Abstract
The gut microbiota (GM) and immune cells (IC) are increasingly recognized as key players in cancer development and progression. This study aimed to explore the potential mediating role of IC in the causal relationship between GM and thyroid cancer (TC) using Mendelian randomization (MR) analysis. Data from genome-wide association studies (GWAS) encompassing 473 GM species, 731 IC types, and TC were utilized. MR analysis identified nine GM species with significant causal relationships to TC, mediated by 10 IC phenotypes such as "Switched Memory AC," "IgD-CD38dim AC," and "EM DN (CD4-CD8-) AC." These findings suggest a complex interplay where specific IC mediate the effects of GM on TC risk. Sensitivity analyses confirmed the robustness of these results, with no evidence of horizontal pleiotropy. This study highlights potential mechanisms linking GM and IC to TC, offering insights that could inform GM-based immunotherapeutic strategies and IC-targeted interventions. However, further experimental research is needed to validate these causal pathways and better understand the underlying biological mechanisms.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-024-01585-x.
Keywords: Gut microbiota, Immune cells, Thyroid cancer, Mendelian randomization, Mediation analysis
Introduction
Since the 1990s, thyroid cancer (TC) has risen dramatically in incidence, ranking fifth in diagnosis and mortality among women diagnosed with cancer [1]. The overall prognosis of TC is generally favorable, however, 6–20% of patients will develop regional or distant metastases, immune system regulation has an impact on TC risk and progression [2]. The development and progression of cancer are influenced by complex molecular mechanisms that underpin tumor behavior and treatment responses. Key signaling pathways and oncoproteins are often implicated in these processes. For example, certain proteins involved in cell cycle regulation and microtubule stability can affect how cancer cells respond to chemotherapeutic agents, particularly in the context of drug resistance [3]. Additionally, pathways such as PI3K/AKT/mTOR have been highlighted for their role in regulating cancer cell growth, survival, and metabolic activity [4]. Recent findings suggest that modulating these pathways may provide adjunctive benefits to standard cancer therapies by enhancing the immune response and targeting tumor progression.
The human gut microbiota (GM) is closely related to the host's metabolism, immunity, and health, playing an important role in the development of adaptive immune systems and the innate, which coordinate and maintain host-microbial symbiosis [5]. The GM modulates cancer immunotherapy and its immune-related adverse reactions. Fecal microbiota transplantation can improve the success rate of immunotherapy in cancer patients [6]. Immune cells (IC) are equally significant due to their central role in anti-tumor immunity and immune surveillance. They mediate responses that can either suppress or promote tumor growth, depending on the context and the interaction with external factors, including the GM. The rationale for targeting GM and IC in this study lies in their synergistic impact: GM-derived metabolites can modulate the activation and differentiation of IC, influencing the immune response against cancer. By focusing on these primary targets, the study aims to uncover the underlying mechanisms that could explain variability in tumor progression and treatment outcomes, potentially leading to innovative therapeutic strategies that enhance immune function through GM manipulation and IC-targeted interventions.
Cancer is a multifaceted disease characterized not only by uncontrolled cell proliferation but also by its interactions with the immune system and the surrounding tumor microenvironment (TME). The TME is composed of various immune cells, signaling molecules, and stromal components that collectively influence tumor growth and the response to therapy. The complex interplay between immune modulation and signaling pathways within the TME can affect both prognosis and therapeutic outcomes [7]. For instance, certain signaling pathways are known to contribute to immune evasion mechanisms and impact the effectiveness of immunotherapy [8]. Moreover, the identification of specific molecular signatures and biomarkers has shown promise in predicting cancer prognosis and tailoring treatment strategies to enhance patient response to therapy. These factors underscore the importance of understanding the dynamic interactions within the TME as a foundation for developing innovative therapeutic approaches.
Recent research on the thyroid-gut axis indicates that intestinal microbiota and its metabolites may influence the thyroid gland by affecting the uptake of intestinal trace elements and immune regulation, thereby enhancing our understanding of the pathogenesis and clinical treatment of thyroid diseases [9]. Beyond thyroid-specific effects, the broader role of GM and IC in cancer treatment outcomes has gained significant attention. The composition and diversity of the gut microbiota can modulate the host's immune response, which is critical for the effectiveness of immunotherapies such as immune checkpoint inhibitors. Certain bacterial species have been linked to better responses to these treatments, as they can promote the activation and infiltration of IC, including T cells, into the TME. Conversely, dysbiosis can impair immune activation, leading to reduced treatment efficacy and increased resistance to therapies. Understanding the interplay between GM, IC, and cancer treatments not only provides insight into patient variability in treatment outcomes but also supports the development of adjunctive therapies, such as probiotics or prebiotics, to enhance the success of cancer immunotherapy and reduce adverse effects.
The symbiotic intestinal microbiota inhabits the gastrointestinal tract, regulates the host's immune response and balance, and affects the metabolism of IC [10]. Dysbiosis of the intestinal microbiota can lead to autoimmune diseases and can be critical in regulating the immune system response [11]. The diversity and abundance of GMs are significantly reduced in individuals with TC [12]. Key mechanisms in GM-IC interactions include microbial metabolites like short-chain fatty acids (SCFAs), which enhance regulatory T cells (Tregs) and maintain immune tolerance, and microbial components like lipopolysaccharides (LPS), which activate immune pathways (e.g., NF-κB) through toll-like receptors (TLRs), modulating cytokine production. Dysbiosis may drive chronic inflammation, promoting tumor progression by creating a pro-inflammatory environment that aids immune evasion. These mechanisms suggest a therapeutic potential in targeting GM to regulate immune responses in TC.
