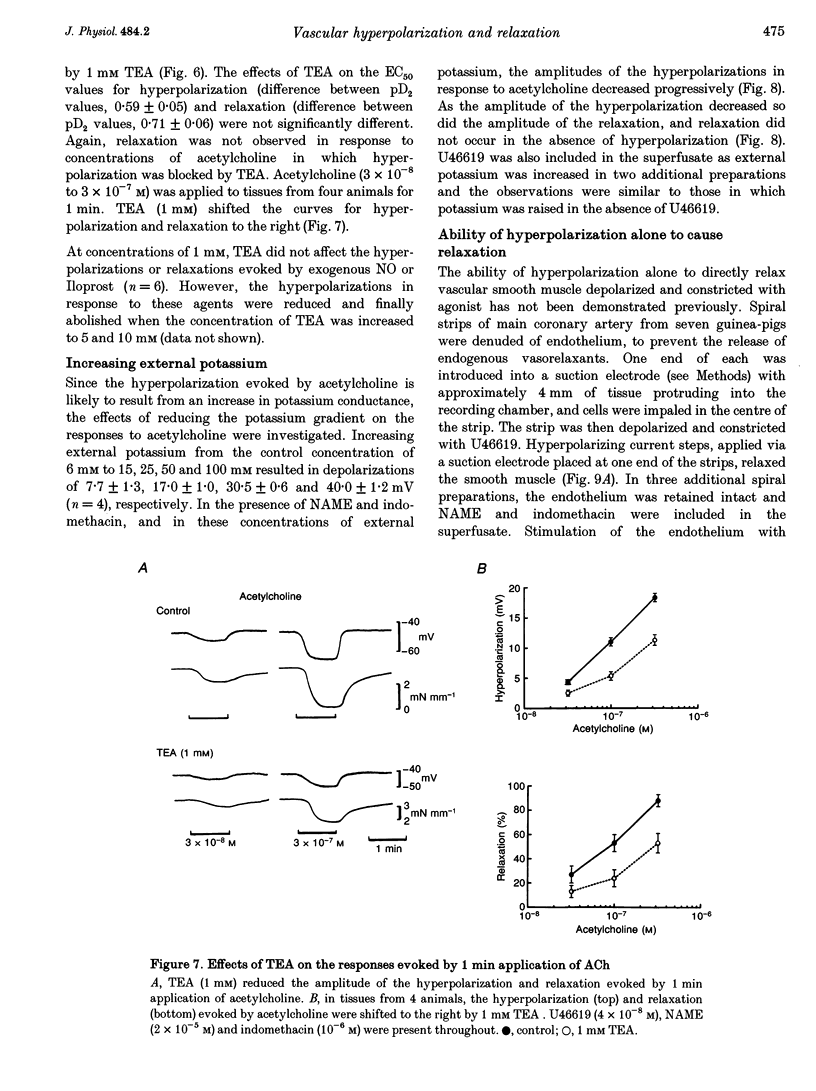
Abstract
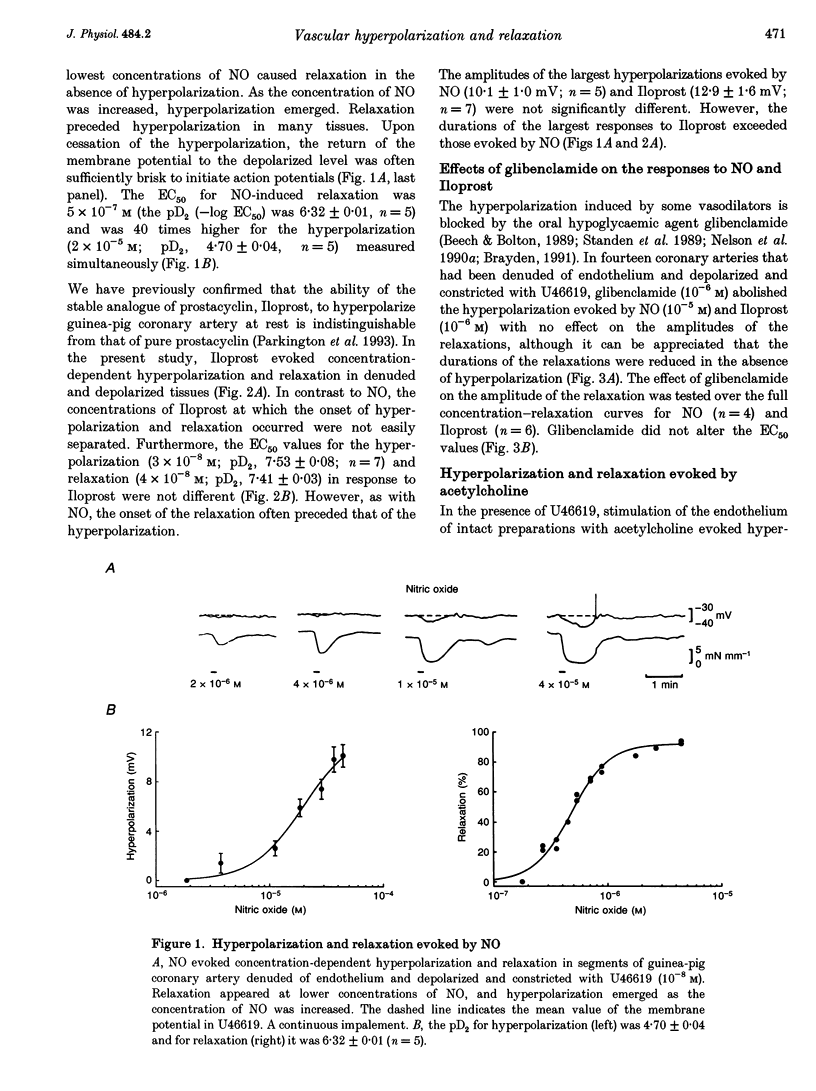
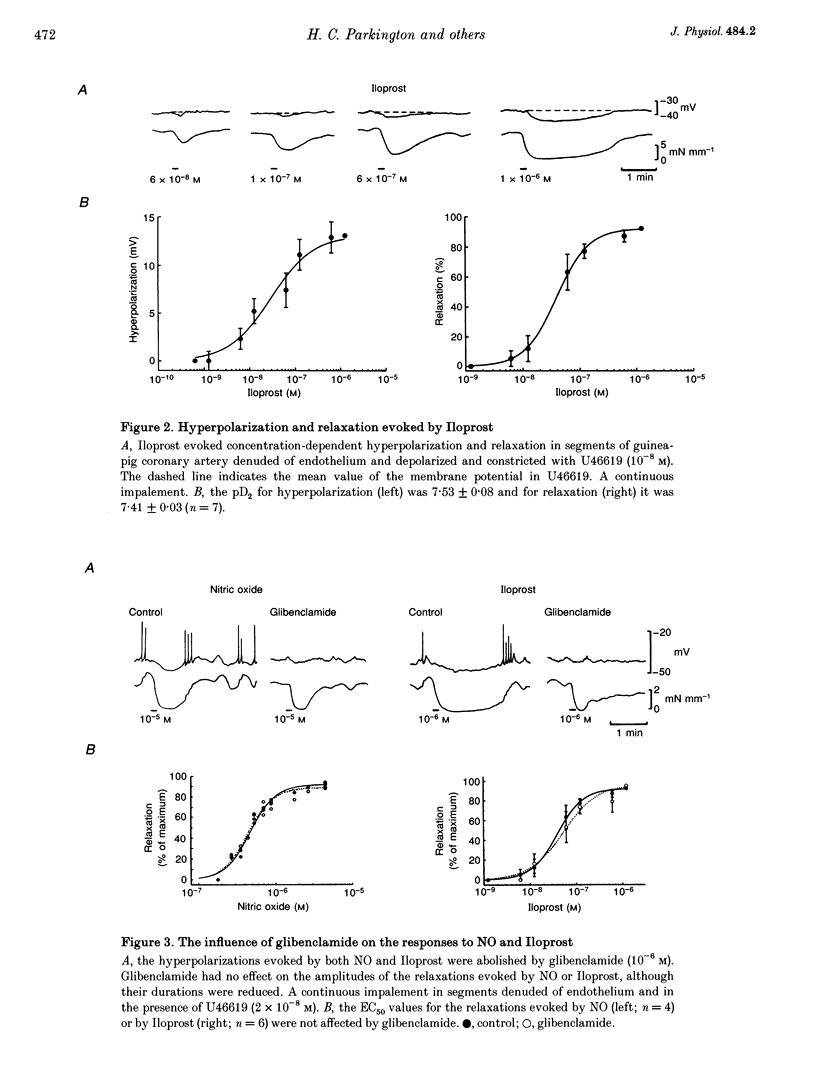
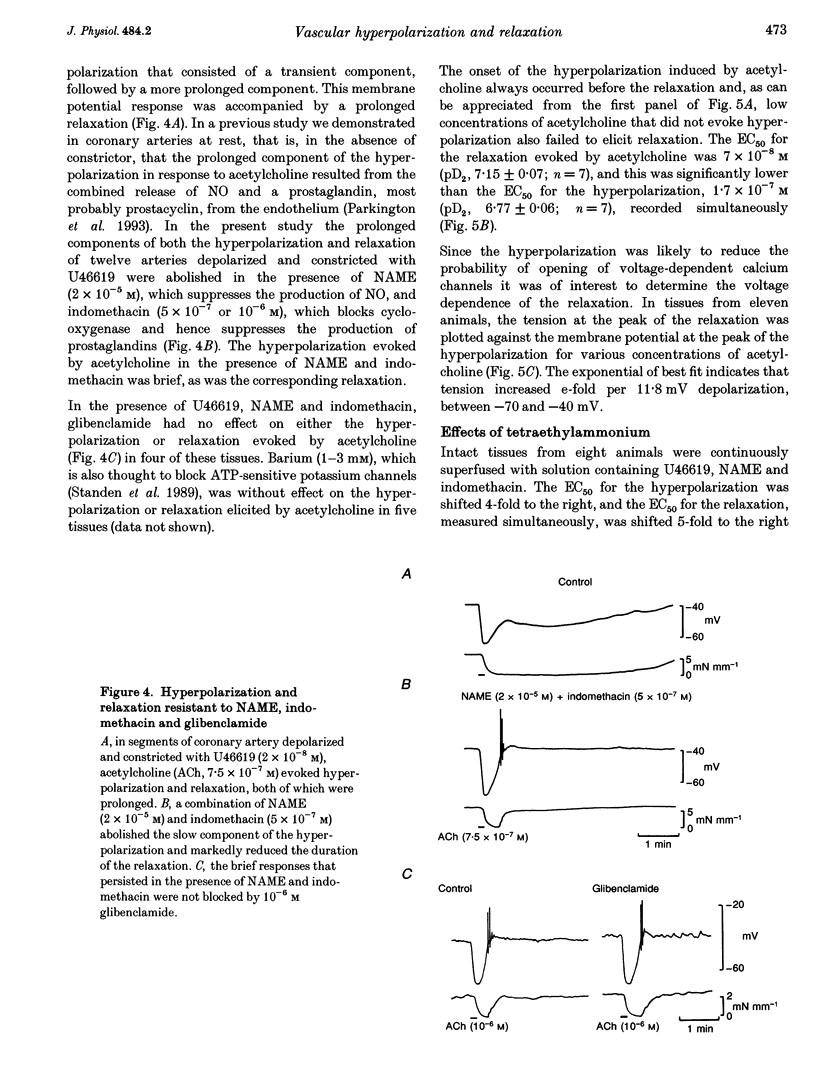
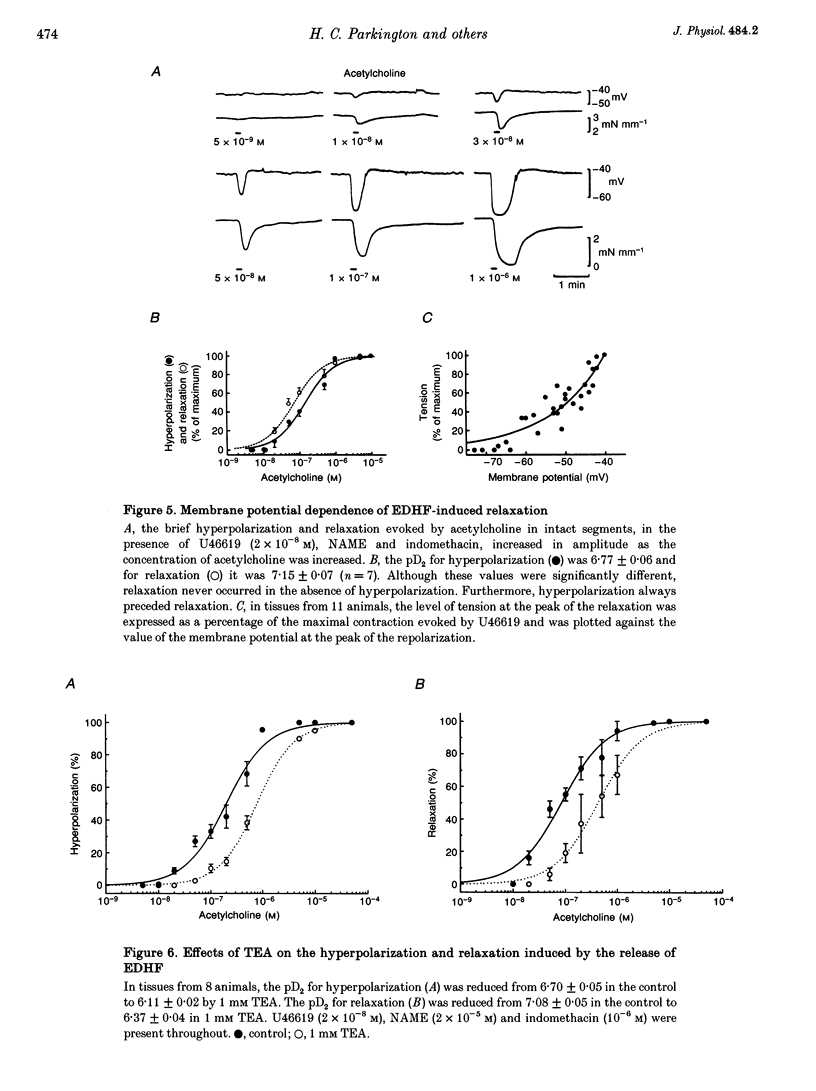
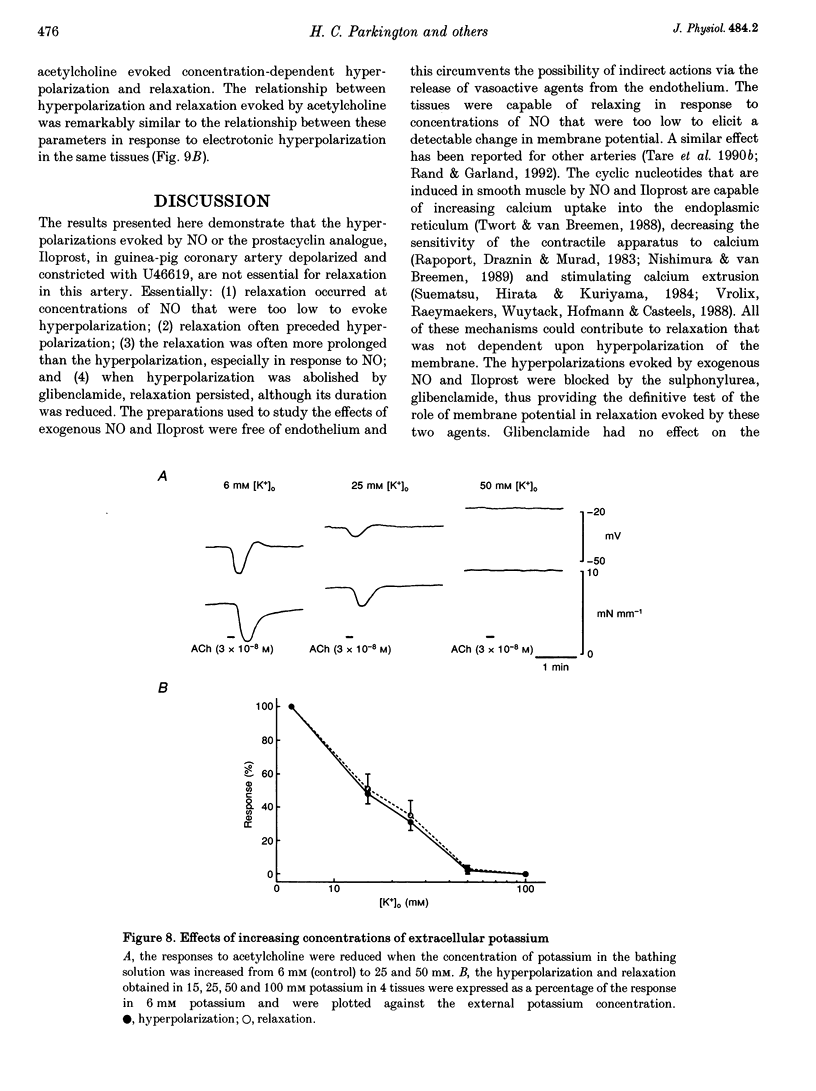
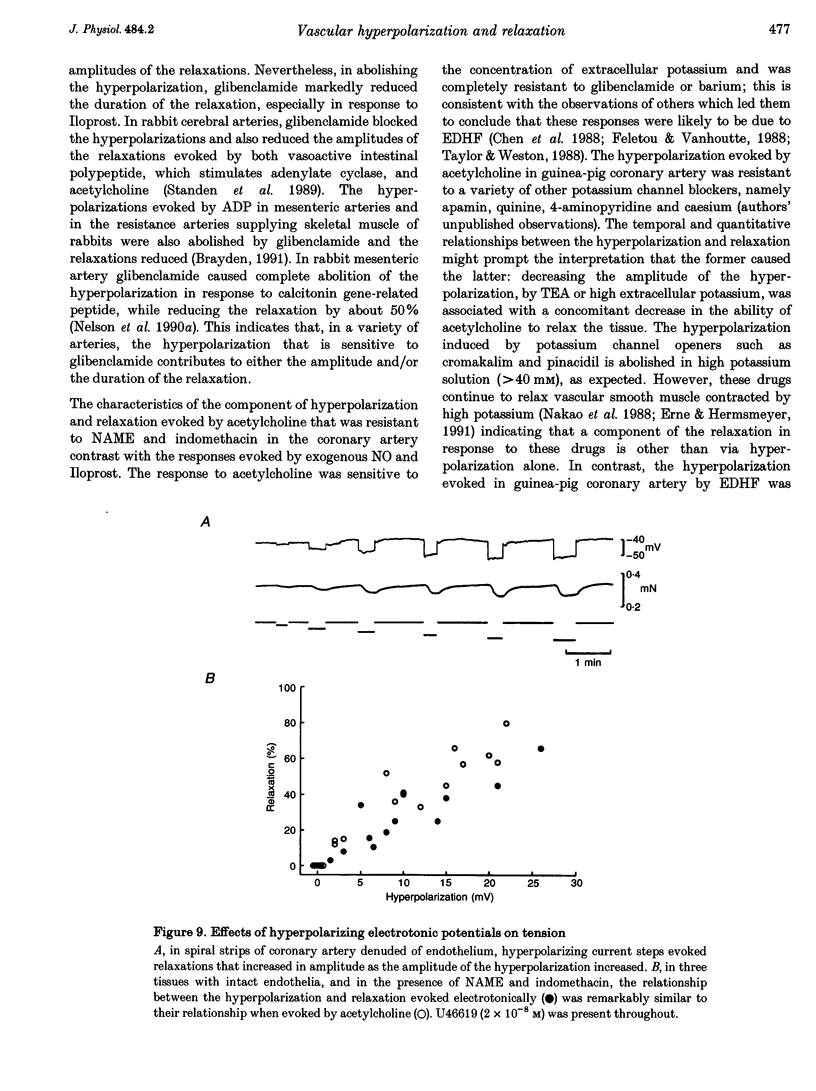
1. Membrane potential and tension were measured simultaneously in ring segments of main coronary artery of guinea-pigs. The synthetic thromboxane A2 analogue U46619 depolarized the tissues from -58 +/- 2 to -40 +/- 1 mV and increased tension by 12 +/- 1 mN mm-1. Nitric oxide (NO) and Iloprost, the stable analogue of prostacyclin, evoked hyperpolarization and relaxation. 2. The concentration of NO required to evoke half-maximal hyperpolarization (EC50 of 2 x 10(-5) M) was 40-fold higher than that which was required to induce relaxation (EC50 of 5 x 10(-7) M). The EC50 for Iloprost-induced hyperpolarization (3 x 10(-8) M) was similar to that for relaxation (4 x 10(-8) M). 3. Glibenclamide (10(-6) M) abolished the hyperpolarization in response to both NO and Iloprost but was without effect on the amplitudes of the relaxations over the complete concentration-response curves. 4. Acetylcholine evoked concentration-dependent hyperpolarization and relaxation in the presence of N omega-nitro-L-arginine methyl ester (NAME; 10(-5) M) and indomethacin (10(-6) M), and these responses were attributed to endothelium-derived hyperpolarizing factor (EDHF). The hyperpolarization produced by EDHF always preceded relaxation, and relaxation never occurred at concentrations of acetylcholine that were insufficient to evoke hyperpolarization. 