Abstract
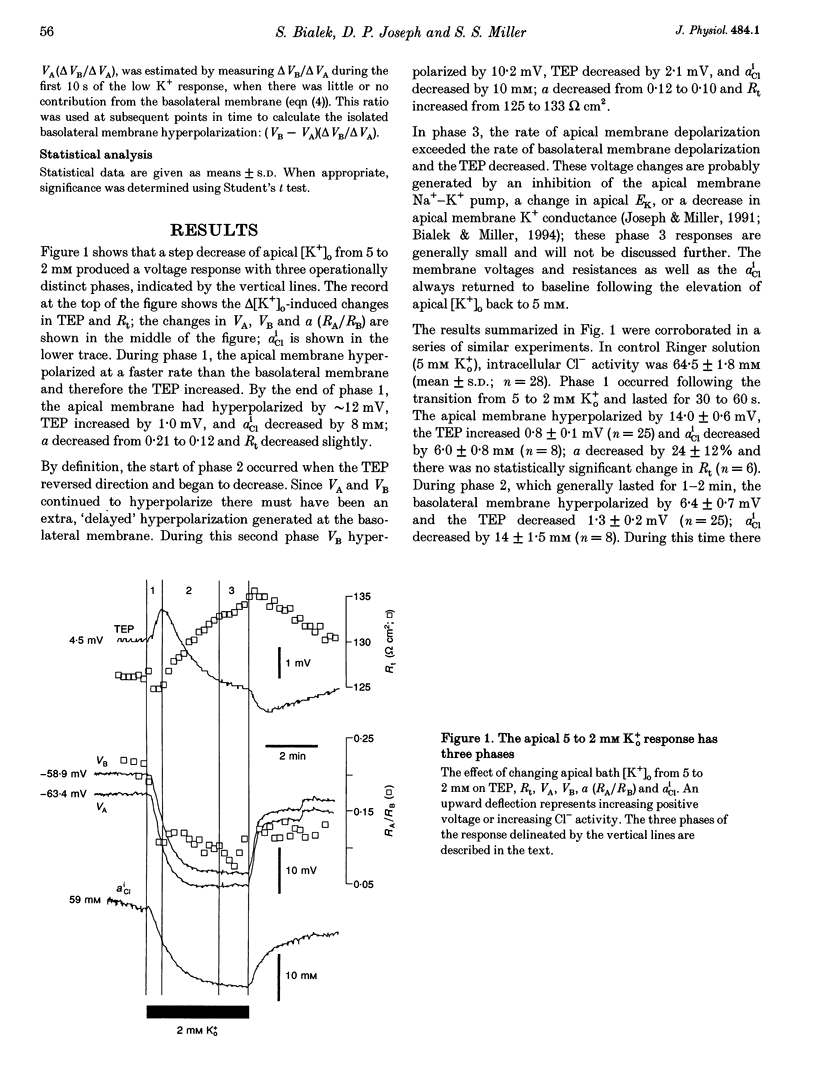
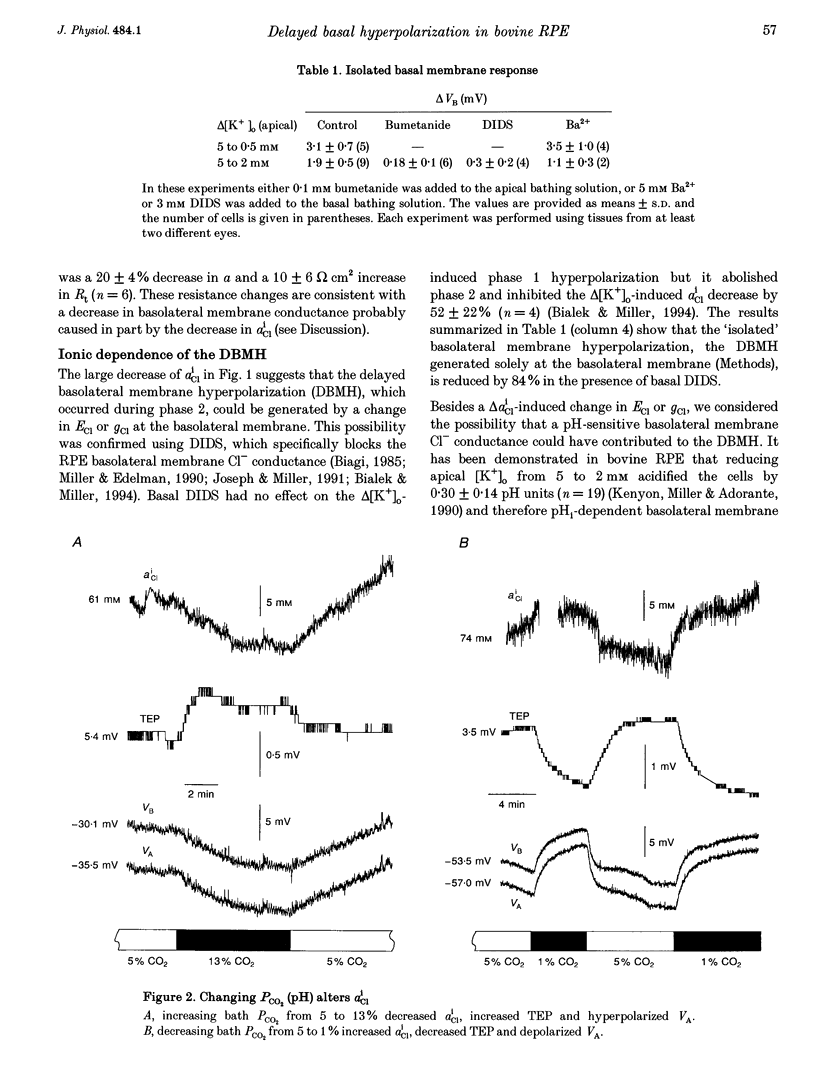
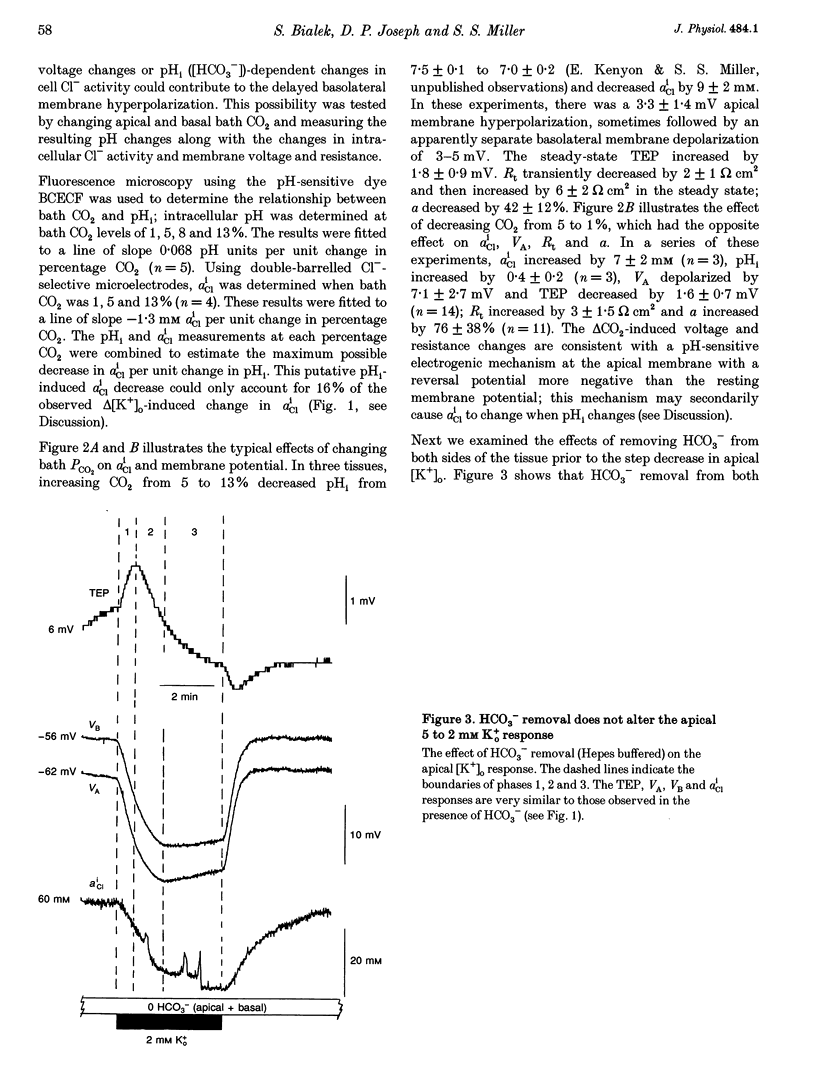
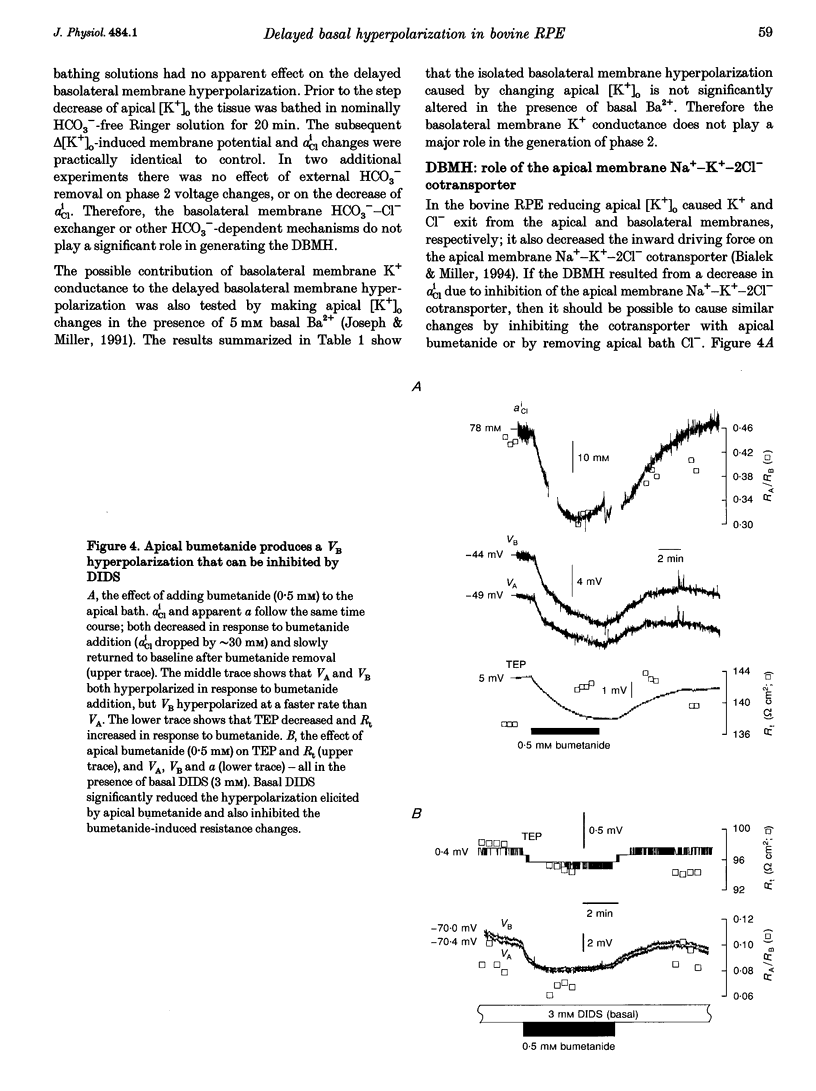
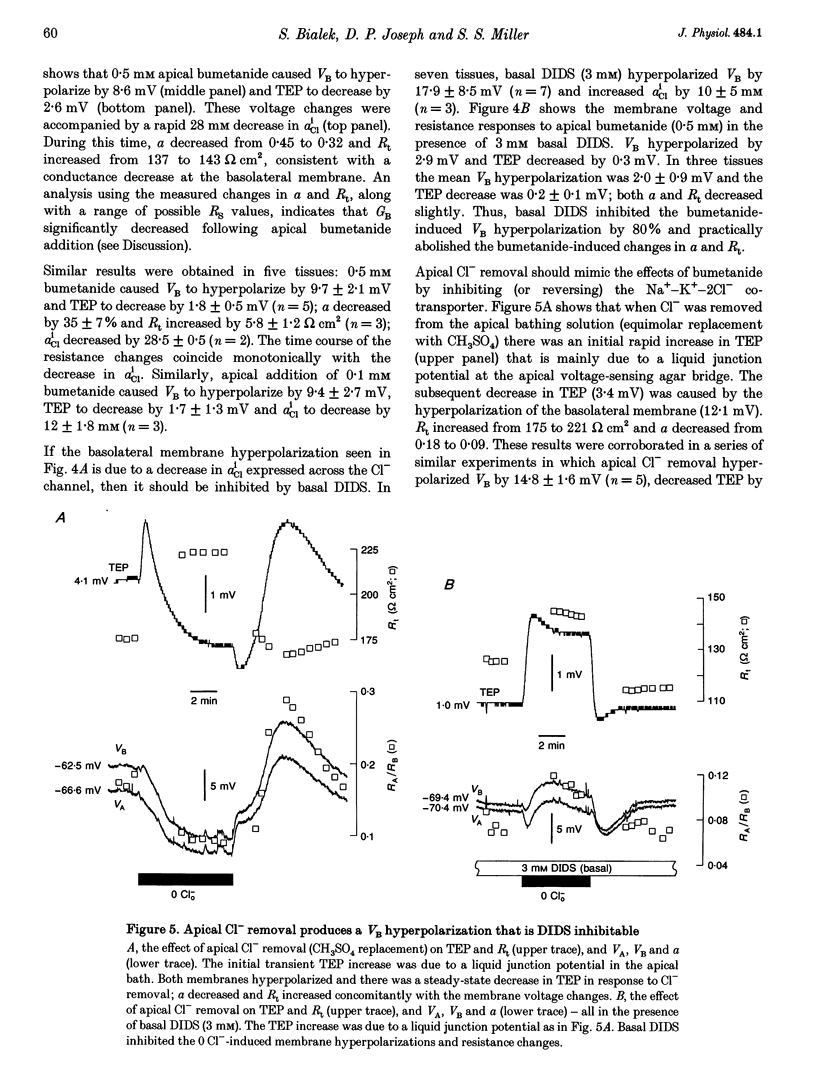
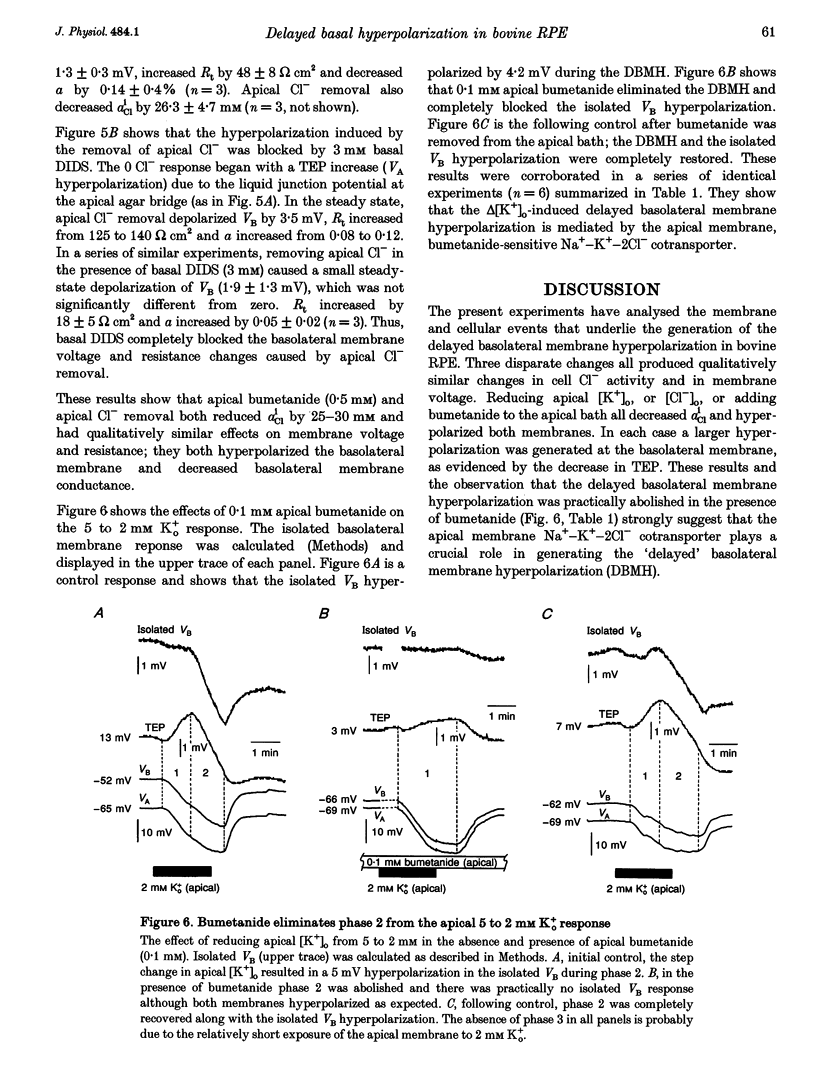
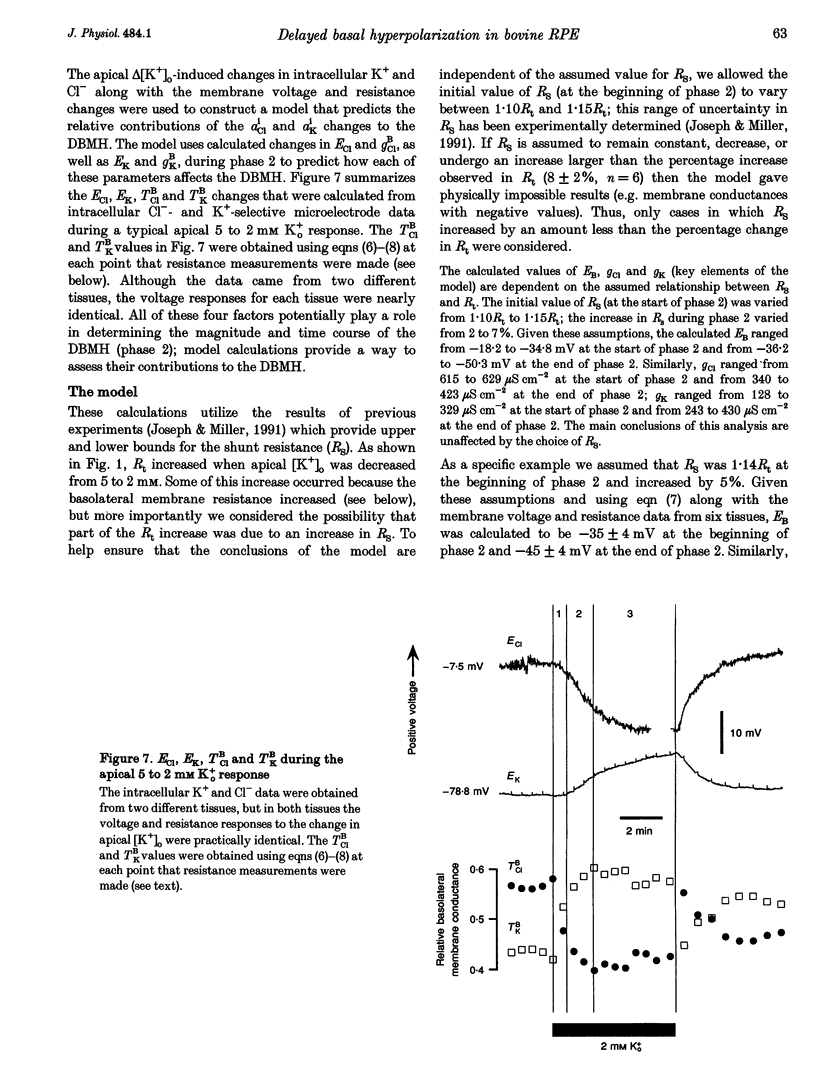
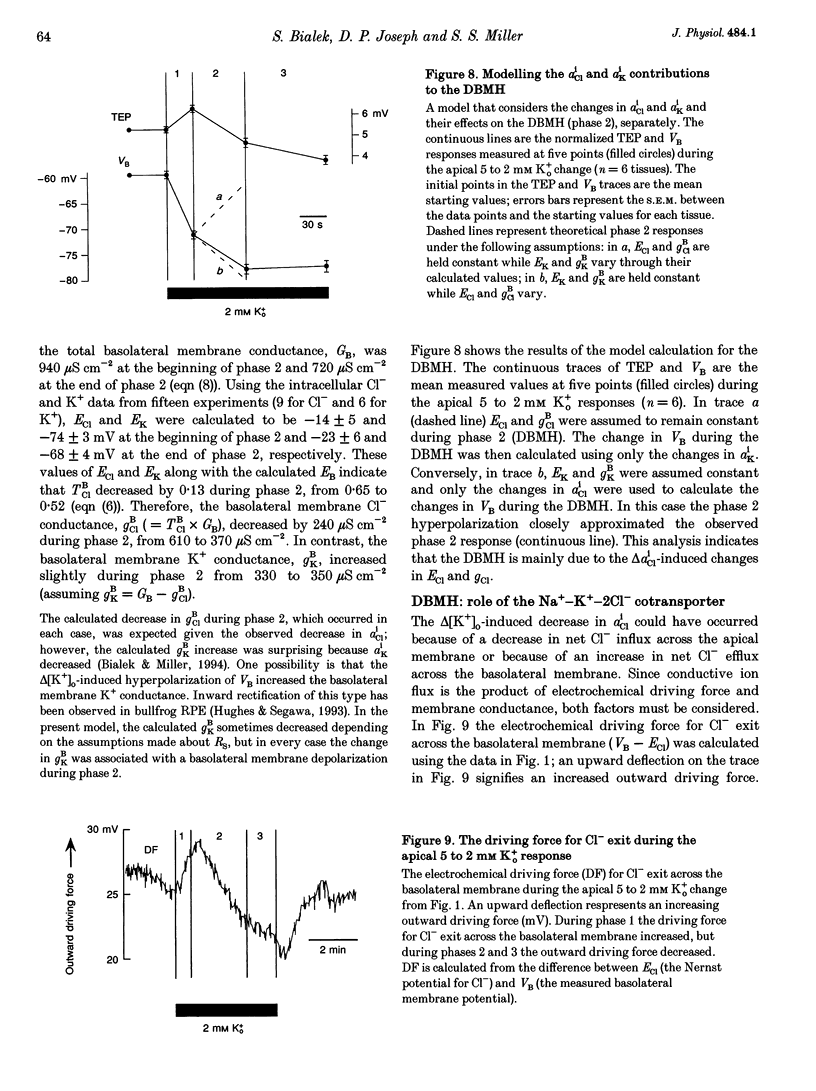
1. Conventional and ion-selective double-barrelled microelectrodes were used in an in vitro preparation of bovine retinal pigment epithelium (RPE)-choroid to measure the changes in membrane voltage, resistance and intracellular Cl- activity (aCli) produced by small, physiological changes in extracellular potassium concentration ([K+]o). These apical [K+]o changes approximate those produced in the extracellular (subretinal) space between the photoreceptors and the RPE following transitions between light and dark. 2. Changing apical [K+]o from 5 to 2 mM in vitro elicited membrane voltage responses with three distinct phases. The first phase was generated by an apical membrane hyperpolarization, followed by a (delayed) basolateral membrane hyperpolarization (DBMH); the third phase was an apical membrane depolarization. The present experiments focus on the membrane and cellular mechanisms that generate phase 2 of the response, the DBMH. 3. The DBMH was abolished in the presence of apical bumetanide (100 microM); this response was completely restored after bumetanide removal. 