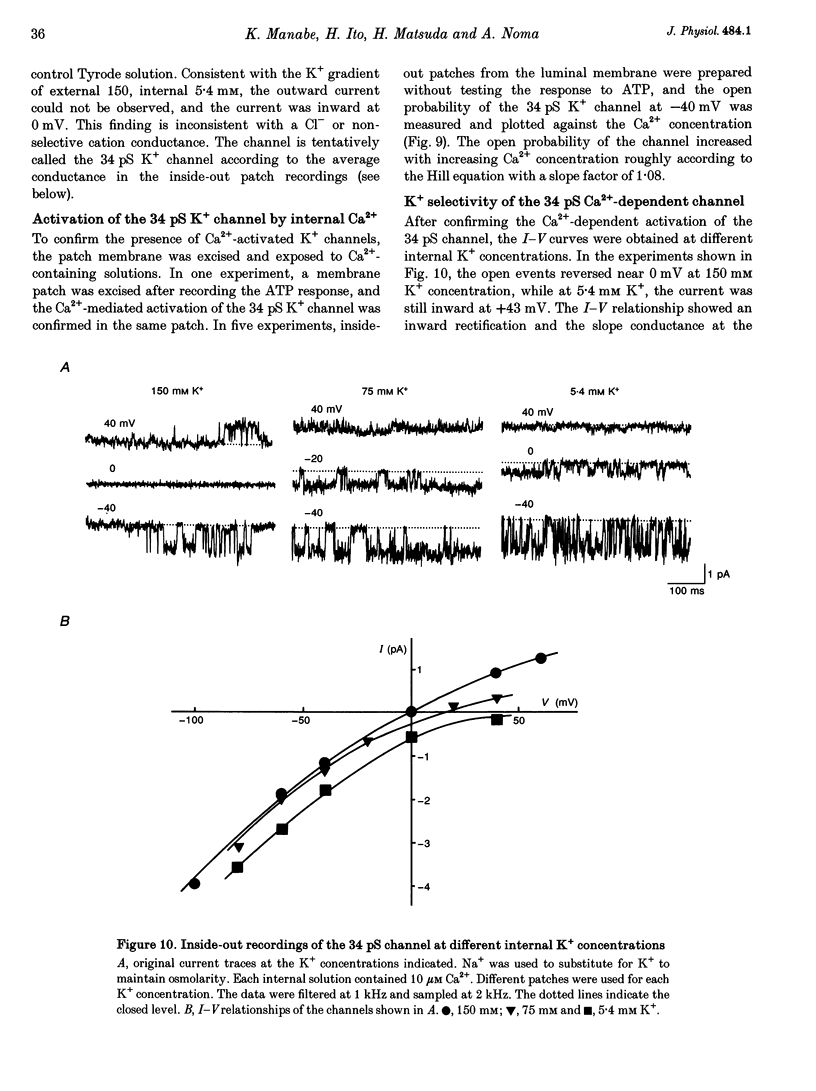
Abstract
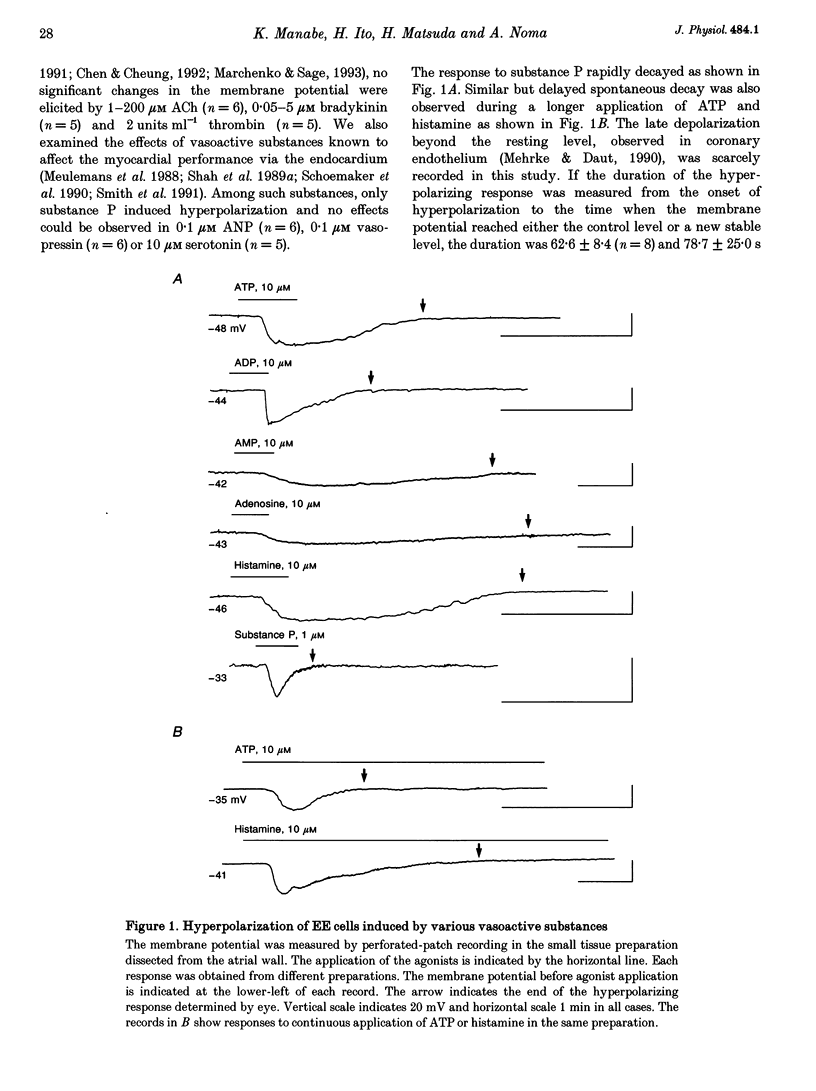
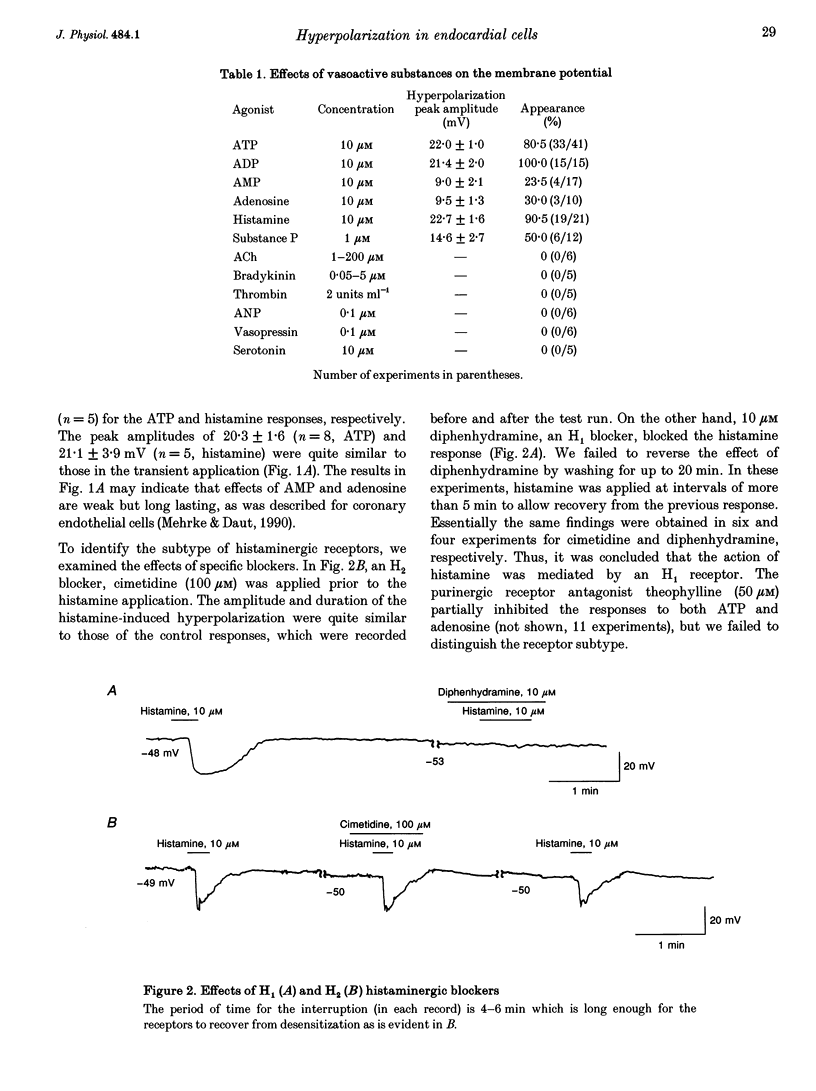
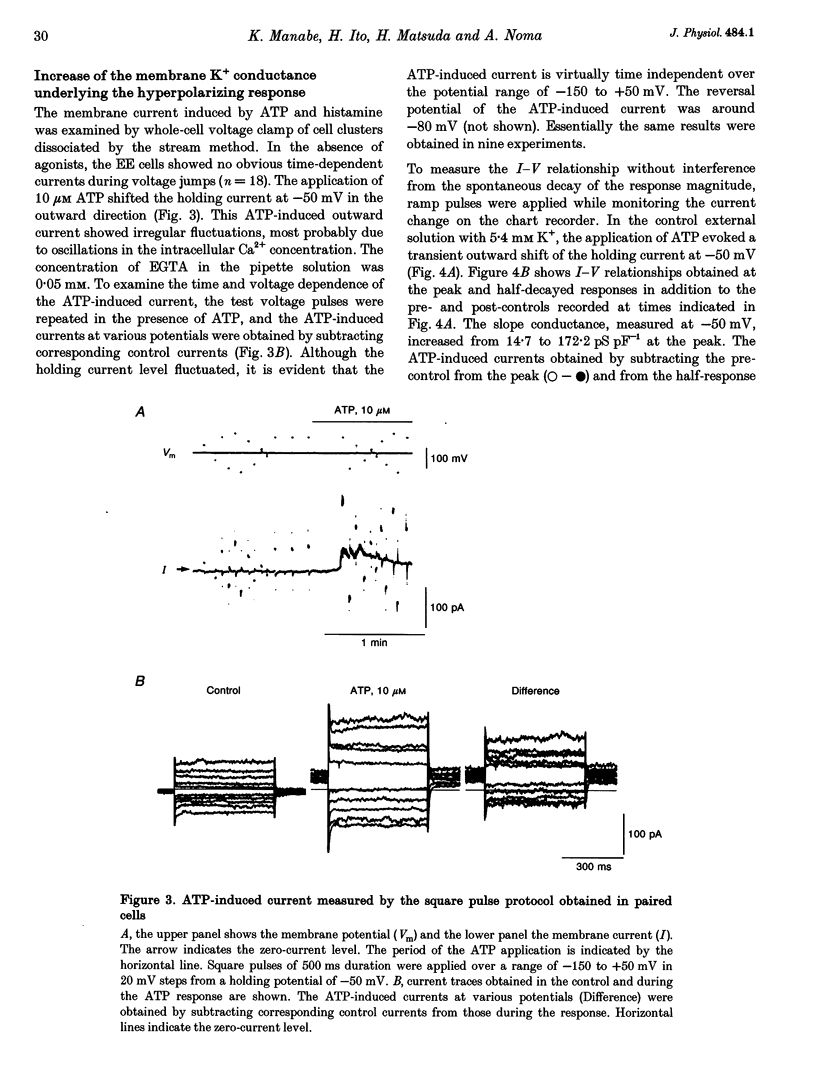
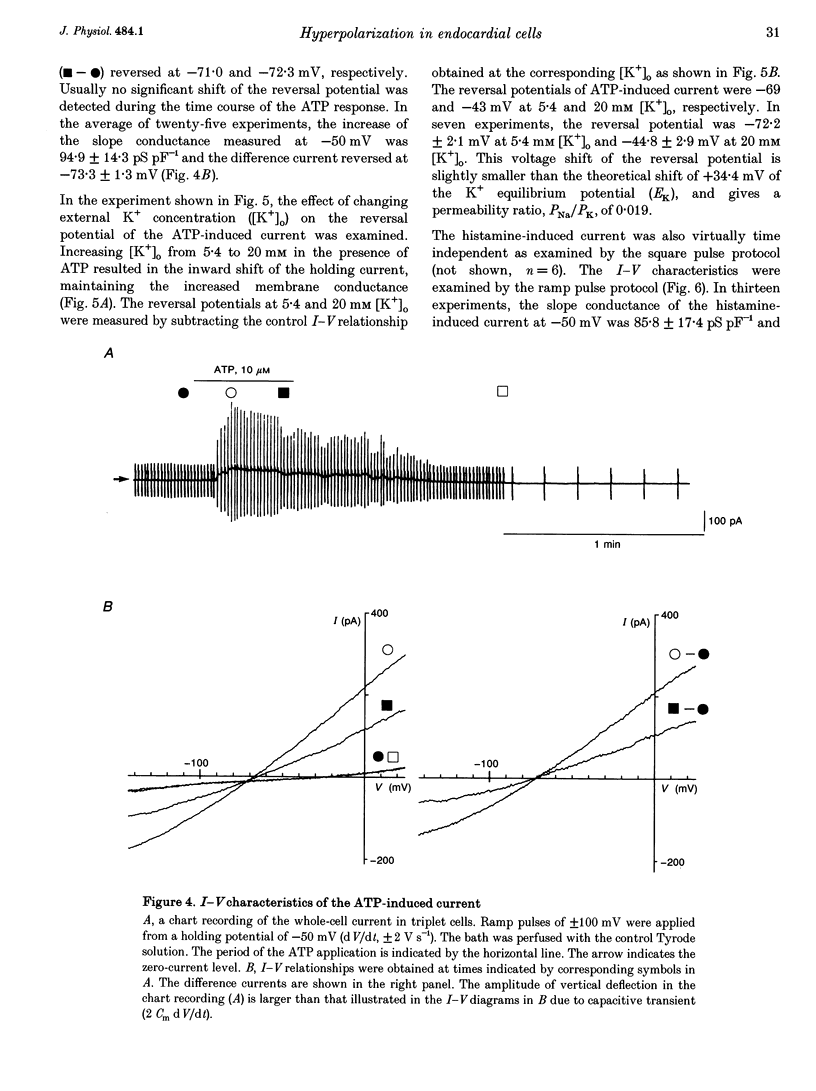
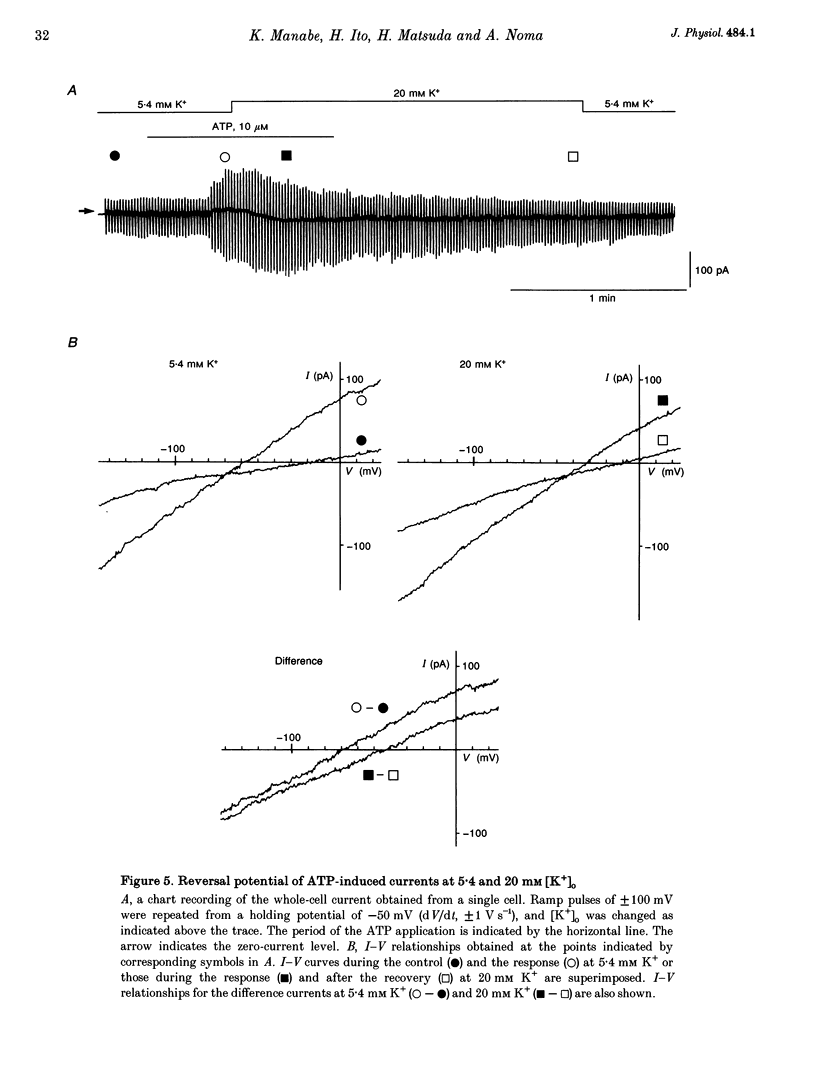
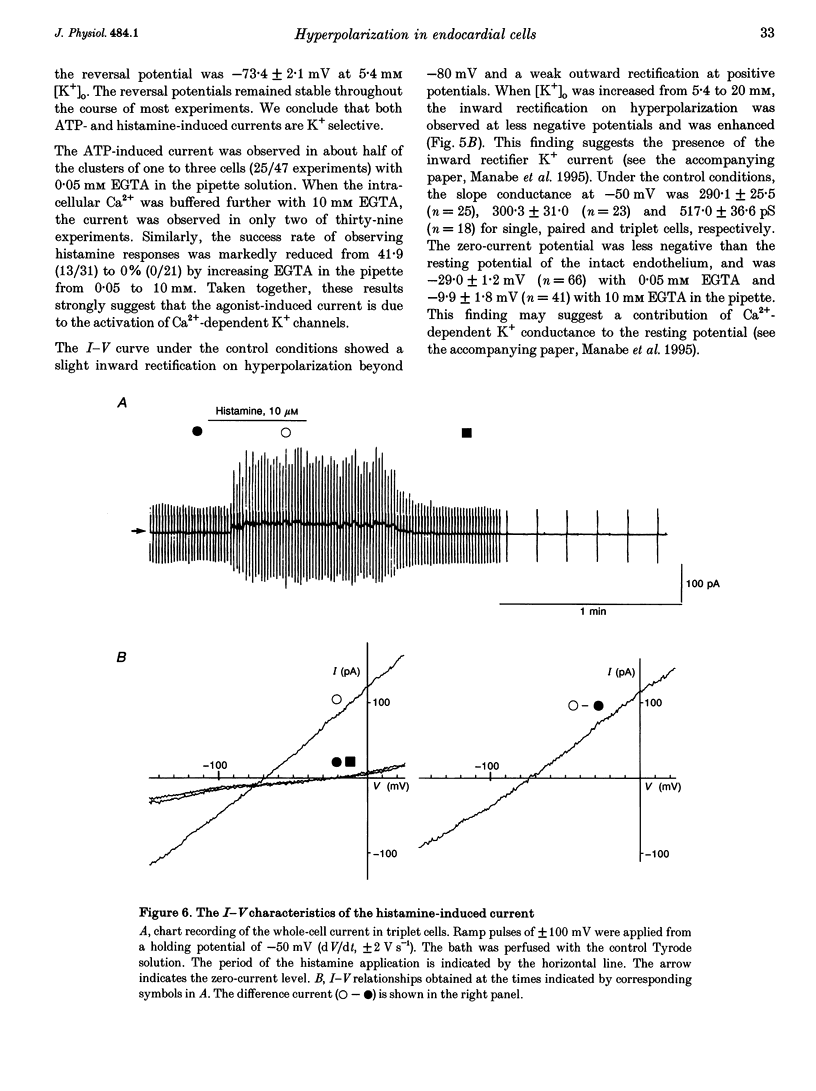
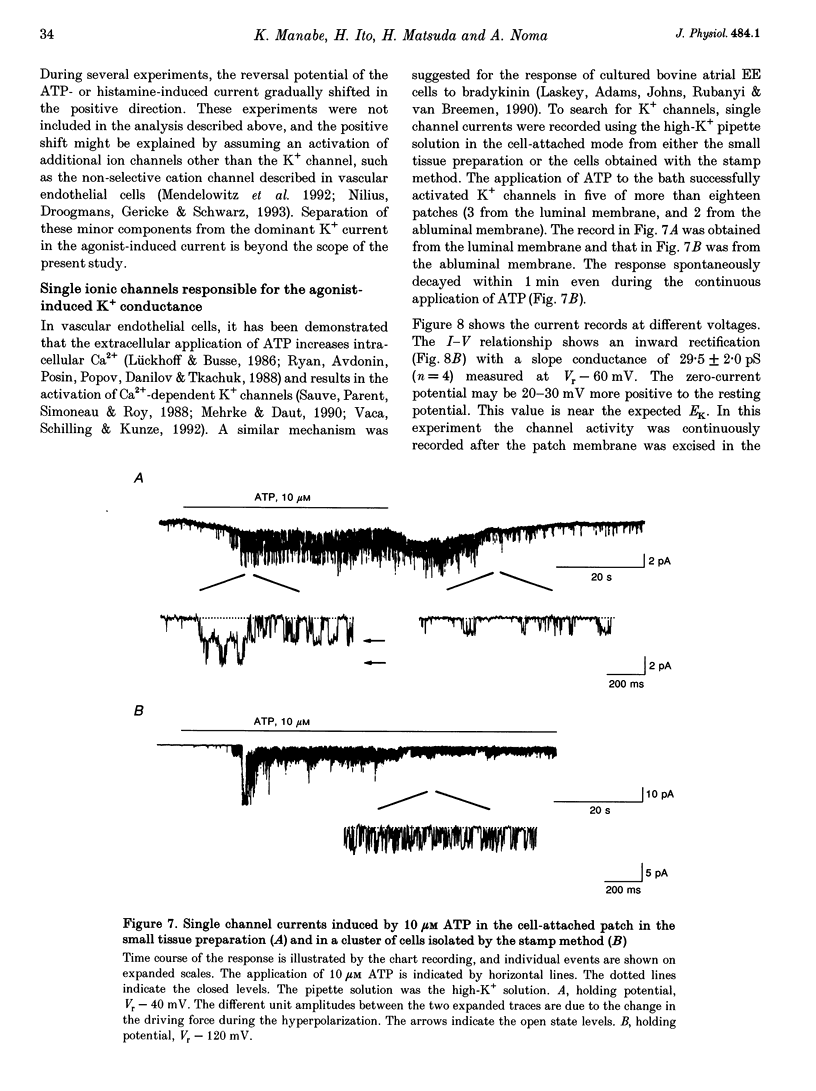
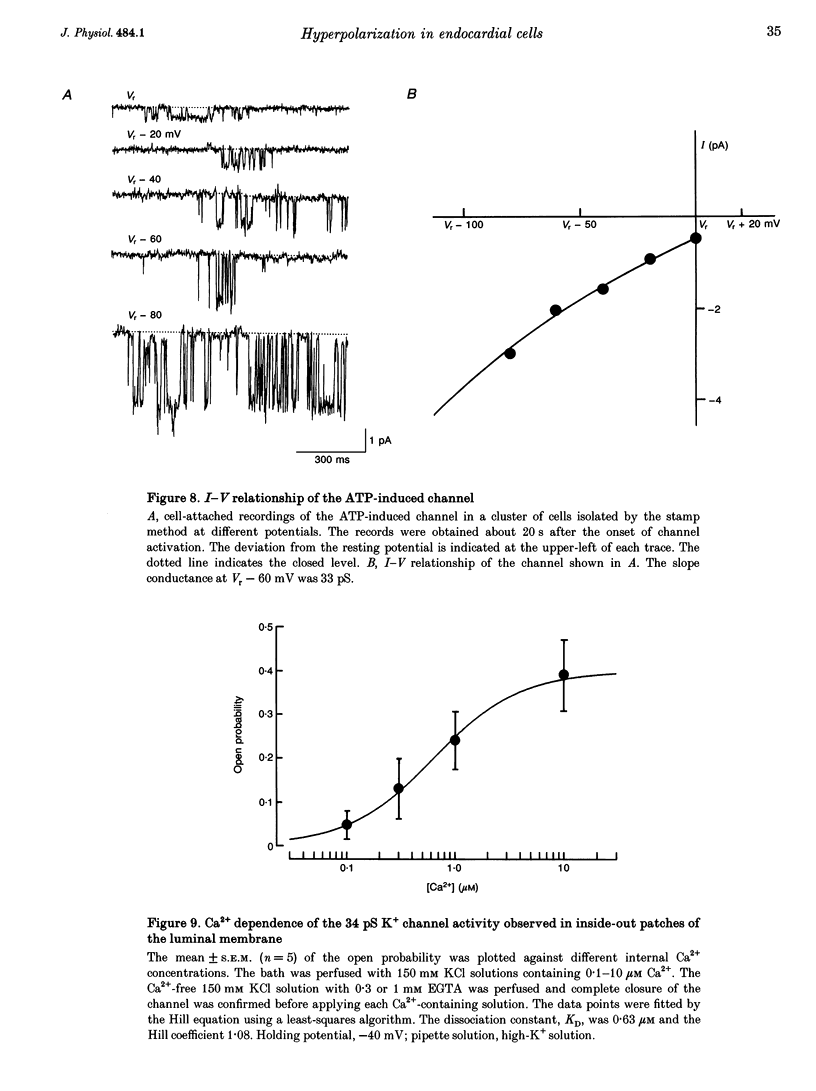
1. The responses of guinea-pig endocardial endothelial (EE) cells to various vasoactive substances were investigated in either the small tissue preparation or freshly isolated cells using the patch clamp technique. 2. The mean resting potential of the EE cell was -44 mV in the small tissue preparation, and applications of ATP, ADP, AMP, adenosine, histamine and substance P induced transient hyperpolarizations of -22, -21, -9, -10, -23 and -15 mV, respectively. The membrane potential of EE cells failed to respond to acetylcholine, bradykinin, thrombin, atrial natriuretic peptide, vasopressin and serotonin. 3. The whole-cell voltage clamp of dissociated cells revealed a transient increase of K+ conductance underlying the ATP and histamine responses. The agonist-induced current showed no time-dependent change during voltage steps. The response was showed no time-dependent change during voltage steps. The response was prevented by adding 10 mM EGTA to the pipette solution. 