5. The concentration-hyperpolarization and concentration-relaxation curves in response to acetylcholine were not affected by glibenclamide or barium (1-3 mM) but were shifted to the right 4- and 5-fold, respectively, by 1 mM tetraethylammonium. The hyperpolarization and relaxation evoked by acetylcholine were also reduced in a parallel manner when the potassium concentration in the superfusate was increased. 6. Hyperpolarizing current steps, applied to spiral strips of coronary artery denuded of endothelium and depolarized and constricted with U46619, caused relaxation. The relationship between hyperpolarization and relaxation evoked electronically was similar to that which was due to EDHF in intact tissues stimulated with acetylcholine. 7. It is concluded that the ability of NO or Iloprost to relax guinea-pig coronary artery does not depend upon hyperpolarization of the smooth muscle. In contrast, hyperpolarization is likely to play a major, if not the only, role in the relaxation in response to EDHF in this tissue.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adeagbo A. S., Triggle C. R. Varying extracellular [K+]: a functional approach to separating EDHF- and EDNO-related mechanisms in perfused rat mesenteric arterial bed. J Cardiovasc Pharmacol. 1993 Mar;21(3):423–429. [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. Properties of the cromakalim-induced potassium conductance in smooth muscle cells isolated from the rabbit portal vein. Br J Pharmacol. 