4. Reducing apical [K+]o, adding apical bumetanide (500 mM), or removing apical Cl- decreased aCli by 25 +/- 6 (n = 8), 28 +/- 1 (n = 2) and 26 +/- 5 mM (n = 3), respectively; adding 100 microM apical bumetanide decreased aCli by 12 +/- 2 mM (n = 3). Adding apical bumetanide or removing apical bath Cl- hyperpolarized the basolateral membrane and decreased the apparent basolateral membrane conductance (GB). 5. DIDS (4,4'-diisothiocyanostilbene-2,2'-disulphonic acid) blocked the RPE basolateral membrane Cl- conductance and inhibited the DBMH and the basolateral membrane hyperpolarization produced by apical bumetanide addition or by removal of apical Cl-o. The present results show that the DBMH is caused by delta[K]o-induced inhibition of the apical membrane Na(+)-K(+)-2Cl- cotransporter; the subsequent decrease in aCli generated a hyperpolarization at the basolateral membrane Cl- channel.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. P., Welsh M. J. Calcium and cAMP activate different chloride channels in the apical membrane of normal and cystic fibrosis epithelia. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6003–6007. doi: 10.1073/pnas.88.14.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi B. A. Effects of the anion transport inhibitor, SITS, on the proximal straight tubule of the rabbit perfused in vitro. J Membr Biol. 1985;88(1):25–31. doi: 10.1007/BF01871210. [DOI] [PubMed] [Google Scholar]
- Bialek S., Miller S. S. K+ and Cl- transport mechanisms in bovine pigment epithelium that could modulate subretinal space volume and composition. J Physiol. 1994 Mar 15;475(3):401–417. doi: 10.1113/jphysiol.1994.sp020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearry A., Burnside B. Light-induced dopamine release from teleost retinas acts as a light-adaptive signal to the retinal pigment epithelium. J Neurochem. 1989 Sep;53(3):870–878. doi: 10.1111/j.1471-4159.1989.tb11785.x. [DOI] [PubMed] [Google Scholar]
- Edelman J. L., Miller S. S. Epinephrine stimulates fluid absorption across bovine retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1991 Nov;32(12):3033–3040. [PubMed] [Google Scholar]
- Gallemore R. P., Steinberg R. H. Light-evoked modulation of basolateral membrane Cl- conductance in chick retinal pigment epithelium: the light peak and fast oscillation. J Neurophysiol. 1993 Oct;70(4):1669–1680. doi: 10.1152/jn.1993.70.4.1669. [DOI] [PubMed] [Google Scholar]