4. In the cell-attached single channel recordings, ATP induced transient K+ channel activities having a slope conductance of 34 pS. In inside-out patches, similar K+ channels were activated by applying Ca2+ of more than 0.1 microM. 5. These findings are consistent with the idea that the Ca(2+)-dependent K+ channel is involved in the hyperpolarizing response of EE cells, as described in vascular endothelial cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Barakeh J., Laskey R., Van Breemen C. Ion channels and regulation of intracellular calcium in vascular endothelial cells. FASEB J. 1989 Oct;3(12):2389–2400. doi: 10.1096/fasebj.3.12.2477294. [DOI] [PubMed] [Google Scholar]
- Brutsaert D. L., Andries L. J. The endocardial endothelium. Am J Physiol. 1992 Oct;263(4 Pt 2):H985–1002. doi: 10.1152/ajpheart.1992.263.4.H985. [DOI] [PubMed] [Google Scholar]
- Brutsaert D. L. The endocardium. Annu Rev Physiol. 1989;51:263–273. doi: 10.1146/annurev.ph.51.030189.001403. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Sage S. O. Bradykinin-evoked changes in cytosolic calcium and membrane currents in cultured bovine pulmonary artery endothelial cells. J Physiol. 1989 Dec;419:555–568. doi: 10.1113/jphysiol.1989.sp017886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. F., Cheung D. W. Characterization of acetylcholine-induced membrane hyperpolarization in endothelial cells. Circ Res. 1992 Feb;70(2):257–263. doi: 10.1161/01.res.70.2.257. [DOI] [PubMed] [Google Scholar]