1989 Nov;98(3):851–864. doi: 10.1111/j.1476-5381.1989.tb14614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Lang R. J., Takewaki T. Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J Physiol. 1984 Jun;351:549–572. doi: 10.1113/jphysiol.1984.sp015262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden J. E. Hyperpolarization and relaxation of resistance arteries in response to adenosine diphosphate. Distribution and mechanism of action. Circ Res. 1991 Nov;69(5):1415–1420. doi: 10.1161/01.res.69.5.1415. [DOI] [PubMed] [Google Scholar]
- Chen G. F., Cheung D. W. Characterization of acetylcholine-induced membrane hyperpolarization in endothelial cells. Circ Res. 1992 Feb;70(2):257–263. doi: 10.1161/01.res.70.2.257. [DOI] [PubMed] [Google Scholar]
- Chen G. F., Suzuki H. Calcium dependency of the endothelium-dependent hyperpolarization in smooth muscle cells of the rabbit carotid artery. J Physiol. 1990 Feb;421:521–534. doi: 10.1113/jphysiol.1990.sp017959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Suzuki H. Some electrical properties of the endothelium-dependent hyperpolarization recorded from rat arterial smooth muscle cells. J Physiol. 1989 Mar;410:91–106. doi: 10.1113/jphysiol.1989.sp017522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Suzuki H., Weston A. H. Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br J Pharmacol. 