Mendelian randomization (MR) adheres to the Mendelian inheritance principle of “random assignment of genetic alleles” in order to infer the causal relations between exposure factors and research outcomes in observational studies [13]. The objective of this study was to identify the IC that mediate the causality between GM and TC. Therefore, we utilized MR analysis to deduce and dissect the causality between GM, IC, and TC.
Materials and methods
Study design
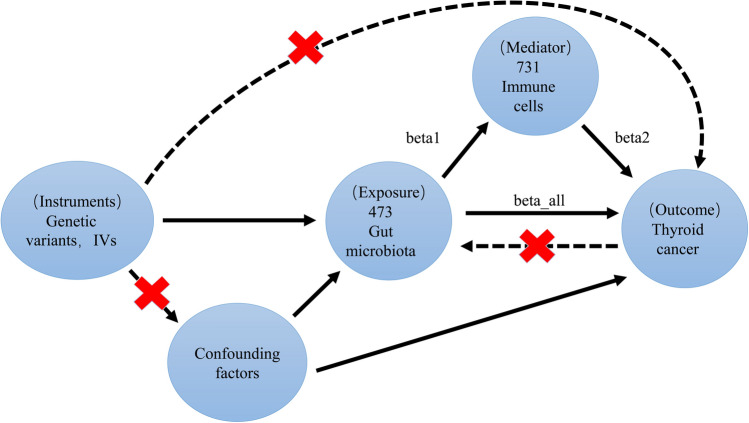
The procedural steps and methodology of MR in this study are detailed below (Fig. 1). Firstly, MR analysis was used to evaluate the causality of GM, TC and IC. Reverse analysis was performed to eliminate the risk of reverse causality. Following the three basic assumptions of MR analysis, the requirements for the selected SNPs are as follows:
They should be strongly related to exposure;
2.The outcome should be affected only by exposure;
Confounding-related SNPs should be eliminated.
Fig. 1.
Specific design and workflow of Mendelian randomization analysis research
Secondly, MR analysis was used to assess the causality of IC on TC.
Finally, we explored the role of IC as a mediator in the pathways between GM and TC, and calculated the effect sizes (beta1, beta2, beta_all) and the proportions for each qualified mediator.
Data sources
The data for 473 GM traits comes from the NHGRI-EBI GWAS, and genome-wide association tests were performed on genetic variants among 5,959 Europeans. A total of 471 genetic taxa were identified [14]. Data on 731 ICs were derived from aggregated GWAS statistics of 3757 Europeans [15]. The 731 immunophenotypes comprised absolute cellularity (AC), morphological parameters, which include CDC and TBNK groups, median fluorescence intensity, and relative cellularity, including TBNK, Treg panel, T cells, bone marrow cells, natural killer cells, monocytes, and CDCs [11]. The data of TC were derived from Global Biobank Project Whole-Genome Genotyping GWAS, including 11,121 Hispanic or Latin American, 25,692 African unspecified, 326,915 East Asian, and 1,376,270 European individuals. The detailed data are shown in Table 1.
Table 1.
Data sources
| Variable | Phenotypes | Cases/controls or sample sizes | Data source | Phenotypes code | Ancestry |
|---|---|---|---|---|---|
| Exposure | Gut microbiota | 5959 | GWAS Catalog | GCST90032172-GCST90032644 | European |
| Mediator | Immune cells | 3757 | GWAS Catalog | GCST90001391-GCST90002121 | European |
| Outcome | Thyroid cancer | 1,620,354/119,644 | GWAS Catalog | GCST90399737 | Global |
Selection of IVs
IVs were selected for subsequent analysis based on stringent criteria to ensure robust and reliable results. First, SNPs were chosen if they had a p-value of less than 5e-8 for their association with GM, IC, and TC, ensuring that only genome-wide significant variants were included. Second, to minimize the risk of linkage disequilibrium (LD) bias, SNPs were filtered using R software to exclude those with an R2 value of less than 0.001 within a 10,000 kb range, which helped maintain the independence of the IVs. Third, SNPs that were strongly associated with the outcome (P < 5×10-5) were excluded to prevent potential confounding influences that could bias the causal inference. Fourth, the F-statistic was calculated for each SNP, and only those with an F-statistic greater than 10 (F > 10) were retained to ensure that the IVs were strong instruments with sufficient power to detect causal relationships.
MR analysis
Among GM, IC, and TC, inverse-variance weighting (IVW) was served as the methodology to evaluate the causality, along with four auxiliary analysis methods [16]. If P < 0.05 in the MR-IVW analysis, there is a causality between the two samples. Set the F > 10 to identify strong instrumental variables [17]. The P-value for the horizontal pleiotropy and heterogeneity tests in MR should be greater than 0.05 to ensure reliability. According to these criteria, intestinal microbiota and IC with a positive causality, but no reverse causality with TC, were screened.
Analysis of intermediate MR method
Follow these steps to find potential IC in the GM-TC mediator pathway (Fig. 1):
We used MR analysis to identify IC causally affected by the GM and calculated the impact value (beta1).
Calculated the impact value (beta2) of the IC on TC.
Calculated the impact value (beta_all) of the identified GM on TC.
Calculated the mediation effect (beta12 = beta1 * beta2).
Calculated the direct effect (beta_dir = beta_all−beta12).
Sensitive MR analysis
In our research, three MR methods were served as sensitivity analysis, namely MR-Egger, MR-PRESSO, and leave-one-out. We assessed heterogeneity and horizontal pleiotropy by calculating Cochran's Q statistic and the MR-Egger regression intercept, respectively. Horizontal pleiotropy in IVs is addressed using MR-PRESSO [18]. Evidence of pleiotropy would invalidate the causality. We used the leave-one-out method to thoroughly investigate the effect of single SNP on IVW and used funnel plots to evaluate potential biases in the study results, ensuring robustness [19].
