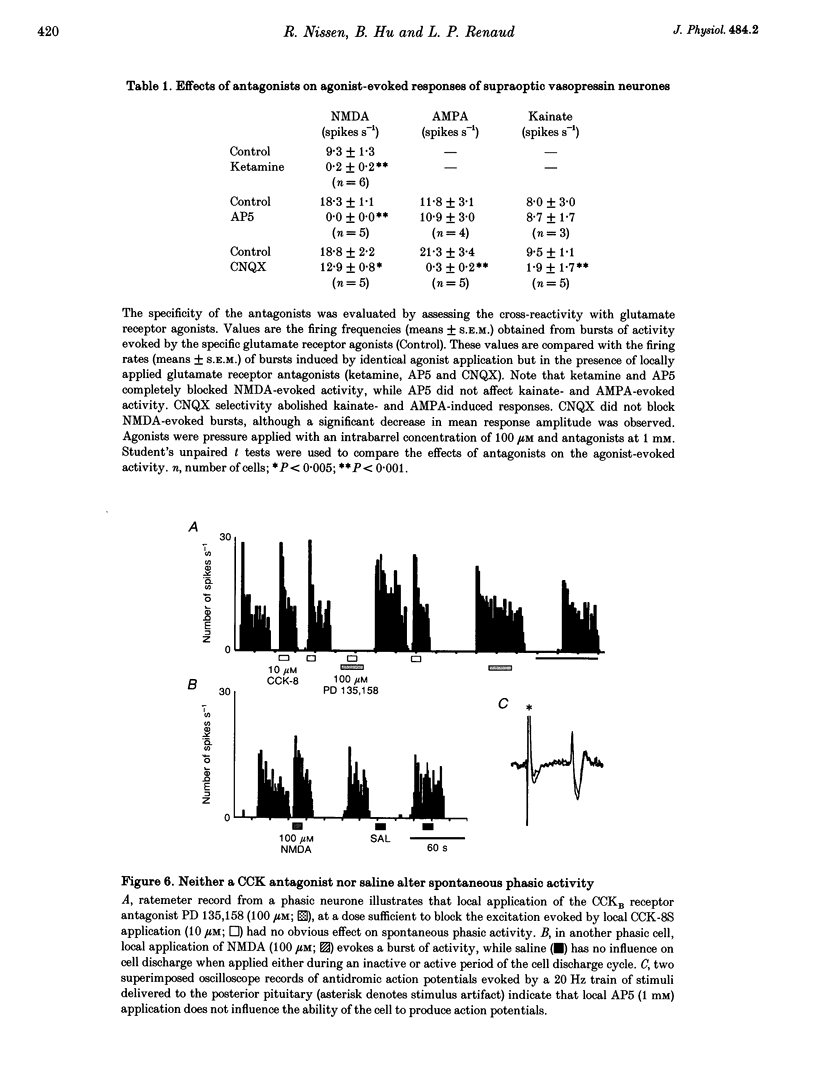
1. Vasopressin-secreting neurones in the rat hypothalamic supraoptic nucleus display patterned spontaneous phasic activity, which is apparently maintained in vivo through yet unidentified neurotransmitter system(s). The present investigation used extracellular recording techniques in anaesthetized Long-Evans rats to evaluate whether the neurotransmitter mechanism underlying phasic firing is provided via a family of ionotropic glutamate receptors. 2. N-Methyl-D-aspartate (NMDA) reliably evoked bursts of activity in twenty-seven of twenty-eight phasic neurones. Amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid (AMPA) and kainate also elicited pronounced excitations in twenty-one of twenty-one and and fourteen of fifteen phasic cells, respectively. 3. A rapid blockade of on-going phasic activity was consistently induced following brief applications of both NMDA and non-NMDA receptor antagonists; extended application of antagonists resulted in prolonged silent periods, during which phasic activity failed to recur for minutes. Neither saline nor a cholecystokinin receptor antagonist influenced cell firing. 4. In contrast to putative vasopressin cells, application of NMDA receptor ligands did not affect the spontaneous activity in most putative oxytocin-secreting neurones, whereas kainate and AMPA potently excited seven of nine and four of five putative oxytocin cells, respectively. 5. These results imply that the maintenance of spontaneous phasic discharges in vivo in supraoptic vasopressin-secreting neurones requires tonic synaptic activation involving both NMDA and non-NMDA glutamate receptors. In putative oxytocin-secreting neurones, spontaneous firing appears to be predominantly regulated by non-NMDA receptors. Glutamatergic innervations may be in a unique position to influence the genesis of patterned electrical activity in supraoptic vasopressin neurones.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrew R. D., Dudek F. E. Burst discharge in mammalian neuroendocrine cells involves an intrinsic regenerative mechanism. Science. 1983 Sep 9;221(4615):1050–1052. doi: 10.1126/science.6879204. [DOI] [PubMed] [Google Scholar]
- Arnauld E., Cirino M., Layton B. S., Renaud L. P. Contrasting actions of amino acids, acetylcholine, noradrenaline and leucine enkephalin on the excitability of supraoptic vasopressin-secreting neurons. A microiontophoretic study in the rat. Neuroendocrinology. 1983;36(3):187–196. doi: 10.1159/000123455. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Crayton J. W., Nicoll R. A. Noradrenaline and acetylcholine responses of supraoptic neurosecretory cells. J Physiol. 1971 Oct;218(1):19–32. doi: 10.1113/jphysiol.1971.sp009602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicknell R. J. Optimizing release from peptide hormone secretory nerve terminals. J Exp Biol. 1988 Sep;139:51–65. doi: 10.1242/jeb.139.1.51. [DOI] [PubMed] [Google Scholar]
- Bioulac B., Gaffori O., Harris M., Vincent J. D. Effects of acetylcholine, sodium glutamate and GABA on the discharge of supraoptic neurons in the rat. Brain Res. 1978 Oct 6;154(1):159–162. doi: 10.1016/0006-8993(78)91064-8. [DOI] [PubMed] [Google Scholar]