- Garcia-Diaz J. F., Stump S., Armstrong W. M. Electronic device for microelectrode recordings in epithelial cells. Am J Physiol. 1984 Mar;246(3 Pt 1):C339–C346. doi: 10.1152/ajpcell.1984.246.3.C339. [DOI] [PubMed] [Google Scholar]
- García D. M., Burnside B. Suppression of cAMP-induced pigment granule aggregation in RPE by organic anion transport inhibitors. Invest Ophthalmol Vis Sci. 1994 Jan;35(1):178–188. [PubMed] [Google Scholar]
- Griff E. R. Potassium-evoked responses from the retinal pigment epithelium of the toad Bufo marinus. Exp Eye Res. 1991 Aug;53(2):219–228. doi: 10.1016/0014-4835(91)90077-r. [DOI] [PubMed] [Google Scholar]
- Griff E. R., Steinberg R. H. Changes in apical [K+] produce delayed basal membrane responses of the retinal pigment epithelium in the gecko. J Gen Physiol. 1984 Feb;83(2):193–211. doi: 10.1085/jgp.83.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griff E. R., Steinberg R. H. Origin of the light peak: in vitro study of Gekko gekko. J Physiol. 1982 Oct;331:637–652. doi: 10.1113/jphysiol.1982.sp014395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes B. A., Adorante J. S., Miller S. S., Lin H. Apical electrogenic NaHCO3 cotransport. A mechanism for HCO3 absorption across the retinal pigment epithelium. J Gen Physiol. 