- Colden-Stanfield M., Cramer E. B., Gallin E. K. Comparison of apical and basal surfaces of confluent endothelial cells: patch-clamp and viral studies. Am J Physiol. 1992 Sep;263(3 Pt 1):C573–C583. doi: 10.1152/ajpcell.1992.263.3.C573. [DOI] [PubMed] [Google Scholar]
- Daut J., Mehrke G., Nees S., Newman W. H. Passive electrical properties and electrogenic sodium transport of cultured guinea-pig coronary endothelial cells. J Physiol. 1988 Aug;402:237–254. doi: 10.1113/jphysiol.1988.sp017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Ito H., Matsuda H., Noma A. Ion channels in the luminal membrane of endothelial cells of the bull-frog heart. Jpn J Physiol. 1993;43(2):191–206. doi: 10.2170/jjphysiol.43.191. [DOI] [PubMed] [Google Scholar]
- Johns A., Lategan T. W., Lodge N. J., Ryan U. S., Van Breemen C., Adams D. J. Calcium entry through receptor-operated channels in bovine pulmonary artery endothelial cells. Tissue Cell. 1987;19(6):733–745. doi: 10.1016/0040-8166(87)90015-2. [DOI] [PubMed] [Google Scholar]
- Kukovetz W. R., Graier W. F., Groschner K. Contribution of agonist-induced hyperpolarization to Ca2+ influx and formation of EDRF in vascular endothelial cells. Jpn J Pharmacol. 1992;58 (Suppl 2):213P–219P. [PubMed] [Google Scholar]
- Laskey R. E., Adams D. J., Johns A., Rubanyi G. M., van Breemen C. Membrane potential and Na(+)-K+ pump activity modulate resting and bradykinin-stimulated changes in cytosolic free calcium in cultured endothelial cells from bovine atria. J Biol Chem. 1990 Feb 15;265(5):2613–2619. [PubMed] [Google Scholar]
- Laskey R. E., Adams D. J., van Breemen C. Cytosolic [Ca2+] measurements in endothelium of rabbit cardiac valves using imaging fluorescence microscopy. Am J Physiol. 1994 May;266(5 Pt 2):H2130–H2135. doi: 10.1152/ajpheart.1994.266.5.H2130. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Calcium influx into endothelial cells and formation of endothelium-derived relaxing factor is controlled by the membrane potential. Pflugers Arch. 1990 May;416(3):305–311. doi: 10.1007/BF00392067. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Increased free calcium in endothelial cells under stimulation with adenine nucleotides. J Cell Physiol. 1986 Mar;126(3):414–420. doi: 10.1002/jcp.1041260312. [DOI] [PubMed] [Google Scholar]