The MR study relied on R software (version 4.3.3) with the software packages: “Variant Annotation” (version 1.48.1), “foreach” (version 1.5.2), “Two Sample MR” (version 0.5.10), “data.table” (version 1.15.4), “ggplot2” (version 3.5.1), and “gwasglue” (version 0.0.0.90).
Results
IVs for exposure
The IVs needed for GM, IC, and TC studies were screened using the aforementioned method. The number of SNPs in GM, IC, and TC we studied ranged from 6 to 22, 12 to 31, and 22, respectively. Additionally, the F-statistic of SNPs screened in this study was at least 15. These data are detailed in Supplementary Material Table V1-V20.
Causality between GM and TC
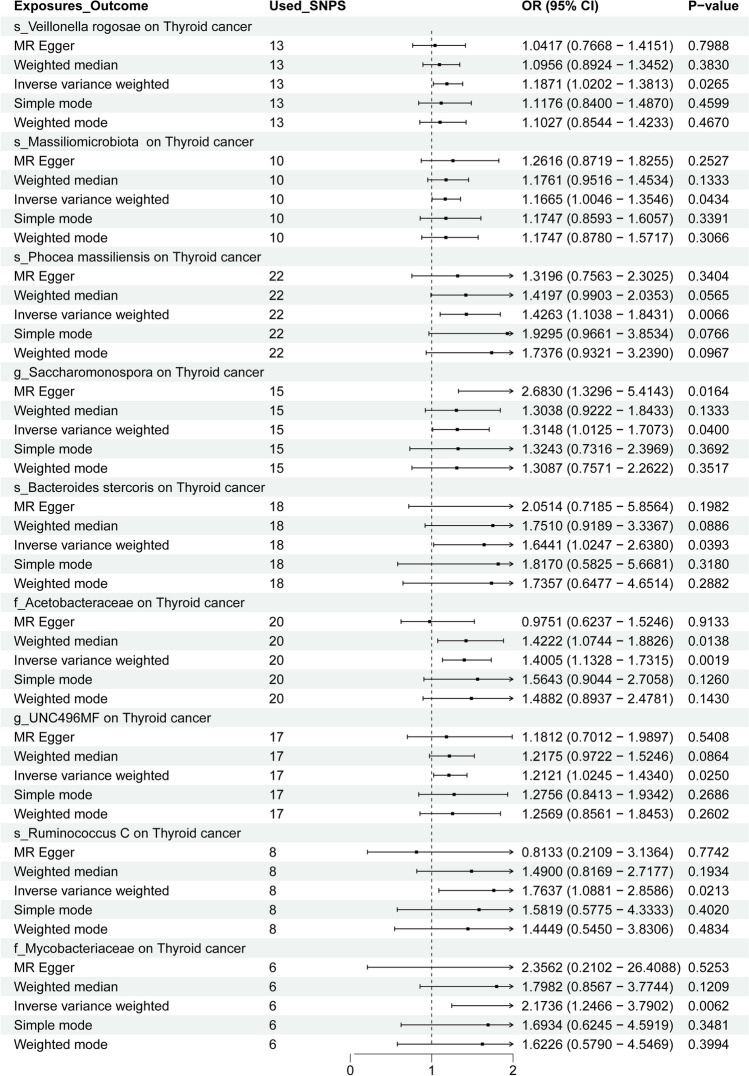
After MR analysis, GM species with a causality (P < 0.05) with TC were screened among 473 GM. Ultimately, 9 GM species were found to have a causal association with TC, with no reverse causality identified (Fig. 2). Using IVW as the primary method, specific GM species demonstrated notable odds ratios (OR), indicating their strength of association with TC. For example, s_Veillonella rogosae had an OR of 1.1871, which implies that an increase in its abundance is associated with an approximately 18.7% higher risk of TC. Similarly, s_Bacteroides stercoris had an OR of 1.6441, suggesting a 64.4% increased risk of TC, highlighting its strong potential role in tumorigenesis. The highest effect was seen with f_Mycobacteriaceae (OR = 2.1736), indicating that its presence could more than double the risk of TC (a 117.4% increase), pointing to a particularly significant influence.
Fig. 2.
MR analysis OR and P value of 9 gut microbiota on thyroid cancer
These effect sizes illustrate the varying degree of impact that different GM species may have on TC. The OR values reflect the potential risk modulation of TC as mediated by GM, providing insight into which species might be prioritized for further research. This highlights the importance of specific GM species not just in terms of their presence but also their quantitative effect on TC risk. The summary results of the MR analysis, including detailed ORs for all GM species, are provided in Fig. 3 and the Supplementary Materials (Figures S1-9, Tables T1-9, U1-9, and V1-9).
Fig. 3.
