Abstract
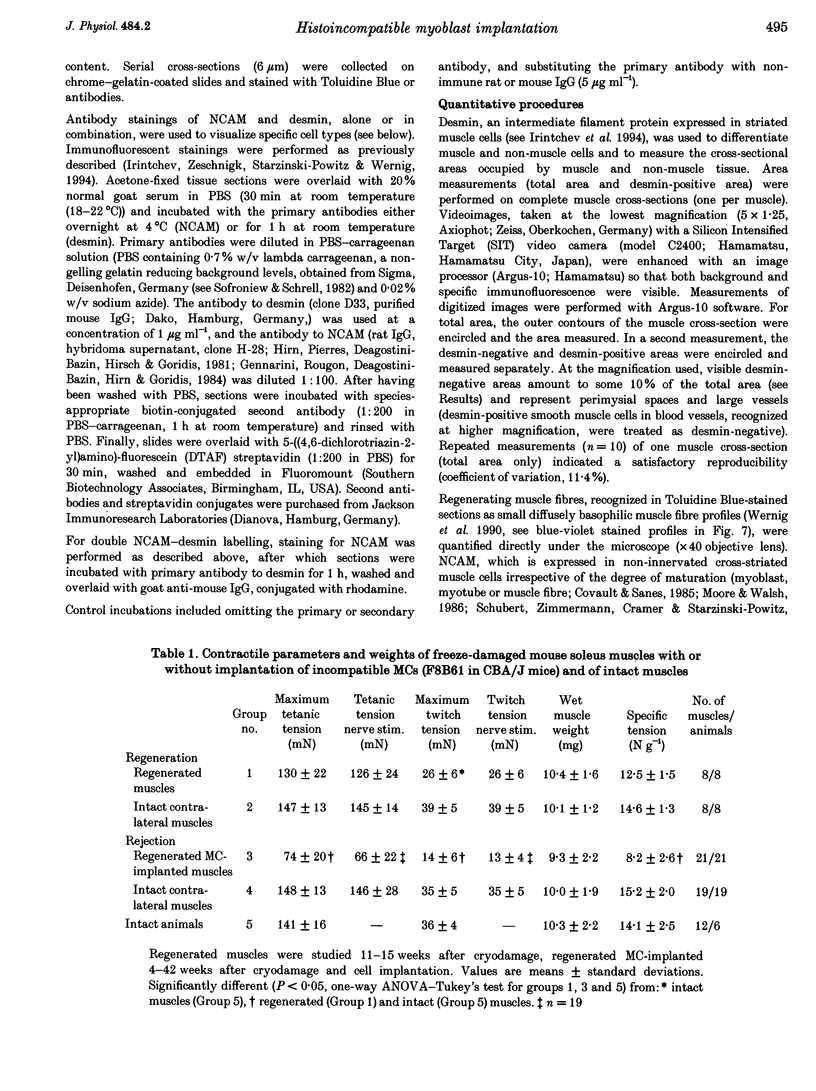
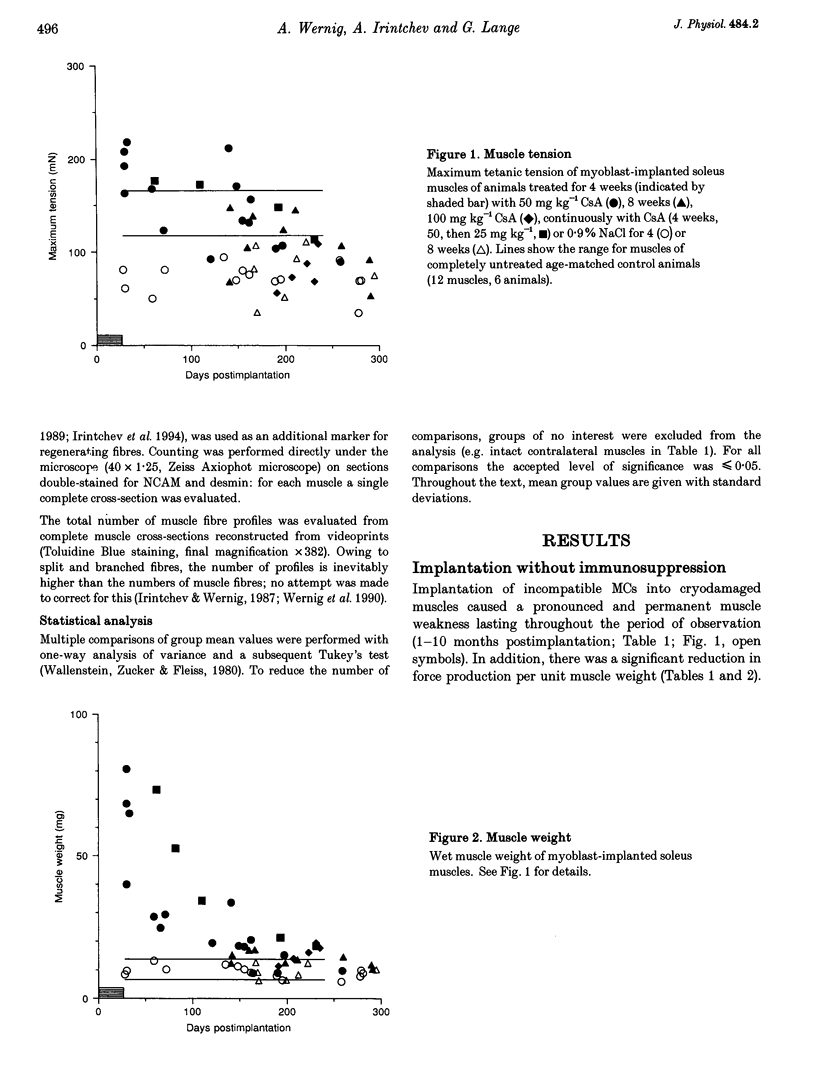
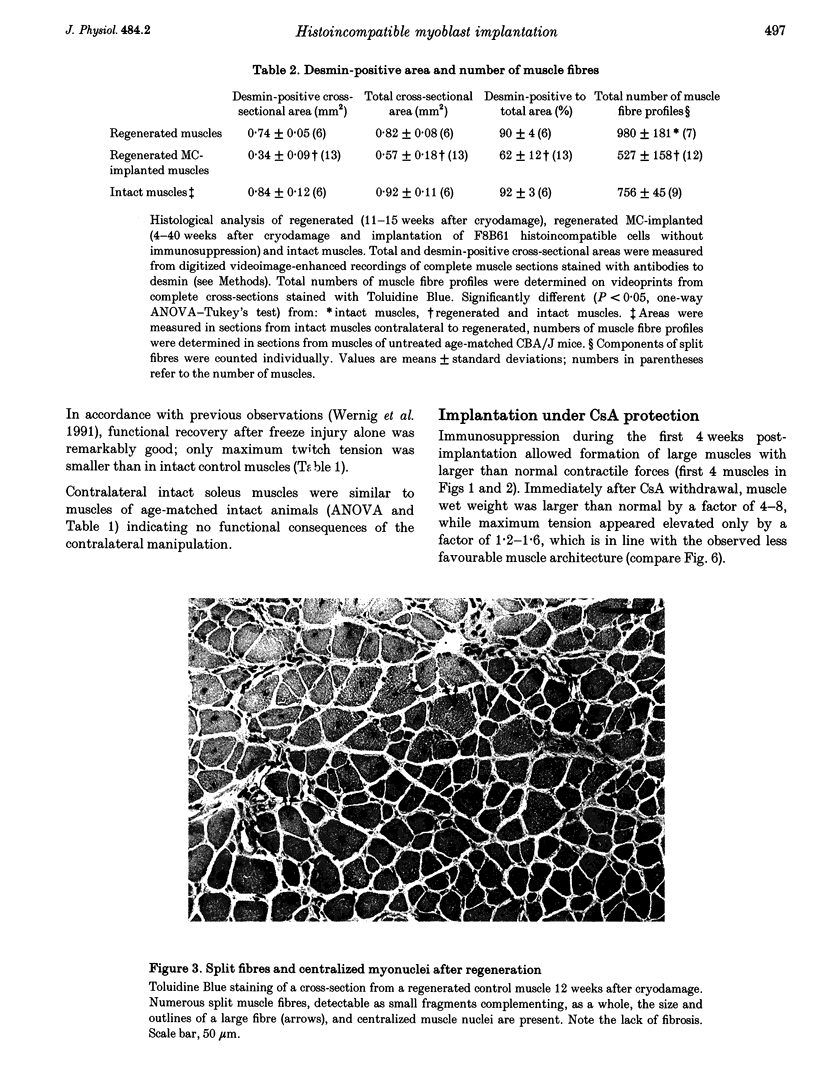
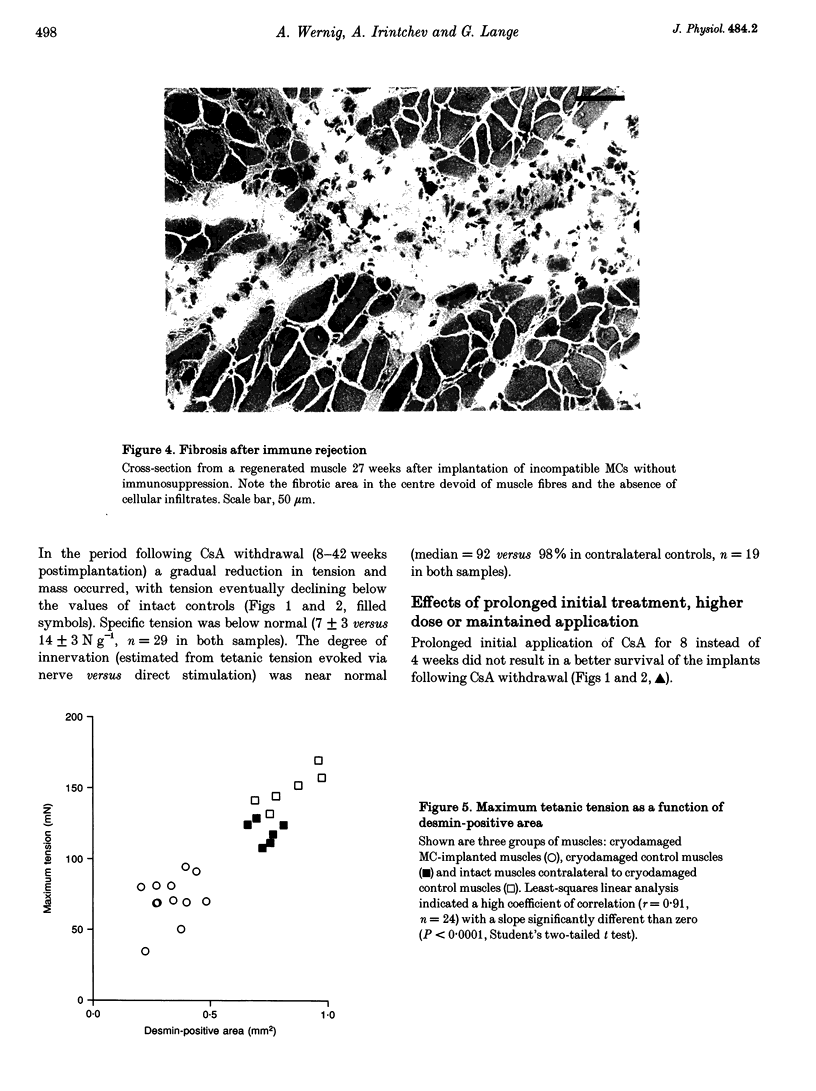
1. The goals of this study were to evaluate the immunogenicity of myogenic cells (MCs) (1) immediately after implantation into regenerating muscles, and (2) following their maturation under initial immunosuppression. Implanted mouse soleus muscles were evaluated by isometric tension recordings in vitro followed by histological investigations on frozen sections. 2. Implantation of non-histocompatible myoblasts into cryodamaged soleus muscles of CBA/J mice induced immune rejection which caused large and permanent deficits in muscle force: 4-42 weeks postimplantation maximal tetanic tension was 50-60% that of intact or regenerated cryodamaged control muscles without tendency for recovery or histological signs of muscle regeneration. Specific tension (force per unit muscle weight) was also significantly reduced. 3. On frozen sections, only 62 +/- 12% of the total area was desmin-positive, that is, occupied by muscle fibres, versus 90 +/- 4% in regenerated and 92 +/- 3% in intact muscles. Also, the total number of muscle fibre profiles was significantly reduced. 4. Under immune suppression with cyclosporin A (CsA), large muscles developed within 4 weeks. Following CsA withdrawal, muscle weight and force, in addition to desmin-positive areas on cross-sections, gradually declined over several months despite continual regeneration, indicating retarded immune rejection. 5. Initial application of CsA for 8 weeks after implantation, instead of 4 weeks, did not result in better survival of the implants, nor did a higher initial dose of CsA (100 instead of 50 mg kg-1 day-1). Prolonged continuous application of a reduced dose (25 mg kg-1 day-1) did not prevent muscle wasting but caused an additional delay. 