Abstract
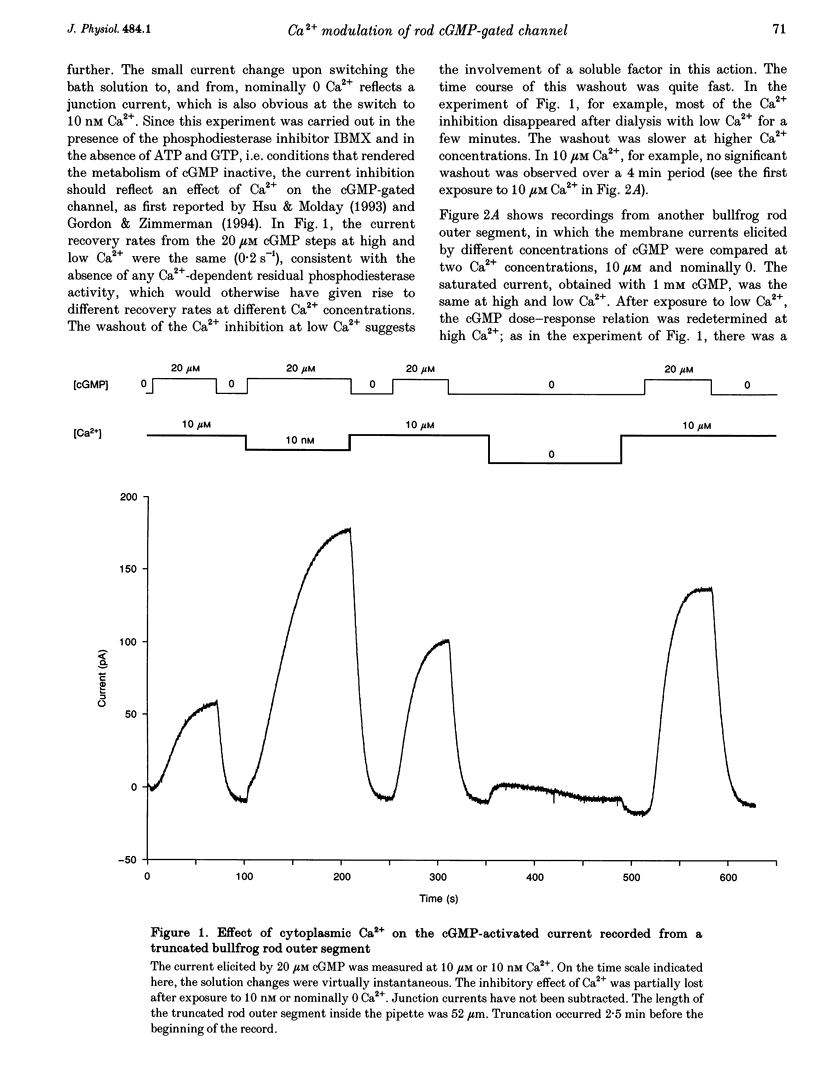
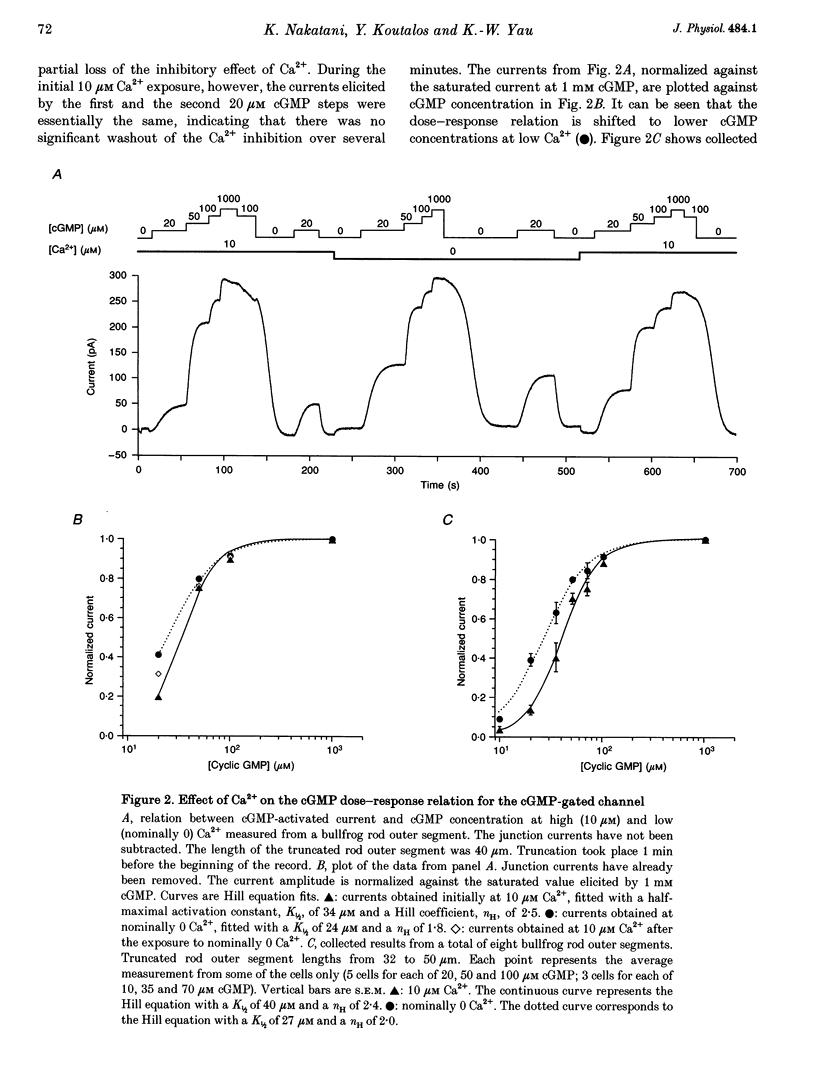
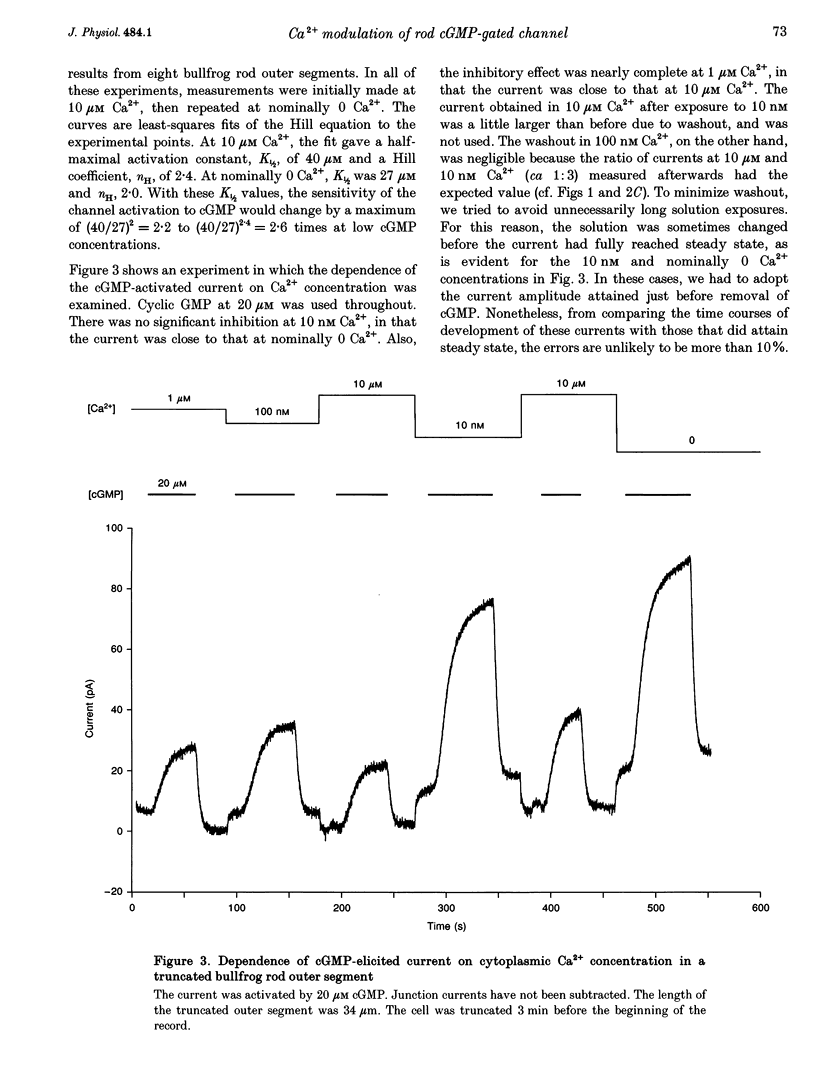
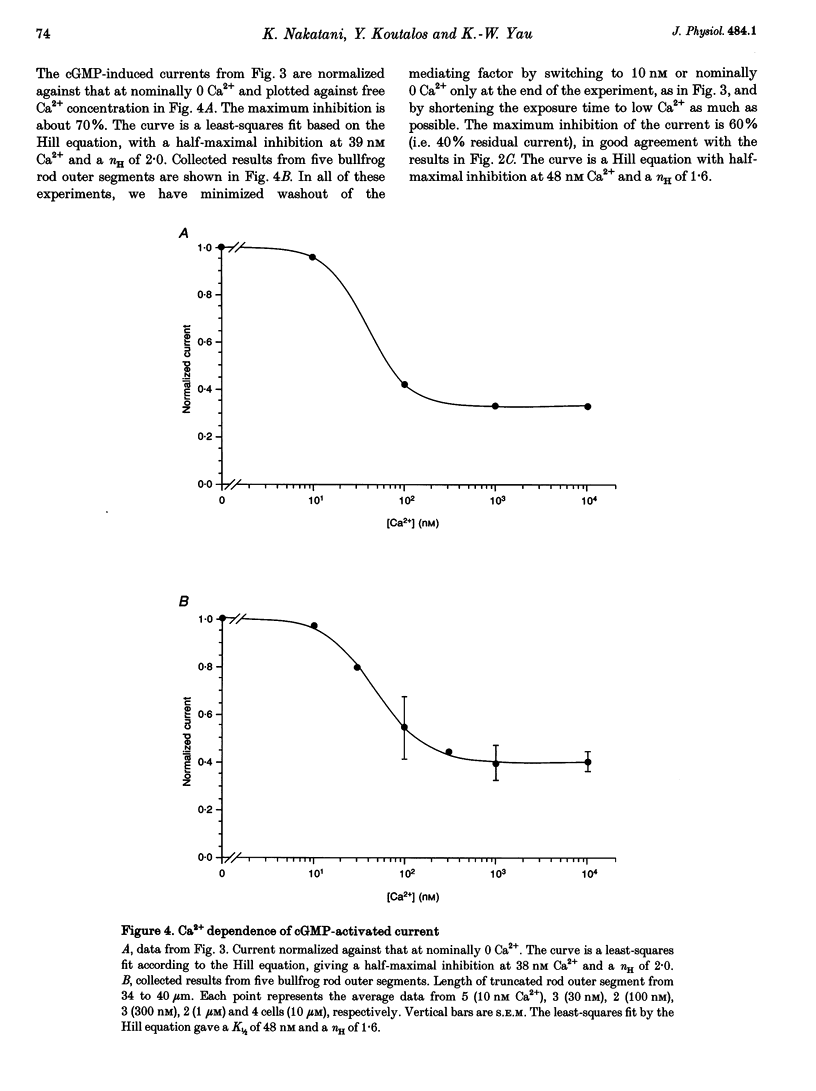
1. The outer segment of an isolated rod photoreceptor from the bullfrog retina was drawn into a pipette containing choline solution for recording membrane current. The rest of the cell was sheared off with a glass probe to allow internal dialysis of the outer segment with a bath potassium solution ('truncated rod outer segment' preparation). The potential between the inside and the outside of the pipette was held at 0 mV. 2. Application of bath cGMP, in the presence of 3-isobutyl-1-methylxanthine (IBMX), gave rise to an outward membrane current. At saturating cGMP concentrations, this current was insensitive to intracellular Ca2+ at concentrations between 0 and 10 microM. At subsaturating cGMP concentrations, however, this current was inhibited by intracellular Ca2+. This sensitivity to Ca2+ declined after dialysis with a low-Ca2+ solution, suggesting the involvement of a soluble factor. 3. At low (nominally 0) Ca2+, the half-maximal activation constant and Hill coefficient for the activation of the cGMP-gated current by cGMP were 27 microM and 2.0, respectively. At high (ca 10 microM) Ca2+, the corresponding values were 40 microM cGMP and 2.4. 4. The inhibition of the current by Ca2+ was characterized at 20 microM cGMP. Ca2+ inhibited the current by up to 60%, with half-maximal inhibition at 48 nM Ca2+ and a Hill coefficient of 1.6.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cervetto L., Lagnado L., Perry R. J., Robinson D. W., McNaughton P. A. Extrusion of calcium from rod outer segments is driven by both sodium and potassium gradients. Nature. 1989 Feb 23;337(6209):740–743. doi: 10.1038/337740a0. [DOI] [PubMed] [Google Scholar]