1988 Dec;95(4):1165–1174. doi: 10.1111/j.1476-5381.1988.tb11752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Yamamoto Y., Miwa K., Suzuki H. Hyperpolarization of arterial smooth muscle induced by endothelial humoral substances. Am J Physiol. 1991 Jun;260(6 Pt 2):H1888–H1892. doi: 10.1152/ajpheart.1991.260.6.H1888. [DOI] [PubMed] [Google Scholar]
- Edwards G., Weston A. H. Potassium channel openers and vascular smooth muscle relaxation. Pharmacol Ther. 1990;48(2):237–258. doi: 10.1016/0163-7258(90)90082-d. [DOI] [PubMed] [Google Scholar]
- Erne P., Hermsmeyer K. Modulation of intracellular calcium by potassium channel openers in vascular muscle. Naunyn Schmiedebergs Arch Pharmacol. 1991 Dec;344(6):706–715. doi: 10.1007/BF00174755. [DOI] [PubMed] [Google Scholar]
- Feletou M., Vanhoutte P. M. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol. 1988 Mar;93(3):515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland C. J., McPherson G. A. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br J Pharmacol. 1992 Feb;105(2):429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Seki N., Suzuki S., Ito S., Kajikuri J., Kuriyama H. Membrane hyperpolarization inhibits agonist-induced synthesis of inositol 1,4,5-trisphosphate in rabbit mesenteric artery. J Physiol. 1992;451:307–328. doi: 10.1113/jphysiol.1992.sp019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauser K., Stekiel W. J., Rubanyi G., Harder D. R. Mechanism of action of EDRF on pressurized arteries: effect on K+ conductance. Circ Res. 1989 Jul;65(1):199–204. doi: 10.1161/01.res.65.1.199. [DOI] [PubMed] [Google Scholar]