- Bourque C. W. Calcium-dependent spike after-current induces burst firing in magnocellular neurosecretory cells. Neurosci Lett. 1986 Oct 8;70(2):204–209. doi: 10.1016/0304-3940(86)90464-7. [DOI] [PubMed] [Google Scholar]
- Cazalis M., Dayanithi G., Nordmann J. J. The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J Physiol. 1985 Dec;369:45–60. doi: 10.1113/jphysiol.1985.sp015887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry M. A., Dyball R. E., Honda K., Wright N. C. The role of interconnection between supraoptic nucleus and anterior third ventricular region in osmoregulation in the rat. J Physiol. 1989 Mar;410:123–135. doi: 10.1113/jphysiol.1989.sp017524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge G. L., Singer W. Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol Sci. 1990 Jul;11(7):290–296. doi: 10.1016/0165-6147(90)90011-v. [DOI] [PubMed] [Google Scholar]
- Day T. A., Renaud L. P. Electrophysiological evidence that noradrenergic afferents selectively facilitate the activity of supraoptic vasopressin neurons. Brain Res. 1984 Jun 15;303(2):233–240. doi: 10.1016/0006-8993(84)91209-5. [DOI] [PubMed] [Google Scholar]
- Day T. A., Renaud L. P., Sibbald J. R. Excitation of supraoptic vasopressin cells by stimulation of the A1 noradrenaline cell group: failure to demonstrate role for established adrenergic or amino acid receptors. Brain Res. 1990 May 14;516(1):91–98. doi: 10.1016/0006-8993(90)90901-m. [DOI] [PubMed] [Google Scholar]
- Dreifuss J. J., Kelly J. S. The activity of identified supraoptic neurones and their response to acetylcholine applied by iontophoresis. J Physiol. 1972 Jan;220(1):105–118. doi: 10.1113/jphysiol.1972.sp009697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton A., Dyball R. E. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol. 1979 May;290(2):433–440. doi: 10.1113/jphysiol.1979.sp012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyball R. E., Prilusky J. Responses of supraoptic neurones in the intact and deafferented rat hypothalamus to injections of hypertonic sodium chloride. J Physiol. 1981 Feb;311:443–452. doi: 10.1113/jphysiol.1981.sp013596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K. R., Ronnekleiv O. K., Kelly M. J. Electrophysiology of guinea-pig supraoptic neurones: role of a hyperpolarization-activated cation current in phasic firing. J Physiol. 1993 Jan;460:407–425. doi: 10.1113/jphysiol.1993.sp019478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribkoff V. K., Dudek F. E. Effects of excitatory amino acid antagonists on synaptic responses of supraoptic neurons in slices of rat hypothalamus. J Neurophysiol. 1990 Jan;63(1):60–71. doi: 10.1152/jn.1990.63.1.60. [DOI] [PubMed] [Google Scholar]
- Gribkoff V. K. Electrophysiological evidence for N-methyl-D-aspartate excitatory amino acid receptors in the rat supraoptic nucleus in vitro. Neurosci Lett. 1991 Oct 14;131(2):260–262. doi: 10.1016/0304-3940(91)90628-7. [DOI] [PubMed] [Google Scholar]