- Manabe K., Ito H., Matsuda H., Noma A., Shibata Y. Classification of ion channels in the luminal and abluminal membranes of guinea-pig endocardial endothelial cells. J Physiol. 1995 Apr 1;484(Pt 1):41–52. doi: 10.1113/jphysiol.1995.sp020646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko S. M., Sage S. O. Electrical properties of resting and acetylcholine-stimulated endothelium in intact rat aorta. J Physiol. 1993 Mar;462:735–751. doi: 10.1113/jphysiol.1993.sp019579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebazaa A., Mayoux E., Maeda K., Martin L. D., Lakatta E. G., Robotham J. L., Shah A. M. Paracrine effects of endocardial endothelial cells on myocyte contraction mediated via endothelin. Am J Physiol. 1993 Nov;265(5 Pt 2):H1841–H1846. doi: 10.1152/ajpheart.1993.265.5.H1841. [DOI] [PubMed] [Google Scholar]
- Mehrke G., Daut J. The electrical response of cultured guinea-pig coronary endothelial cells to endothelium-dependent vasodilators. J Physiol. 1990 Nov;430:251–272. doi: 10.1113/jphysiol.1990.sp018290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrke G., Pohl U., Daut J. Effects of vasoactive agonists on the membrane potential of cultured bovine aortic and guinea-pig coronary endothelium. J Physiol. 1991 Aug;439:277–299. doi: 10.1113/jphysiol.1991.sp018667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelowitz D., Bacal K., Kunze D. L. Bradykinin-activated calcium influx pathway in bovine aortic endothelial cells. Am J Physiol. 1992 Apr;262(4 Pt 2):H942–H948. doi: 10.1152/ajpheart.1992.262.4.H942. [DOI] [PubMed] [Google Scholar]