- Komori K., Suzuki H. Electrical responses of smooth muscle cells during cholinergic vasodilation in the rabbit saphenous artery. Circ Res. 1987 Oct;61(4):586–593. doi: 10.1161/01.res.61.4.586. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Suzuki H. The effects of acetylcholine on the membrane and contractile properties of smooth muscle cells of the rabbit superior mesenteric artery. Br J Pharmacol. 1978 Dec;64(4):493–501. doi: 10.1111/j.1476-5381.1978.tb17310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc N., Wilde D. W., Keef K. D., Hume J. R. Electrophysiological mechanisms of minoxidil sulfate-induced vasodilation of rabbit portal vein. Circ Res. 1989 Oct;65(4):1102–1111. doi: 10.1161/01.res.65.4.1102. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Activators of potassium channels enhance calcium influx into endothelial cells as a consequence of potassium currents. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jul;342(1):94–99. doi: 10.1007/BF00178979. [DOI] [PubMed] [Google Scholar]
- Marchenko S. M., Sage S. O. Electrical properties of resting and acetylcholine-stimulated endothelium in intact rat aorta. J Physiol. 1993 Mar;462:735–751. doi: 10.1113/jphysiol.1993.sp019579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F. The role of hyperpolarization in the relaxation of smooth muscle of monkey coronary artery. J Physiol. 