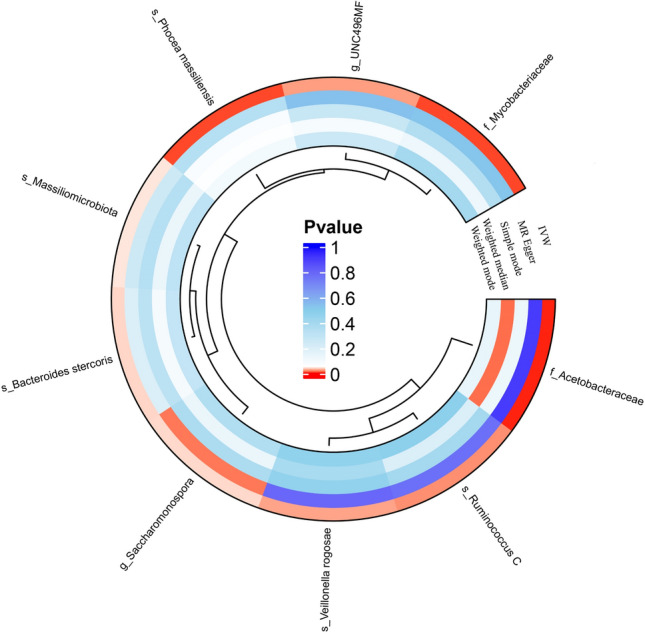
MR analysis P value of 9 gut microbiota on thyroid cancer
Causality between IC and TC
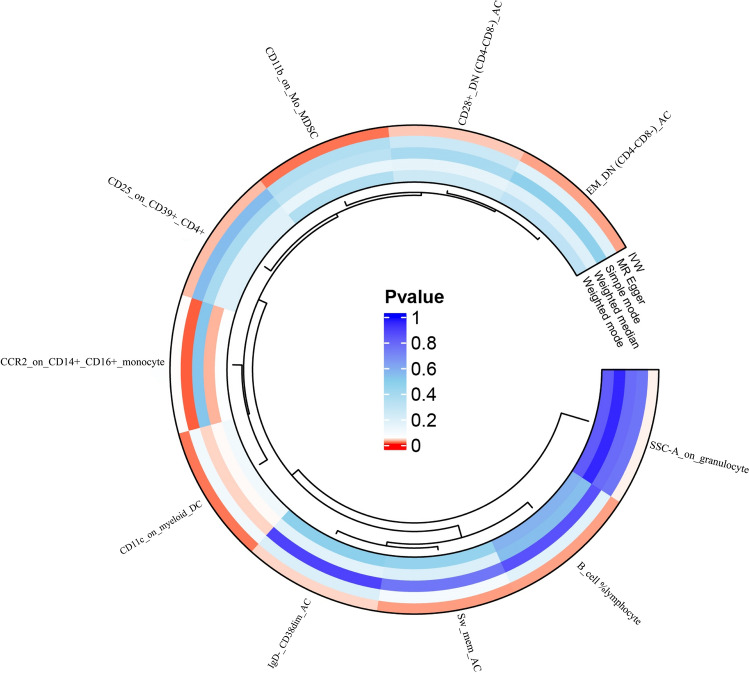
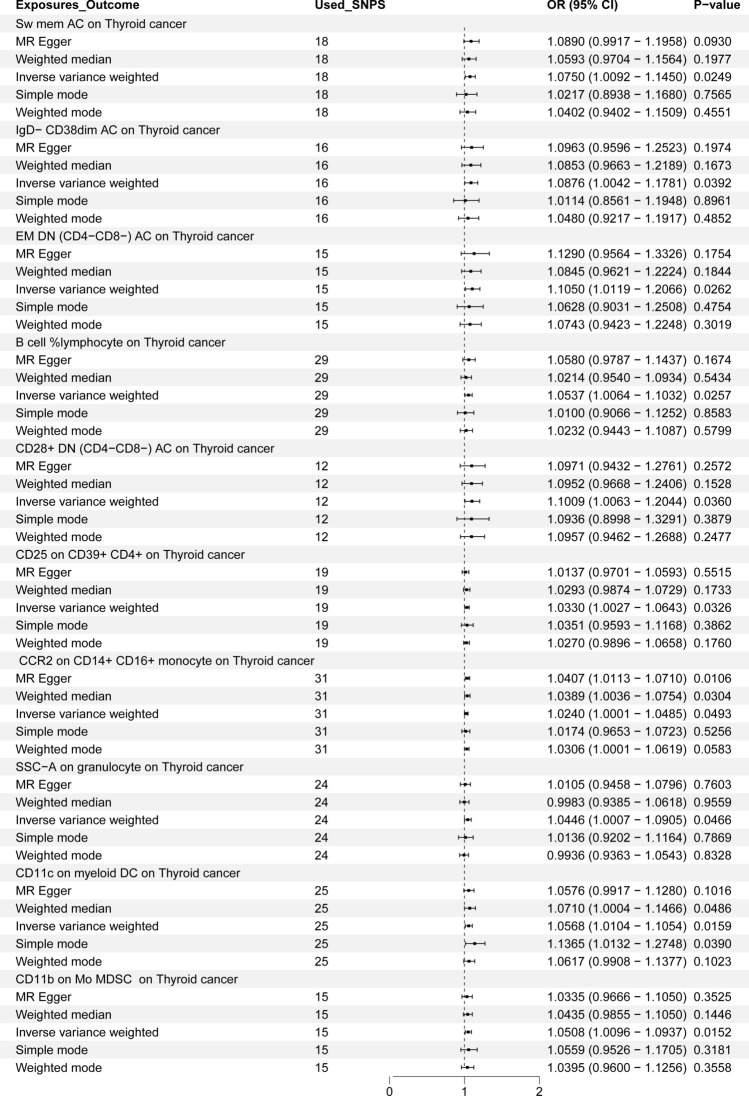
After conducting MR analysis, we screened 731 IC to identify those causally related to TC based on GM (P < 0.05), ultimately identifying 10 types that were causally related to TC (Fig. 4). When IVW serves as the primary method to evaluate the causality of IC on TC in the MR analysis, Switched Memory AC (OR = 1.075), CD25 on CD39 + CD4 + (OR = 1.033), IgD- CD38dim AC (OR = 1.0876), Effector Memory Double Negative (EMDN) (CD4-CD8-) AC (OR = 1.105), B cell %lymphocyte (OR = 1.0537), CD28 + DN (CD4-CD8-) AC (OR = 1.1009), CCR2 on CD14 + CD16 + monocyte (OR = 1.024), SSC-A on granulocyte, the expression of CD11c on myeloid Dendritic Cells (DC) (OR = 1.0568), and CD11b on Mo MDSC (OR = 1.0508) were correlated with TC. The detailed results of all MR analyses are shown in Fig. 5 and Supplementary Figures (S21-30, T21-30, U21-30, and V10-19).