- Meulemans A. L., Sipido K. R., Sys S. U., Brutsaert D. L. Atriopeptin III induces early relaxation of isolated mammalian papillary muscle. Circ Res. 1988 Jun;62(6):1171–1174. doi: 10.1161/01.res.62.6.1171. [DOI] [PubMed] [Google Scholar]
- Mistry D. K., Hablitz J. J. Nystatin-perforated patch recordings disclose NMDA-induced outward currents in cultured neocortical neurons. Brain Res. 1990 Dec 10;535(2):318–322. doi: 10.1016/0006-8993(90)91616-o. [DOI] [PubMed] [Google Scholar]
- Newby A. C., Henderson A. H. Stimulus-secretion coupling in vascular endothelial cells. Annu Rev Physiol. 1990;52:661–674. doi: 10.1146/annurev.ph.52.030190.003305. [DOI] [PubMed] [Google Scholar]
- Nilius B., Droogmans G., Gericke M., Schwarz G. Nonselective ion pathways in human endothelial cells. EXS. 1993;66:269–280. doi: 10.1007/978-3-0348-7327-7_21. [DOI] [PubMed] [Google Scholar]
- Olesen S. P., Davies P. F., Clapham D. E. Muscarinic-activated K+ current in bovine aortic endothelial cells. Circ Res. 1988 Jun;62(6):1059–1064. doi: 10.1161/01.res.62.6.1059. [DOI] [PubMed] [Google Scholar]