1986 Feb;371:257–265. doi: 10.1113/jphysiol.1986.sp015972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvany M. J., Nilsson H., Flatman J. A. Role of membrane potential in the response of rat small mesenteric arteries to exogenous noradrenaline stimulation. J Physiol. 1982 Nov;332:363–373. doi: 10.1113/jphysiol.1982.sp014418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao K., Okabe K., Kitamura K., Kuriyama H., Weston A. H. Characteristics of cromakalim-induced relaxations in the smooth muscle cells of guinea-pig mesenteric artery and vein. Br J Pharmacol. 1988 Nov;95(3):795–804. doi: 10.1111/j.1476-5381.1988.tb11707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. T., Huang Y., Brayden J. E., Hescheler J., Standen N. B. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature. 1990 Apr 19;344(6268):770–773. doi: 10.1038/344770a0. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Patlak J. B., Worley J. F., Standen N. B. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990 Jul;259(1 Pt 1):C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- Nelson M. T., Standen N. B., Brayden J. E., Worley J. F., 3rd Noradrenaline contracts arteries by activating voltage-dependent calcium channels. Nature. 1988 Nov 24;336(6197):382–385. doi: 10.1038/336382a0. [DOI] [PubMed] [Google Scholar]
- Nilius B., Schwartz G., Oike M., Droogmans G. Histamine-activated, non-selective cation currents and Ca2+ transients in endothelial cells from human umbilical vein. Pflugers Arch. 1993 Aug;424(3-4):285–293. doi: 10.1007/BF00384354. [DOI] [PubMed] [Google Scholar]