- Chen T. Y., Illing M., Molday L. L., Hsu Y. T., Yau K. W., Molday R. S. Subunit 2 (or beta) of retinal rod cGMP-gated cation channel is a component of the 240-kDa channel-associated protein and mediates Ca(2+)-calmodulin modulation. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11757–11761. doi: 10.1073/pnas.91.24.11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detwiler P. B., Gray-Keller M. P. Some unresolved issues in the physiology and biochemistry of phototransduction. Curr Opin Neurobiol. 1992 Aug;2(4):433–438. doi: 10.1016/0959-4388(92)90176-l. [DOI] [PubMed] [Google Scholar]
- Gray-Keller M. P., Polans A. S., Palczewski K., Detwiler P. B. The effect of recoverin-like calcium-binding proteins on the photoresponse of retinal rods. Neuron. 1993 Mar;10(3):523–531. doi: 10.1016/0896-6273(93)90339-s. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J., Yau K. W. Effect of ions on retinal rods from Bufo marinus. J Physiol. 1984 May;350:649–680. doi: 10.1113/jphysiol.1984.sp015223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y. T., Molday R. S. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature. 1993 Jan 7;361(6407):76–79. doi: 10.1038/361076a0. [DOI] [PubMed] [Google Scholar]
- Kawamura S., Murakami M. Calcium-dependent regulation of cyclic GMP phosphodiesterase by a protein from frog retinal rods. Nature. 1991 Jan 31;349(6308):420–423. doi: 10.1038/349420a0. [DOI] [PubMed] [Google Scholar]