6. It is concluded that histoincompatible myoblasts are highly immunogenic and that immune rejection causes large and permanent muscle deficits indicating elimination of host muscle tissue. Initial transient immunosuppression protects the incompatible cells, but after withdrawal, prolonged immune rejection and retarded muscle wasting occur.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard S. T., Dunn M. J., Dubowitz V., Rose M. L. Increased expression of HLA ABC class I antigens by muscle fibres in Duchenne muscular dystrophy, inflammatory myopathy, and other neuromuscular disorders. Lancet. 1985 Feb 16;1(8425):361–363. doi: 10.1016/s0140-6736(85)91384-4. [DOI] [PubMed] [Google Scholar]
- Covault J., Sanes J. R. Neural cell adhesion molecule (N-CAM) accumulates in denervated and paralyzed skeletal muscles. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4544–4548. doi: 10.1073/pnas.82.13.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarini G., Rougon G., Deagostini-Bazin H., Hirn M., Goridis C. Studies on the transmembrane disposition of the neural cell adhesion molecule N-CAM. A monoclonal antibody recognizing a cytoplasmic domain and evidence for the presence of phosphoserine residues. Eur J Biochem. 1984 Jul 2;142(1):57–64. doi: 10.1111/j.1432-1033.1984.tb08250.x. [DOI] [PubMed] [Google Scholar]
- Goodman M. N. Tumor necrosis factor induces skeletal muscle protein breakdown in rats. Am J Physiol. 1991 May;260(5 Pt 1):E727–E730. doi: 10.1152/ajpendo.1991.260.5.E727. [DOI] [PubMed] [Google Scholar]
- Green C. J. Neuroleptanalgesic drug combinations in the anaesthetic management of small laboratory animals. Lab Anim. 1975 Jul;9(3):161–178. doi: 10.1258/002367775780994574. [DOI] [PubMed] [Google Scholar]