- Nishimura J., van Breemen C. Direct regulation of smooth muscle contractile elements by second messengers. Biochem Biophys Res Commun. 1989 Sep 15;163(2):929–935. doi: 10.1016/0006-291x(89)92311-5. [DOI] [PubMed] [Google Scholar]
- Parkington H. C., Tare M., Tonta M. A., Coleman H. A. Stretch revealed three components in the hyperpolarization of guinea-pig coronary artery in response to acetylcholine. J Physiol. 1993 Jun;465:459–476. doi: 10.1113/jphysiol.1993.sp019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand V. E., Garland C. J. Endothelium-dependent relaxation to acetylcholine in the rabbit basilar artery: importance of membrane hyperpolarization. Br J Pharmacol. 1992 May;106(1):143–150. doi: 10.1111/j.1476-5381.1992.tb14307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport R. M., Draznin M. B., Murad F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature. 1983 Nov 10;306(5939):174–176. doi: 10.1038/306174a0. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989 Jul 14;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Suematsu E., Hirata M., Kuriyama H. Effects of cAMP- and cGMP-dependent protein kinases, and calmodulin on Ca2+ uptake by highly purified sarcolemmal vesicles of vascular smooth muscle. Biochim Biophys Acta. 1984 Jun 13;773(1):83–90. doi: 10.1016/0005-2736(84)90552-2. [DOI] [PubMed] [Google Scholar]