- Kawamura S. Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature. 1993 Apr 29;362(6423):855–857. doi: 10.1038/362855a0. [DOI] [PubMed] [Google Scholar]
- Kilhoffer M. C., Haiech J., Demaille J. G. Ion binding to calmodulin. A comparison with other intracellular calcium-binding proteins. Mol Cell Biochem. 1983;51(1):33–54. doi: 10.1007/BF00215584. [DOI] [PubMed] [Google Scholar]
- Koch K. W., Stryer L. Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature. 1988 Jul 7;334(6177):64–66. doi: 10.1038/334064a0. [DOI] [PubMed] [Google Scholar]
- Kohnken R. E., Chafouleas J. G., Eadie D. M., Means A. R., McConnell D. G. Calmodulin in bovine rod outer segments. J Biol Chem. 1981 Dec 10;256(23):12517–12522. [PubMed] [Google Scholar]
- Koutalos Y., Nakatani K., Yau K. W. Cyclic GMP diffusion coefficient in rod photoreceptor outer segments. Biophys J. 1995 Jan;68(1):373–382. doi: 10.1016/S0006-3495(95)80198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutalos Y., Yau K. W. A rich complexity emerges in phototransduction. Curr Opin Neurobiol. 1993 Aug;3(4):513–519. doi: 10.1016/0959-4388(93)90049-5. [DOI] [PubMed] [Google Scholar]
- Lagnado L., Baylor D. A. Calcium controls light-triggered formation of catalytically active rhodopsin. Nature. 1994 Jan 20;367(6460):273–277. doi: 10.1038/367273a0. [DOI] [PubMed] [Google Scholar]
- Lagnado L., Baylor D. Signal flow in visual transduction. Neuron. 1992 Jun;8(6):995–1002. doi: 10.1016/0896-6273(92)90122-t. [DOI] [PubMed] [Google Scholar]
- Matthews H. R., Murphy R. L., Fain G. L., Lamb T. D. Photoreceptor light adaptation is mediated by cytoplasmic calcium concentration. Nature. 1988 Jul 7;334(6177):67–69. doi: 10.1038/334067a0. [DOI] [PubMed] [Google Scholar]