Fig. 4.
MR analysis P value of 10 immune cell on thyroid cancer
Fig. 5.
MR analysis OR and P value of 10 immune cell on thyroid cancer
Causal relationship between GM and IC
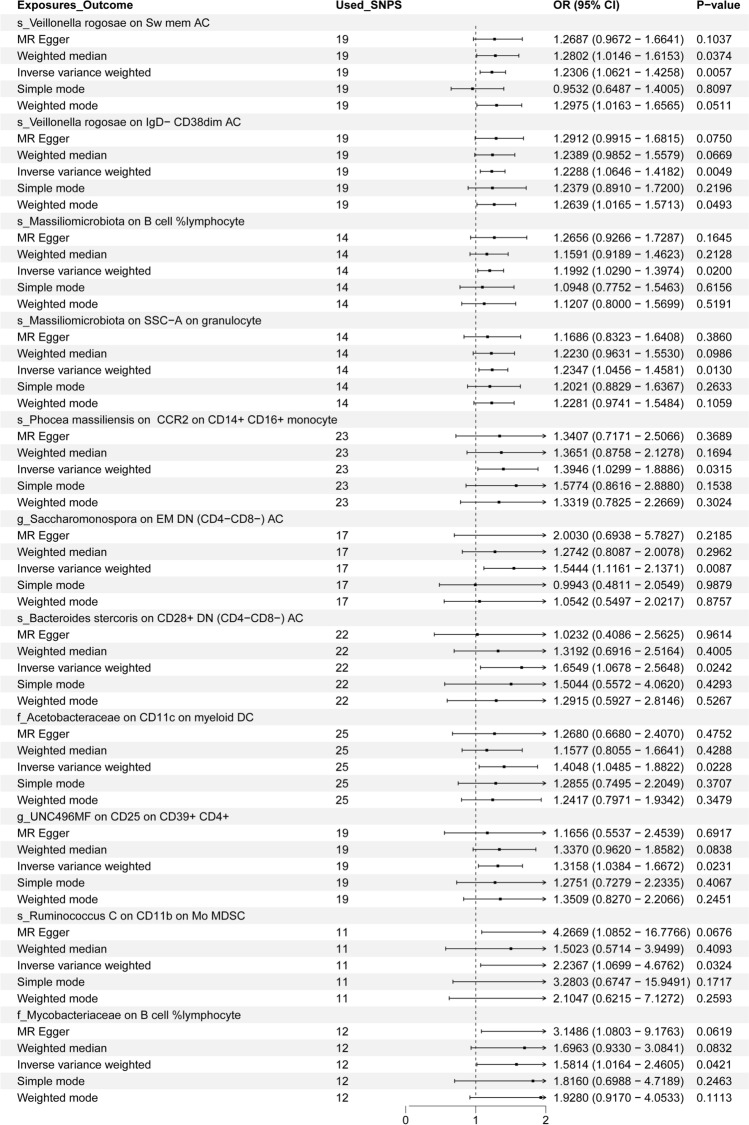
When IVW serves as the primary method to evaluate the causality of GM on IC in the MR analysis, s_Veillonella rogosae on Switched Memory AC (OR = 1.2306), s_Veillonella rogosae on IgD- CD38dim AC (OR = 1.2288), s_Massiliomicrobiota on B cell % lymphocyte (OR = 1.1992), s_Massiliomicrobiota on SSC-A on granulocyte (OR = 1.2347), s_Phocea massiliensis on CCR2 on CD14 + CD16 + monocyte (OR = 1.3946), and g_Saccharomonospora on EM DN (CD4-CD8-) AC (OR = 1.5444). The above results revealed that GM had a positive correlation with IC. Detailed results of all MR analyses are shown in Fig. 6 and Supplementary Figures (S10-20, T10-20, U10-20, and V20).
Fig. 6.
MR analysis OR and P value of 9 gut microbiota on 10 immune cells
Analysis of potential IC mediation
MR analysis revealed that 10 IC mediated the causality between 9 GM and TC. Notably, 'EM DN (CD4-CD8-) AC' exhibited the highest mediation ratio (beta_P of 15.9%) in the pathway between g_Saccharomonospora and TC, suggesting that this immune cell type may play a particularly significant role in influencing tumor progression. The strong mediation by 'Switched Memory AC' (beta_P of 8.7%) and 'IgD-CD38dim AC' (beta_P of 10.1%) in the relationship between s_Veillonella rogosae and TC highlights the potential importance of adaptive immune memory responses in modulating cancer outcomes. These cell types are known for their roles in sustaining long-term immune surveillance and response, which could explain their notable mediation effects.
In contrast, 'CCR2 on CD14 + CD16 + monocyte' had a lower mediation ratio (beta_P of 2.2%) in the pathway between s_Phocea massiliensis and TC, possibly reflecting a more specialized or context-dependent role of this monocyte subtype in the immune response to tumorigenesis. The involvement of 'CD28 + DN (CD4-CD8-) AC' (beta_P of 9.7%) in mediating the effect of s_Bacteroides stercoris on TC may indicate a crucial role in immune regulation, given the dual functions of CD28 in co-stimulatory signaling for T cells.
