Abstract
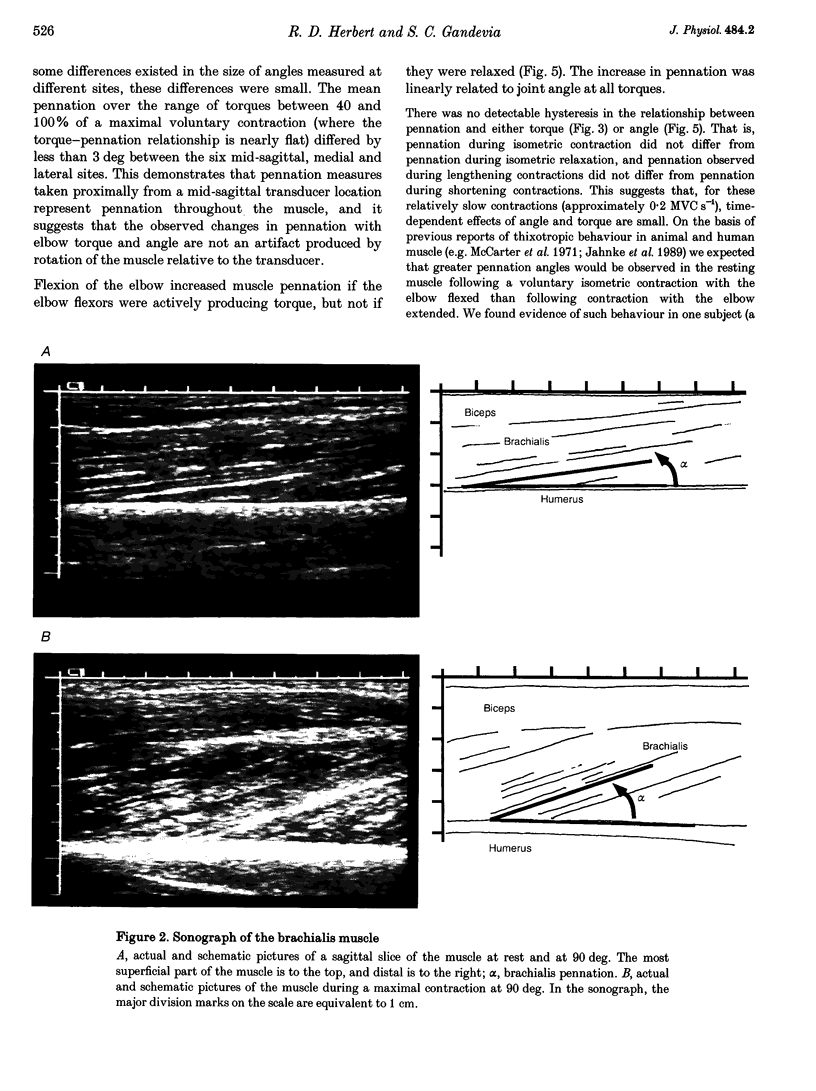
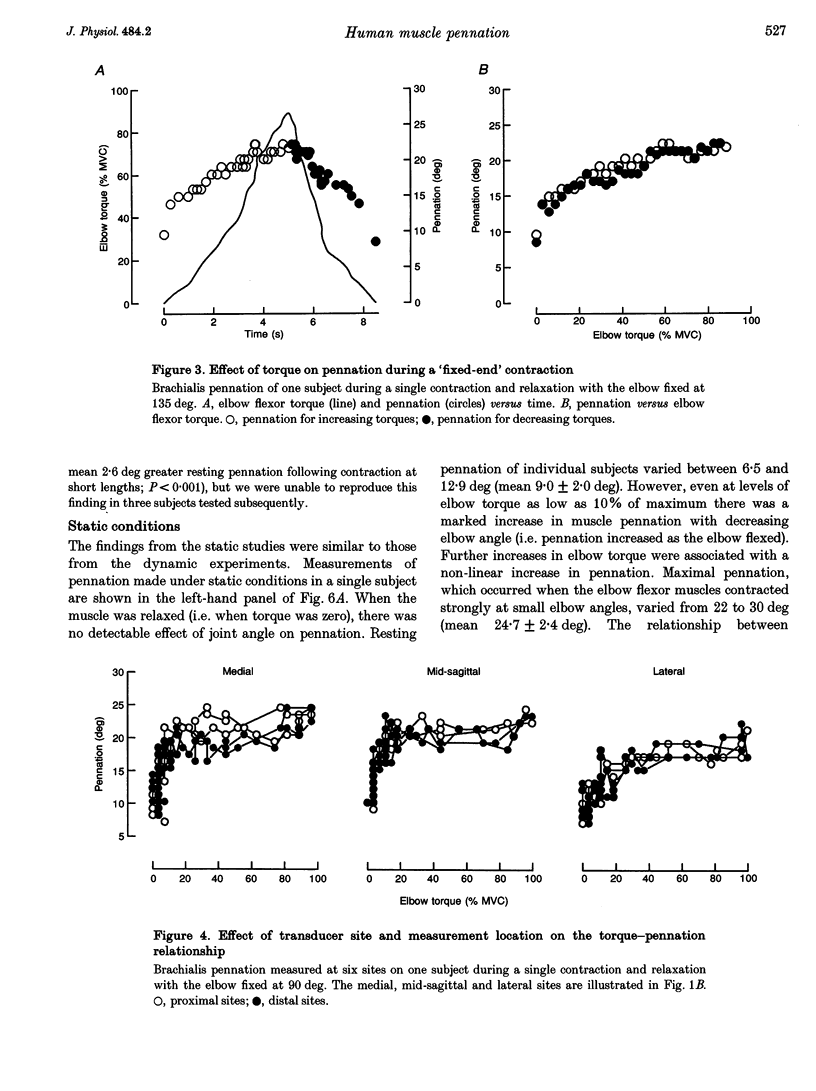
1. Estimates of pennation in human muscles are usually obtained from cadavers. In this study, pennation of human brachialis was measured in vivo using sonography. Effects of static and dynamic changes in elbow angle and torque were investigated. 2. Pennation was measured in eight subjects using an 80 mm, 5 MHz, linear-array ultrasound transducer to generate sagittal images of the brachialis during maximal and submaximal isometric contractions at various elbow angles. It was shown that estimates of pennation were reproducible, representative of measurements made throughout the belly of the muscle and not distorted by compression of the muscle with the transducer or rotation of the muscle out of the plane of the transducer. 3. Mean resting pennation was 9.0 +/- 2.0 deg (S.D., range 6.5-12.9 deg). When the muscle was relaxed there was no effect of elbow angle on pennation. However, during a maximal isometric contraction (MVC), with the elbow flexed to 90 deg, pennation increased non-linearly with elbow torque to between 22 and 30 deg (mean 24.7 +/- 2.4 deg). The effect of increasing torque was small when the elbow was fully extended. The relationship between elbow angle, elbow torque and brachialis pennation suggests that the relaxed brachialis muscle is slack over much of its physiological range of lengths. 4. There was no hysteresis in the relationship between torque and pennation during slow isometric contractions (0.2 MVC s-1), and the relationship between elbow angle and pennation was similar during slow shortening and lengthening contractions. 5. Two consequences follow from these findings. Firstly, intramuscular mechanics are complex and simple planar models of muscles underestimate the increases in pennation which occur during muscle contraction. Second, spindle afferents from relaxed muscles may not encode joint angle over the full range of movement.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An K. N., Hui F. C., Morrey B. F., Linscheid R. L., Chao E. Y. Muscles across the elbow joint: a biomechanical analysis. J Biomech. 1981;14(10):659–669. doi: 10.1016/0021-9290(81)90048-8. [DOI] [PubMed] [Google Scholar]
- Baskin R. J., Paolini P. J. Volume change and pressure development in muscle during contraction. Am J Physiol. 1967 Oct;213(4):1025–1030. doi: 10.1152/ajplegacy.1967.213.4.1025. [DOI] [PubMed] [Google Scholar]
- Crosbie J., Eisenhuth J. Transducer for the measurement of linear displacement of body segments. Med Biol Eng Comput. 1993 Jul;31(4):430–432. doi: 10.1007/BF02446701. [DOI] [PubMed] [Google Scholar]