- Patlak J. B. Sodium channel subconductance levels measured with a new variance-mean analysis. J Gen Physiol. 1988 Oct;92(4):413–430. doi: 10.1085/jgp.92.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan U. S., Avdonin P. V., Posin E. Y., Popov E. G., Danilov S. M., Tkachuk V. A. Influence of vasoactive agents on cytoplasmic free calcium in vascular endothelial cells. J Appl Physiol (1985) 1988 Nov;65(5):2221–2227. doi: 10.1152/jappl.1988.65.5.2221. [DOI] [PubMed] [Google Scholar]
- Sauve R., Parent L., Simoneau C., Roy G. External ATP triggers a biphasic activation process of a calcium-dependent K+ channel in cultured bovine aortic endothelial cells. Pflugers Arch. 1988 Oct;412(5):469–481. doi: 10.1007/BF00582535. [DOI] [PubMed] [Google Scholar]
- Schoemaker I. E., Meulemans A. L., Andries L. J., Brutsaert D. L. Role of endocardial endothelium in positive inotropic action of vasopressin. Am J Physiol. 1990 Oct;259(4 Pt 2):H1148–H1151. doi: 10.1152/ajpheart.1990.259.4.H1148. [DOI] [PubMed] [Google Scholar]
- Shah A. M., Meulemans A. L., Brutsaert D. L. Myocardial inotropic responses to aggregating platelets and modulation by the endocardium. Circulation. 1989 Jun;79(6):1315–1323. doi: 10.1161/01.cir.79.6.1315. [DOI] [PubMed] [Google Scholar]
- Smith J. A., Shah A. M., Lewis M. J. Factors released from endocardium of the ferret and pig modulate myocardial contraction. J Physiol. 