- Nagao S., Yamazaki A., Bitensky M. W. Calmodulin and calmodulin binding proteins in amphibian rod outer segments. Biochemistry. 1987 Mar 24;26(6):1659–1665. doi: 10.1021/bi00380a026. [DOI] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Calcium and light adaptation in retinal rods and cones. Nature. 1988 Jul 7;334(6177):69–71. doi: 10.1038/334069a0. [DOI] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Calcium and magnesium fluxes across the plasma membrane of the toad rod outer segment. J Physiol. 1988 Jan;395:695–729. doi: 10.1113/jphysiol.1988.sp016942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K., Yau K. W. Guanosine 3',5'-cyclic monophosphate-activated conductance studied in a truncated rod outer segment of the toad. J Physiol. 1988 Jan;395:731–753. doi: 10.1113/jphysiol.1988.sp016943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh E. N., Jr, Lamb T. D. Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta. 1993 Mar 1;1141(2-3):111–149. doi: 10.1016/0005-2728(93)90038-h. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Baylor D. A. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 1984 Oct 18;311(5987):661–663. doi: 10.1038/311661a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., Nakatani K. Light-suppressible, cyclic GMP-sensitive conductance in the plasma membrane of a truncated rod outer segment. Nature. 1985 Sep 19;317(6034):252–255. doi: 10.1038/317252a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W. Phototransduction mechanism in retinal rods and cones. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1994 Jan;35(1):9–32. [PubMed] [Google Scholar]