The varying mediation ratios observed suggest that some IC, such as 'EM DN (CD4-CD8-) AC' and 'Switched Memory AC,' may have broader and more impactful roles in the interplay between GM and TC, potentially due to their involvement in maintaining immune homeostasis and response modulation. Meanwhile, mediators like 'CD11b on Mo MDSC' (beta_P of 7.0%) and 'CD11c on myeloid DC' (beta_P of 5.6%) underscore the influence of myeloid lineage cells in immune suppression and tumor immune escape. These differences emphasize the complexity of the immune response and the varied influence of different IC in mediating GM's effects on TC, warranting further experimental validation to uncover the mechanisms driving these interactions. These data are detailed in Table 2.
Table 2.
Mediating effect on thyroid cancer
| Gut microbiota | Immune cells | beta all | beta dir | beta 1 | beta 12 | beta P (%) | beta 2 |
|---|---|---|---|---|---|---|---|
| s_Veillonella rogosae | Sw mem AC | 0.172 | 0.157 | 0.208 | 0.015 | 8.7 | 0.072 |
| s_Veillonella rogosae | IgD- CD38dim AC | 0.172 | 0.154 | 0.206 | 0.017 | 10.1 | 0.084 |
| s_Massiliomicrobiota | SSC-A on granulocyte | 0.154 | 0.145 | 0.211 | 0.009 | 6.0 | 0.044 |
| s_Massiliomicrobiota | B cell %lymphocyte | 0.154 | 0.145 | 0.182 | 0.009 | 6.2 | 0.052 |
| s_Phocea massiliensis | CCR2 on CD14 + CD16 + monocyte | 0.355 | 0.347 | 0.333 | 0.008 | 2.2 | 0.024 |
| g_Saccharomonospora | EM DN (CD4-CD8-) AC | 0.274 | 0.23 | 0.435 | 0.043 | 15.9 | 0.1 |
| s_Bacteroides stercoris | CD28 + DN (CD4-CD8-) AC | 0.497 | 0.449 | 0.504 | 0.048 | 9.7 | 0.106 |
| f_Acetobacteraceae | CD11c on myeloid DC | 0.337 | 0.318 | 0.34 | 0.019 | 5.6 | 0.055 |
| g_UNC496MF | CD25 on CD39 + CD4 + | 0.192 | 0.183 | 0.274 | 0.009 | 4.6 | 0.033 |
| s_Ruminococcus C | CD11b on Mo MDSC | 0.567 | 0.527 | 0.805 | 0.04 | 7.0 | 0.05 |
| f_Mycobacteriaceae | B cell %lymphocyte | 0.776 | 0.752 | 0.458 | 0.024 | 3.1 | 0.052 |
beta all: Total effect of gut microbiota on thyroid cancer
beta dir: Direct effects of gut microbiota on thyroid cancer
beta 1: Effects of gut microbiota on immune cells
beta 12: Mediating effect of gut microbiota on thyroid cancer
beta P: Percentage of mediating effects of gut microbiota on thyroid cancer
beta 2: Effects of immune cells on thyroid cancer
MR sensitivity analysis
This MR study found no evidence to support the existence of horizontal pleiotropy, as indicated by the MR-Egger and MR-PRESSO. Leave-one-out study revealed that independent SNPs didn’t significantly influence the overall effect in the MR analyses of GM-TC and TC-GM (Supplementary Material Figure U1-U30). Overall, the findings of the MR study were confirmed to be robust through the conducted sensitivity analyses.
Discussion
Our research identified that 9 specific GM species (s_Veillonella rogosae, s_Massiliomicrobiota, s_Phocea massiliensis, g_Saccharomonospora, s_Bacteroides stercoris, f_Acetobacteraceae, g_UNC496MF, s_Ruminococcus C, and f_Mycobacteriaceae) were causally related to TC and might be mediated by 10 IC phenotypes (EM DN (CD4-CD8-) AC, Switched Memory AC, B cell % lymphocyte, IgD-CD38dim AC, CD28+(DN) (CD4-CD8-) AC, CD25 on CD39+CD4+cells, CD11c on myeloid DC, CCR2 on CD14+CD16+monocytes, SSC-A on granulocytes, and CD11b on Mo MDSC). These findings elucidate potential immune-mediated mechanisms between GM and TC, providing new insights into GM-based immunotherapeutic strategies and IC-targeted interventions for TC. This study highlights the complex interplay between specific gut microbiota and immune cells, emphasizing the potential for targeted approaches that leverage these interactions to modulate the immune environment and improve therapeutic outcomes in thyroid cancer.
The GM is crucial for the progression, differentiation, as well as maturation of the human immune system and has a disproportionate impact on triggering thyroid autoimmune diseases [20]. In vitro studies using mature monocytes treated with antiretroviral therapy have shown that HIV + CD14 + CD16 + monocytes are the first to migrate [21]. Our study suggests that the IC phenotype “CCR2 on CD14 + CD16 + ” may mediate the positive effects of “Phocea massiliensis” on TC. Whole genome expression analysis using RNA sequencing showed that Ess2 deficiency altered the expression of immune-related genes and Myc target genes in CD4 single-positive thymocytes [22]. Our findings indicated that the IC phenotype “EM DN (CD4-CD8-) AC” mediates the positive effects of “s_Phocea massiliensis” on TC. Our study found that the IC phenotype “CD28 + DN (CD4-CD8-) AC” mediates the positive effect of “s_Bacteroides stercoris” on TC. CD4 + CD25 + Tregs have significant impact on preventing immune attacks and exert immune surveillance by modulating the function of antigen-presenting cells [23]. Our study found that the phenotype “CD25 on CD39 + CD4 + ” mediates the positive effect of g_UNC496MF on TC.
MDSCs are cells that suppress anti-tumor immunity, including CD11b + Gr1 + ly6 PMN-MDSCs and CD11b + Gr1 + ly6 Mo-MDSCs [24]. Our study found that the IC phenotype “CD11b on Mo MDSC” mediates the positive influence of “s_Ruminococcus C” on TC.