- Hu B., Bourque C. W. NMDA receptor-mediated rhythmic bursting activity in rat supraoptic nucleus neurones in vitro. J Physiol. 1992 Dec;458:667–687. doi: 10.1113/jphysiol.1992.sp019440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester R. A., Quarum M. L., Parker J. D., Weber E., Jahr C. E. Interaction of 6-cyano-7-nitroquinoxaline-2,3-dione with the N-methyl-D-aspartate receptor-associated glycine binding site. Mol Pharmacol. 1989 May;35(5):565–570. [PubMed] [Google Scholar]
- Meeker R. B., Swanson D. J., Hayward J. N. Light and electron microscopic localization of glutamate immunoreactivity in the supraoptic nucleus of the rat hypothalamus. Neuroscience. 1989;33(1):157–167. doi: 10.1016/0306-4522(89)90318-7. [DOI] [PubMed] [Google Scholar]
- Nissen R., Hu B., Renaud L. P. N-methyl-D-aspartate receptor antagonist ketamine selectively attenuates spontaneous phasic activity of supraoptic vasopressin neurons in vivo. Neuroscience. 1994 Mar;59(1):115–120. doi: 10.1016/0306-4522(94)90103-1. [DOI] [PubMed] [Google Scholar]
- Palkovits M., Láng T., Patthy A., Elekes I. Distribution and stress-induced increase of glutamate and aspartate levels in discrete brain nuclei of rats. Brain Res. 1986 May 14;373(1-2):252–257. doi: 10.1016/0006-8993(86)90339-2. [DOI] [PubMed] [Google Scholar]
- Petralia R. S., Yokotani N., Wenthold R. J. Light and electron microscope distribution of the NMDA receptor subunit NMDAR1 in the rat nervous system using a selective anti-peptide antibody. J Neurosci. 1994 Feb;14(2):667–696. doi: 10.1523/JNEUROSCI.14-02-00667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle J. C., Bourque C. W., Renaud L. P. Alpha 1-adrenergic receptor activation depolarizes rat supraoptic neurosecretory neurons in vitro. Am J Physiol. 1986 Sep;251(3 Pt 2):R569–R574. doi: 10.1152/ajpregu.1986.251.3.R569. [DOI] [PubMed] [Google Scholar]
- Renaud L. P., Bourque C. W. Neurophysiology and neuropharmacology of hypothalamic magnocellular neurons secreting vasopressin and oxytocin. Prog Neurobiol. 1991;36(2):131–169. doi: 10.1016/0301-0082(91)90020-2. [DOI] [PubMed] [Google Scholar]
- Sah P., Hestrin S., Nicoll R. A. Tonic activation of NMDA receptors by ambient glutamate enhances excitability of neurons. Science. 1989 Nov 10;246(4931):815–818. doi: 10.1126/science.2573153. [DOI] [PubMed] [Google Scholar]
- Smithson K. G., Weiss M. L., Hatton G. I. Supraoptic nucleus afferents from the main olfactory bulb--I. Anatomical evidence from anterograde and retrograde tracers in rat. Neuroscience. 1989;31(2):277–287. doi: 10.1016/0306-4522(89)90373-4. [DOI] [PubMed] [Google Scholar]
- Yang C. R., Senatorov V. V., Renaud L. P. Organum vasculosum lamina terminalis-evoked postsynaptic responses in rat supraoptic neurones in vitro. J Physiol. 1994 May 15;477(Pt 1):59–74. doi: 10.1113/jphysiol.1994.sp020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol A. N. Glutamate and aspartate immunoreactivity in hypothalamic presynaptic axons. J Neurosci. 1991 Jul;11(7):2087–2101. doi: 10.1523/JNEUROSCI.11-07-02087.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