1989 Jul;94(1):125–150. doi: 10.1085/jgp.94.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue I. Voltage-dependent chloride conductance of the squid axon membrane and its blockade by some disulfonic stilbene derivatives. J Gen Physiol. 1985 Apr;85(4):519–537. doi: 10.1085/jgp.85.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C., Finkbeiner W. E., Widdicombe J. H., McCray P. B., Jr, Miller S. S. Altered fluid transport across airway epithelium in cystic fibrosis. Science. 1993 Oct 15;262(5132):424–427. doi: 10.1126/science.8211164. [DOI] [PubMed] [Google Scholar]
- Joseph D. P., Miller S. S. Alpha-1-adrenergic modulation of K and Cl transport in bovine retinal pigment epithelium. J Gen Physiol. 1992 Feb;99(2):263–290. doi: 10.1085/jgp.99.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph D. P., Miller S. S. Apical and basal membrane ion transport mechanisms in bovine retinal pigment epithelium. J Physiol. 1991 Apr;435:439–463. doi: 10.1113/jphysiol.1991.sp018518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon E., Yu K., La Cour M., Miller S. S. Lactate transport mechanisms at apical and basolateral membranes of bovine retinal pigment epithelium. Am J Physiol. 1994 Dec;267(6 Pt 1):C1561–C1573. doi: 10.1152/ajpcell.1994.267.6.C1561. [DOI] [PubMed] [Google Scholar]
- Lin H., Miller S. S. pHi regulation in frog retinal pigment epithelium: two apical membrane mechanisms. Am J Physiol. 1991 Jul;261(1 Pt 1):C132–C142. doi: 10.1152/ajpcell.1991.261.1.C132. [DOI] [PubMed] [Google Scholar]
- Lin H., Miller S. S. pHi-dependent Cl-HCO3 exchange at the basolateral membrane of frog retinal pigment epithelium. Am J Physiol. 1994 Apr;266(4 Pt 1):C935–C945. doi: 10.1152/ajpcell.1994.266.4.C935. [DOI] [PubMed] [Google Scholar]
- Linsenmeier R. A., Steinberg R. H. Delayed basal hyperpolarization of cat retinal pigment epithelium and its relation to the fast oscillation of the DC electroretinogram. J Gen Physiol. 1984 Feb;83(2):213–232. doi: 10.1085/jgp.83.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmeier R. A., Steinberg R. H. Origin and sensitivity of the light peak in the intact cat eye. J Physiol. 1982 Oct;331:653–673. doi: 10.1113/jphysiol.1982.sp014396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. S., Edelman J. L. Active ion transport pathways in the bovine retinal pigment epithelium. J Physiol. 