- Gussoni E., Pavlath G. K., Lanctot A. M., Sharma K. R., Miller R. G., Steinman L., Blau H. M. Normal dystrophin transcripts detected in Duchenne muscular dystrophy patients after myoblast transplantation. Nature. 1992 Apr 2;356(6368):435–438. doi: 10.1038/356435a0. [DOI] [PubMed] [Google Scholar]
- Hirn M., Pierres M., Deagostini-Bazin H., Hirsch M., Goridis C. Monoclonal antibody against cell surface glycoprotein of neurons. Brain Res. 1981 Jun 15;214(2):433–439. doi: 10.1016/0006-8993(81)91208-7. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Engel A. G. Lysis of myotubes by alloreactive cytotoxic T cells and natural killer cells. Relevance to myoblast transplantation. J Clin Invest. 1990 Jul;86(1):370–374. doi: 10.1172/JCI114711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard J., Bouchard J. P., Roy R., Labrecque C., Dansereau G., Lemieux B., Tremblay J. P. Myoblast transplantation produced dystrophin-positive muscle fibres in a 16-year-old patient with Duchenne muscular dystrophy. Clin Sci (Lond) 1991 Aug;81(2):287–288. doi: 10.1042/cs0810287. [DOI] [PubMed] [Google Scholar]
- Huard J., Roy R., Bouchard J. P., Malouin F., Richards C. L., Tremblay J. P. Human myoblast transplantation between immunohistocompatible donors and recipients produces immune reactions. Transplant Proc. 1992 Dec;24(6):3049–3051. [PubMed] [Google Scholar]
- Irintchev A., Draguhn A., Wernig A. Reinnervation and recovery of mouse soleus muscle after long-term denervation. Neuroscience. 1990;39(1):231–243. doi: 10.1016/0306-4522(90)90236-w. [DOI] [PubMed] [Google Scholar]
- Irintchev A., Salvini T. F., Faissner A., Wernig A. Differential expression of tenascin after denervation, damage or paralysis of mouse soleus muscle. J Neurocytol. 1993 Nov;22(11):955–965. doi: 10.1007/BF01218353. [DOI] [PubMed] [Google Scholar]
- Irintchev A., Wernig A. Muscle damage and repair in voluntarily running mice: strain and muscle differences. Cell Tissue Res. 1987 Sep;249(3):509–521. doi: 10.1007/BF00217322. [DOI] [PubMed] [Google Scholar]