1991 Aug;439:1–14. doi: 10.1113/jphysiol.1991.sp018653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Klepper M. Voltage-dependent and agonist-activated ionic currents in vascular endothelial cells: a review. Blood Vessels. 1990;27(2-5):169–183. doi: 10.1159/000158808. [DOI] [PubMed] [Google Scholar]
- Tracey W. R., Peach M. J. Differential muscarinic receptor mRNA expression by freshly isolated and cultured bovine aortic endothelial cells. Circ Res. 1992 Feb;70(2):234–240. doi: 10.1161/01.res.70.2.234. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta. 1980 Jul;599(2):623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Vaca L., Schilling W. P., Kunze D. L. G-protein-mediated regulation of a Ca(2+)-dependent K+ channel in cultured vascular endothelial cells. Pflugers Arch. 1992 Oct;422(1):66–74. doi: 10.1007/BF00381515. [DOI] [PubMed] [Google Scholar]
- Wang J., Morgan J. P. Endocardial endothelium modulates myofilament Ca2+ responsiveness in aequorin-loaded ferret myocardium. Circ Res. 1992 Apr;70(4):754–760. doi: 10.1161/01.res.70.4.754. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Chen G., Miwa K., Suzuki H. Permeability and Mg2+ blockade of histamine-operated cation channel in endothelial cells of rat intrapulmonary artery. J Physiol. 1992 May;450:395–408. doi: 10.1113/jphysiol.1992.sp019133. [DOI] [PMC free article] [PubMed] [Google Scholar]