- Tare M., Parkington H. C., Coleman H. A., Neild T. O., Dusting G. J. Hyperpolarization and relaxation of arterial smooth muscle caused by nitric oxide derived from the endothelium. Nature. 1990 Jul 5;346(6279):69–71. doi: 10.1038/346069a0. [DOI] [PubMed] [Google Scholar]
- Taylor S. G., Southerton J. S., Weston A. H., Baker J. R. Endothelium-dependent effects of acetylcholine in rat aorta: a comparison with sodium nitroprusside and cromakalim. Br J Pharmacol. 1988 Jul;94(3):853–863. doi: 10.1111/j.1476-5381.1988.tb11597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. G., Weston A. H. Endothelium-derived hyperpolarizing factor: a new endogenous inhibitor from the vascular endothelium. Trends Pharmacol Sci. 1988 Aug;9(8):272–274. doi: 10.1016/0165-6147(88)90003-x. [DOI] [PubMed] [Google Scholar]
- Twort C. H., van Breemen C. Cyclic guanosine monophosphate-enhanced sequestration of Ca2+ by sarcoplasmic reticulum in vascular smooth muscle. Circ Res. 1988 May;62(5):961–964. doi: 10.1161/01.res.62.5.961. [DOI] [PubMed] [Google Scholar]
- Videbaek L. M., Aalkjaer C., Hughes A. D., Mulvany M. J. Effect of pinacidil on ion permeability in resting and contracted resistance vessels. Am J Physiol. 1990 Jul;259(1 Pt 2):H14–H22. doi: 10.1152/ajpheart.1990.259.1.H14. [DOI] [PubMed] [Google Scholar]
- Vrolix M., Raeymaekers L., Wuytack F., Hofmann F., Casteels R. Cyclic GMP-dependent protein kinase stimulates the plasmalemmal Ca2+ pump of smooth muscle via phosphorylation of phosphatidylinositol. Biochem J. 1988 Nov 1;255(3):855–863. doi: 10.1042/bj2550855. [DOI] [PMC free article] [PubMed] [Google Scholar]