- Irintchev A., Zeschnigk M., Starzinski-Powitz A., Wernig A. Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev Dyn. 1994 Apr;199(4):326–337. doi: 10.1002/aja.1001990407. [DOI] [PubMed] [Google Scholar]
- Karpati G., Ajdukovic D., Arnold D., Gledhill R. B., Guttmann R., Holland P., Koch P. A., Shoubridge E., Spence D., Vanasse M. Myoblast transfer in Duchenne muscular dystrophy. Ann Neurol. 1993 Jul;34(1):8–17. doi: 10.1002/ana.410340105. [DOI] [PubMed] [Google Scholar]
- Karpati G., Pouliot Y., Carpenter S. Expression of immunoreactive major histocompatibility complex products in human skeletal muscles. Ann Neurol. 1988 Jan;23(1):64–72. doi: 10.1002/ana.410230111. [DOI] [PubMed] [Google Scholar]
- Klaus G. G., Kunkl A. Effects of cyclosporine on the immune system of the mouse. II. Cyclosporine inhibits the effector function of primary T helper cells, but not helper cell priming. Transplantation. 1983 Jul;36(1):80–84. doi: 10.1097/00007890-198307000-00016. [DOI] [PubMed] [Google Scholar]
- Law P. K., Bertorini T. E., Goodwin T. G., Chen M., Fang Q. W., Li H. J., Kirby D. S., Florendo J. A., Herrod H. G., Golden G. S. Dystrophin production induced by myoblast transfer therapy in Duchenne muscular dystrophy. Lancet. 1990 Jul 14;336(8707):114–115. doi: 10.1016/0140-6736(90)91628-n. [DOI] [PubMed] [Google Scholar]
- Law P. K., Goodwin T. G., Fang Q., Duggirala V., Larkin C., Florendo J. A., Kirby D. S., Deering M. B., Li H. J., Chen M. Feasibility, safety, and efficacy of myoblast transfer therapy on Duchenne muscular dystrophy boys. Cell Transplant. 1992;1(2-3):235–244. doi: 10.1177/0963689792001002-305. [DOI] [PubMed] [Google Scholar]
- Law P. K., Goodwin T. G., Li H. J. Histoincompatible myoblast injection improves muscle structure and function of dystrophic mice. Transplant Proc. 1988 Jun;20(3 Suppl 3):1114–1119. [PubMed] [Google Scholar]