- Elek J., Prochazka A., Hulliger M., Vincent S. In-series compliance of gastrocnemius muscle in cat step cycle: do spindles signal origin-to-insertion length? J Physiol. 1990 Oct;429:237–258. doi: 10.1113/jphysiol.1990.sp018254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom C. M., Loeb G. E., Reid J. G., Forrest W. J., Avruch L. Morphometry of the human thigh muscles. A comparison between anatomical sections and computer tomographic and magnetic resonance images. J Anat. 1991 Jun;176:139–156. [PMC free article] [PubMed] [Google Scholar]
- Ettema G. J., Huijing P. A. Properties of the tendinous structures and series elastic component of EDL muscle-tendon complex of the rat. J Biomech. 1989;22(11-12):1209–1215. doi: 10.1016/0021-9290(89)90223-6. [DOI] [PubMed] [Google Scholar]
- Gans C., Bock W. J. The functional significance of muscle architecture--a theoretical analysis. Ergeb Anat Entwicklungsgesch. 1965;38:115–142. [PubMed] [Google Scholar]
- Griffiths R. I. Shortening of muscle fibres during stretch of the active cat medial gastrocnemius muscle: the role of tendon compliance. J Physiol. 1991 May;436:219–236. doi: 10.1113/jphysiol.1991.sp018547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth K. E., Nordin M., Bongiovanni L. G. After-effects on stiffness and stretch reflexes of human finger flexor muscles attributed to muscle thixotropy. J Physiol. 1995 Jan 1;482(Pt 1):215–223. doi: 10.1113/jphysiol.1995.sp020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson-Larsén K., Wretling M. L., Lorentzon R., Oberg L. Do muscle fibre size and fibre angulation correlate in pennated human muscles? Eur J Appl Physiol Occup Physiol. 1992;64(1):68–72. doi: 10.1007/BF00376443. [DOI] [PubMed] [Google Scholar]
- Hill D. K. Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation. J Physiol. 1968 Dec;199(3):637–684. doi: 10.1113/jphysiol.1968.sp008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnke M. T., Proske U., Struppler A. Measurements of muscle stiffness, the electromyogram and activity in single muscle spindles of human flexor muscles following conditioning by passive stretch or contraction. Brain Res. 1989 Jul 24;493(1):103–112. doi: 10.1016/0006-8993(89)91004-4. [DOI] [PubMed] [Google Scholar]
- McCarter R. J., Nabarro F. R., Wyndham C. H. Reversibility of the passive length-tension relation in mammalian skeletal muscle. Arch Int Physiol Biochim. 1971 Aug;79(3):469–479. doi: 10.3109/13813457109085331. [DOI] [PubMed] [Google Scholar]
- McKenzie D. K., Gandevia S. C., Gorman R. B., Southon F. C. Dynamic changes in the zone of apposition and diaphragm length during maximal respiratory efforts. Thorax. 1994 Jul;49(7):634–638. doi: 10.1136/thx.49.7.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. L., Prochazka A., Proske U. The after-effects of stretch and fusimotor stimulation on the responses of primary endings of cat muscle spindles. J Physiol. 1984 Nov;356:465–477. doi: 10.1113/jphysiol.1984.sp015477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhl Z. F. Active length-tension relation and the effect of muscle pinnation on fiber lengthening. J Morphol. 1982 Sep;173(3):285–292. doi: 10.1002/jmor.1051730305. [DOI] [PubMed] [Google Scholar]