1990 May;424:283–300. doi: 10.1113/jphysiol.1990.sp018067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. S., Steinberg R. H. Passive ionic properties of frog retinal pigment epithelium. J Membr Biol. 1977 Sep 15;36(4):337–372. doi: 10.1007/BF01868158. [DOI] [PubMed] [Google Scholar]
- Newman E. A. Membrane physiology of retinal glial (Müller) cells. J Neurosci. 1985 Aug;5(8):2225–2239. doi: 10.1523/JNEUROSCI.05-08-02225.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B., 2nd, Miller S. S., Steinberg R. H. Effect of intracellular potassium upon the electrogenic pump of frog retinal pigment epithelium. J Membr Biol. 1978 Dec 29;44(3-4):281–307. doi: 10.1007/BF01944225. [DOI] [PubMed] [Google Scholar]
- Quinn R. H., Miller S. S. Ion transport mechanisms in native human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1992 Dec;33(13):3513–3527. [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982 Oct;95(1):189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki H., Oakley B., 2nd Reaccumulation of [K+]o in the toad retina during maintained illumination. J Gen Physiol. 1984 Sep;84(3):475–504. doi: 10.1085/jgp.84.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg R. H., Schmidt R., Brown K. T. Intracellular responses to light from cat pigment epithelium: origin of the electroretinogram c-wave. Nature. 1970 Aug 15;227(5259):728–730. doi: 10.1038/227728a0. [DOI] [PubMed] [Google Scholar]
- Stutts M. J., Chinet T. C., Mason S. J., Fullton J. M., Clarke L. L., Boucher R. C. Regulation of Cl- channels in normal and cystic fibrosis airway epithelial cells by extracellular ATP. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1621–1625. doi: 10.1073/pnas.89.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeton J. M., van Norren D. Intraretinal recordings of slow electrical responses to steady illumination in monkey: isolation of receptor responses and the origin of the light peak. Vision Res. 1982;22(3):393–399. doi: 10.1016/0042-6989(82)90155-9. [DOI] [PubMed] [Google Scholar]
- Weleber R. G. Fast and slow oscillations of the electro-oculogram in Best's macular dystrophy and retinitis pigmentosa. Arch Ophthalmol. 1989 Apr;107(4):530–537. doi: 10.1001/archopht.1989.01070010544028. [DOI] [PubMed] [Google Scholar]
- Yamamoto F., Borgula G. A., Steinberg R. H. Effects of light and darkness on pH outside rod photoreceptors in the cat retina. Exp Eye Res. 1992 May;54(5):685–697. doi: 10.1016/0014-4835(92)90023-l. [DOI] [PubMed] [Google Scholar]