- Moore S. E., Walsh F. S. Nerve dependent regulation of neural cell adhesion molecule expression in skeletal muscle. Neuroscience. 1986 Jun;18(2):499–505. doi: 10.1016/0306-4522(86)90170-3. [DOI] [PubMed] [Google Scholar]
- Morgan J. E., Coulton G. R., Partridge T. A. Muscle precursor cells invade and repopulate freeze-killed muscles. J Muscle Res Cell Motil. 1987 Oct;8(5):386–396. doi: 10.1007/BF01578428. [DOI] [PubMed] [Google Scholar]
- Morgan J. E., Partridge T. A. Cell transplantation and gene therapy in muscular dystrophy. Bioessays. 1992 Sep;14(9):641–645. doi: 10.1002/bies.950140913. [DOI] [PubMed] [Google Scholar]
- Partridge T. A. Invited review: myoblast transfer: a possible therapy for inherited myopathies? Muscle Nerve. 1991 Mar;14(3):197–212. doi: 10.1002/mus.880140302. [DOI] [PubMed] [Google Scholar]
- Partridge T. A., Morgan J. E., Coulton G. R., Hoffman E. P., Kunkel L. M. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989 Jan 12;337(6203):176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- Ponder B. A., Wilkinson M. M., Wood M., Westwood J. H. Immunohistochemical demonstration of H2 antigens in mouse tissue sections. J Histochem Cytochem. 1983 Jul;31(7):911–919. doi: 10.1177/31.7.6343482. [DOI] [PubMed] [Google Scholar]
- Roy R., Tremblay J. P., Huard J., Richards C., Malouin F., Bouchard J. P. Antibody formation after myoblast transplantation in Duchenne-dystrophic patients, donor HLA compatible. Transplant Proc. 1993 Feb;25(1 Pt 2):995–997. [PubMed] [Google Scholar]
- Schubert W., Zimmermann K., Cramer M., Starzinski-Powitz A. Lymphocyte antigen Leu-19 as a molecular marker of regeneration in human skeletal muscle. Proc Natl Acad Sci U S A. 1989 Jan;86(1):307–311. doi: 10.1073/pnas.86.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal S. S., Faulkner J. A. Temperature-dependent physiological stability of rat skeletal muscle in vitro. Am J Physiol. 1985 Mar;248(3 Pt 1):C265–C270. doi: 10.1152/ajpcell.1985.248.3.C265. [DOI] [PubMed] [Google Scholar]