- Neering I. R., Quesenberry L. A., Morris V. A., Taylor S. R. Nonuniform volume changes during muscle contraction. Biophys J. 1991 Apr;59(4):926–933. doi: 10.1016/S0006-3495(91)82306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U., Morgan D. L., Gregory J. E. Thixotropy in skeletal muscle and in muscle spindles: a review. Prog Neurobiol. 1993 Dec;41(6):705–721. doi: 10.1016/0301-0082(93)90032-n. [DOI] [PubMed] [Google Scholar]
- Rutherford O. M., Jones D. A. Measurement of fibre pennation using ultrasound in the human quadriceps in vivo. Eur J Appl Physiol Occup Physiol. 1992;65(5):433–437. doi: 10.1007/BF00243510. [DOI] [PubMed] [Google Scholar]
- Scott S. H., Engstrom C. M., Loeb G. E. Morphometry of human thigh muscles. Determination of fascicle architecture by magnetic resonance imaging. J Anat. 1993 Apr;182(Pt 2):249–257. [PMC free article] [PubMed] [Google Scholar]
- Spoor C. W., van Leeuwen J. L., van der Meulen W. J., Huson A. Active force-length relationship of human lower-leg muscles estimated from morphological data: a comparison of geometric muscle models. Eur J Morphol. 1991;29(3):137–160. [PubMed] [Google Scholar]
- Wei J. Y., Simon J., Randić M., Burgess P. R. Joint angle signaling by muscle spindle receptors. Brain Res. 1986 Apr 2;370(1):108–118. doi: 10.1016/0006-8993(86)91110-8. [DOI] [PubMed] [Google Scholar]
- Woittiez R. D., Huijing P. A., Boom H. B., Rozendal R. H. A three-dimensional muscle model: a quantified relation between form and function of skeletal muscles. J Morphol. 1984 Oct;182(1):95–113. doi: 10.1002/jmor.1051820107. [DOI] [PubMed] [Google Scholar]
- Zajac F. E. Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Eng. 1989;17(4):359–411. [PubMed] [Google Scholar]
- Zuurbier C. J., Everard A. J., van der Wees P., Huijing P. A. Length-force characteristics of the aponeurosis in the passive and active muscle condition and in the isolated condition. J Biomech. 1994 Apr;27(4):445–453. doi: 10.1016/0021-9290(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Zuurbier C. J., Huijing P. A. Changes in geometry of actively shortening unipennate rat gastrocnemius muscle. J Morphol. 1993 Nov;218(2):167–180. doi: 10.1002/jmor.1052180206. [DOI] [PubMed] [Google Scholar]
- Zuurbier C. J., Huijing P. A. Influence of muscle geometry on shortening speed of fibre, aponeurosis and muscle. J Biomech. 1992 Sep;25(9):1017–1026. doi: 10.1016/0021-9290(92)90037-2. [DOI] [PubMed] [Google Scholar]