The GM regulates the efficacy of cancer immunotherapy and its immune-related adverse reactions. Fecal microbiota transplantation or dietary intervention may be clinically employed to enhance the success rate of immunotherapy in cancer patients [6]. Consuming a diet that triggers inflammation has been found to be associated with higher levels of “Veillonella rogosae” in individuals with inflammatory bowel disease who are in remission, which suggests that the food we choose to eat can impact the composition of the GM and the level of inflammation experienced by these patients [25]. According to our analysis results, the IC phenotypes " Switched Memory AC" and "IgD-CD38dim AC" acted as mediators, both of which may mediate the positive correlation between “s_Veillonella rogosae” and TC. Enhancing the effectiveness of immunotherapy for TC by maintaining a favorable microbiota profile can enhance the clinical outcomes and the quality of survival sufferers with TC [26]. These findings underscore the potential for integrating gut microbiota modulation into clinical practice as an adjunctive approach to existing cancer therapies. Personalized dietary interventions, probiotics, or fecal microbiota transplantation could be tailored to individual patients to optimize their microbiota composition, thus potentially improving immune response and reducing adverse reactions. Additionally, understanding the specific immune cell phenotypes involved could aid in the development of targeted immunotherapies that harness the body’s natural defenses more effectively. This approach opens new avenues for precision medicine, where treatment strategies are adapted based on a patient’s microbiome and immune cell profile to enhance therapeutic success and minimize side effects.
Immune elimination and escape may partly rely on bacteria to shape immunity by mediating host immune regulation, and the mutual regulation of host-microbiome provides a novel therapeutic strategy to enhance the efficacy of anti-cancer treatment [27]. Dietary regulation of the intestinal microbiota directly influences the microbial metabolites produced in the intestinal mucosa and their impact on IC [28]. However, it is important to note that not all GM or IC species demonstrated significant associations with thyroid cancer in our MR analysis. This could be due to several factors, such as insufficient statistical power for certain less abundant microbial or immune cell types, potential confounding variables not accounted for, or the complex interplay of genetic and environmental factors that may mask specific relationships. Future studies should explore these null results in greater detail to understand if they indicate true non-associations or are influenced by limitations in data or methodology.
In the adaptive immune system, thyroid hormone may activate T lymphocytes through multiple potential mechanisms, including mediation of NF-κB signaling pathways, as well as β-adrenergic receptors, resulting in increased T lymphocyte activation [29]. Our results suggest that "SSC-A on granulocyte" and "B cell % lymphocyte" as IC phenotypes may mediate s_Massiliomicrobiota, while "B cell % lymphocyte" may mediate f_Mycobacteriaceae, all exhibiting positive mediating effects on TC. CD11c + CD8 + T cells, when activated, can potentially stop the development of autoimmune colitis through adoptive transfer; meanwhile, in specific viral and cancer models, they function as immune effectors, enhancing immune potential [30].
The intestinal microbiota is considered to be an important factor affecting thyroid homeostasis, and low abundance of Faecalibacterium may lead to GM dysbiosis before or after the development of TC [31]. The GM can interact with the host's colon epithelial cells and IC by releasing a variety of metabolites, thereby regulating the development of colorectal cancer [32].
Limitation
This study utilized a large GWAS dataset, encompassing summary data from 473 GM, 731 IC, and TC. The MR analysis method not only identifies statistically significant findings but also guarantees strong statistical efficacy. Secondly, through MR analysis, this study identified 10 IC phenotypes as mediating factors in the causality from 9 types of GM to TC. However, this study has several limitations. The causality between GM, IC, and TC determined by MR analysis may be influenced by potential confounders, including environmental factors such as diet, lifestyle, and microbiome exposure, as well as genetic background, which add complexity to these relationships. Additionally, while MR analysis helps mitigate some biases present in observational studies, it cannot fully account for all unmeasured confounding variables or the intricacies of indirect pathways in the GM-IC-TC axis. The use of GWAS summary data limits the ability to explore interactions at an individual level and may introduce biases related to population-specific genetic structures. To address these challenges, future research should include experimental studies such as cell culture and animal models to validate the causal pathways suggested by MR analysis and provide deeper insight into the biological interactions among GM, IC, and thyroid cancer. Such approaches would enhance the understanding of these complex interactions and help identify potential confounding factors more precisely.
Conclusion
This Mendelian randomization study provided an in-depth exploration of the causal relationships between GM, IC, and TC. We identified nine GM species that demonstrated a causal association with TC, mediated by ten distinct IC phenotypes, including "Switched Memory AC," "IgD-CD38dim AC," and "CD28+DN (CD4-CD8-)." These findings illuminate the complex interplay between GM and IC in influencing tumor progression and suggest potential avenues for GM-based immunotherapies and IC-targeted treatments. While this study advances our understanding of the GM-IC-TC axis, further experimental validation is necessary. Future research should focus on in vitro and in vivo studies to corroborate these mediating effects and unravel the underlying molecular mechanisms. Such work could bridge the gap between statistical associations and biological causality, enabling the development of innovative therapeutic interventions.
Supplementary Information
Acknowledgements
None
Author contributions
JD designed the study. JD, YG, MY, YL, and LQ performed data analysis. JD drafted the manuscript and revised the manuscript. All authors read and approved the final manuscript.
Funding
None.