- Sofroniew M. V., Schrell U. Long-term storage and regular repeated use of diluted antisera in glass staining jars for increased sensitivity, reproducibility, and convenience of single- and two-color light microscopic immunocytochemistry. J Histochem Cytochem. 1982 Jun;30(6):504–511. doi: 10.1177/30.6.6178778. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Watt D. J., Morgan J. E., Partridge T. A. Allografts of muscle precursor cells persist in the non-tolerized host. Neuromuscul Disord. 1991;1(5):345–355. doi: 10.1016/0960-8966(91)90121-8. [DOI] [PubMed] [Google Scholar]
- Watt D. J., Morgan J. E., Partridge T. A. Long term survival of allografted muscle precursor cells following a limited period of treatment with cyclosporin A. Clin Exp Immunol. 1984 Feb;55(2):419–426. [PMC free article] [PubMed] [Google Scholar]
- Watt D. J., Partridge T. A., Sloper J. C. Cyclosporin A as a means of preventing rejection of skeletal muscle allografts in mice. Transplantation. 1981 Apr;31(4):266–271. doi: 10.1097/00007890-198104000-00007. [DOI] [PubMed] [Google Scholar]
- Wernig A., Irintchev A., Härtling A., Stephan G., Zimmermann K., Starzinski-Powitz A. Formation of new muscle fibres and tumours after injection of cultured myogenic cells. J Neurocytol. 1991 Dec;20(12):982–997. doi: 10.1007/BF01187916. [DOI] [PubMed] [Google Scholar]
- Wernig A., Irintchev A., Weisshaupt P. Muscle injury, cross-sectional area and fibre type distribution in mouse soleus after intermittent wheel-running. J Physiol. 1990 Sep;428:639–652. doi: 10.1113/jphysiol.1990.sp018232. [DOI] [PMC free article] [PubMed] [Google Scholar]