Data availablity
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang H, et al. Epigenetic signature associated with thyroid cancer progression and metastasis. Semin Cancer Biol. 2022;83:261–8. [DOI] [PubMed] [Google Scholar]
- 2.Shobab L, Burman KD, Wartofsky L. Sex differences in differentiated thyroid cancer. Thyroid. 2022;32(3):224–35. [DOI] [PubMed] [Google Scholar]
- 3.Lin X, et al. Regulation of oncoprotein 18/Stathmin signaling by ERK concerns the resistance to taxol in nonsmall cell lung cancer cells. Cancer Biother Radiopharm. 2016;31(2):37–43. [DOI] [PubMed] [Google Scholar]
- 4.Tong G, et al. Effects of GLP-1 receptor agonists on biological behavior of colorectal cancer cells by regulating PI3K/AKT/mTOR signaling pathway. Front Pharmacol. 2022;13: 901559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma PJ, Wang MM, Wang Y. Gut microbiota: a new insight into lung diseases. Biomed Pharmacother. 2022;155: 113810. [DOI] [PubMed] [Google Scholar]
- 6.Fernandes MR, et al. Targeting the gut microbiota for cancer therapy. Nat Rev Cancer. 2022;22(12):703–22. [DOI] [PubMed] [Google Scholar]
- 7.Duan WW, et al. A TGF-β signaling-related lncRNA signature for prediction of glioma prognosis, immune microenvironment, and immunotherapy response. CNS Neurosci Ther. 2024;30(4): e14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lou Y, et al. Simultaneous quantification of mirabegron and vibegron in human plasma by HPLC-MS/MS and its application in the clinical determination in patients with tumors associated with overactive bladder. J Pharm Biomed Anal. 2024;240: 115937. [DOI] [PubMed] [Google Scholar]
- 9.Jiang W, et al. The relationships between the gut microbiota and its metabolites with thyroid diseases. Front Endocrinol (Lausanne). 2022;13: 943408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, et al. Gut-microbiota-derived metabolites maintain gut and systemic immune homeostasis. Cells. 2023;12(5):793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belvoncikova P, Maronek M, Gardlik R. Gut dysbiosis and fecal microbiota transplantation in autoimmune diseases. Int J Mol Sci. 2022;23(18):10729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu G, et al. Alterations of gut microbiome and metabolite profiles associated with anabatic lipid dysmetabolism in thyroid cancer. Front Endocrinol (Lausanne). 2022;13: 893164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang M, et al. No evidence of a genetic causal relationship between ankylosing spondylitis and gut microbiota: a two-sample mendelian randomization study. Nutrients. 2023;15(4):1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin Y, et al. Combined effects of host genetics and diet on human gut microbiota and incident disease in a single population cohort. Nat Genet. 2022;54(2):134–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orrù V, et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat Genet. 2020;52(10):1036–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S, et al. The impact of serum 25-hydroxyvitamin D, calcium, and parathyroid hormone levels on the risk of coronary artery disease in patients with diabetes: a Mendelian randomization study. Nutr J. 2021;20(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao X, et al. Causal linkage between type 2 diabetes mellitus and inflammatory bowel disease: an integrated Mendelian randomization study and bioinformatics analysis. Front Endocrinol (Lausanne). 2024;15:1275699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhuang Y, et al. Unraveling genetic causality between metformin and myocardial infarction on the basis of Mendelian randomization. Front Endocrinol (Lausanne). 2024;15:1376464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu F, et al. Mendelian randomization study of inflammatory bowel disease and bone mineral density. BMC Med. 2020;18(1):312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Legakis I, Chrousos GP, Chatzipanagiotou S. Thyroid diseases and intestinal microbiome. Horm Metab Res. 2023;55(12):813–8. [DOI] [PubMed] [Google Scholar]
- 21.León-Rivera R, et al. Central nervous system (CNS) viral seeding by mature monocytes and potential therapies to reduce CNS viral reservoirs in the cART Era. mBio. 2021;12(2):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takada I, et al. Transcriptional coregulator Ess2 controls survival of post-thymic CD4(+) T cells through the Myc and IL-7 signaling pathways. J Biol Chem. 2022;298(9): 102342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.André S, et al. Surveillance of antigen-presenting cells by CD4+ CD25+ regulatory T cells in autoimmunity: immunopathogenesis and therapeutic implications. Am J Pathol. 2009;174(5):1575–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karin N, Razon H. The role of CCR5 in directing the mobilization and biological function of CD11b(+)Gr1(+)Ly6C(low) polymorphonuclear myeloid cells in cancer. Cancer Immunol Immunother. 2018;67(12):1949–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rocha I, et al. Pro-inflammatory diet is correlated with high Veillonellarogosae, gut inflammation and clinical relapse of inflammatory bowel disease. Nutrients. 2023;15(19):4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie Z, et al. Clinical potential of microbiota in thyroid cancer therapy. Biochim Biophys Acta Mol Basis Dis. 2024;1870(2): 166971. [DOI] [PubMed] [Google Scholar]
- 27.Zhou CB, Zhou YL, Fang JY. Gut microbiota in cancer immune response and immunotherapy. Trends Cancer. 2021;7(7):647–60. [DOI] [PubMed] [Google Scholar]
- 28.Cayres LCF, et al. Detection of alterations in the gut microbiota and intestinal permeability in patients with Hashimoto thyroiditis. Front Immunol. 2021;12: 579140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han Z, et al. The role of thyroid hormone in the renal immune microenvironment. Int Immunopharmacol. 2023;119: 110172. [DOI] [PubMed] [Google Scholar]
- 30.Vinay DS, Kwon BS. CD11c+CD8+ T cells: two-faced adaptive immune regulators. Cell Immunol. 2010;264(1):18–22. [DOI] [PubMed] [Google Scholar]
- 31.Yu X, et al. Gut microbiota changes and its potential relations with thyroid carcinoma. J Adv Res. 2022;35:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong CC, Yu J. Gut microbiota in colorectal cancer development and therapy. Nat Rev Clin Oncol. 2023;20(7):429–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.