Abstract
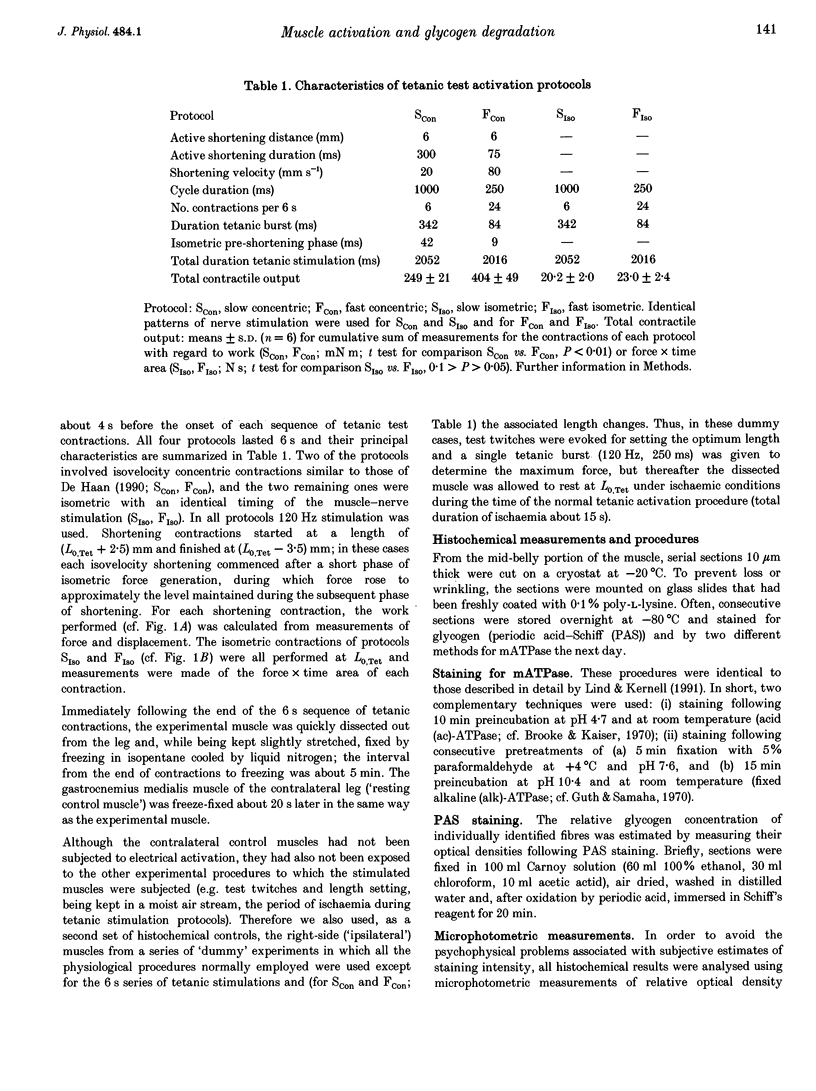
1. The relative degree of glycogen degradation, caused in different fibre types by supramaximal electrical activation of the muscle nerve, was investigated in m. gastrocnemius medialis of young adult rats under general pentobarbitone anaesthesia. Four different protocols of intermittent maximal tetanic activation were used, each lasting 6 s (33% duty cycle; fast and slow isovelocity concentric (shortening) contractions, brief- and long-burst isometric contractions; 6 rats per group). All contractions were evoked under ischaemic conditions. 2. Work output finally dropped to 29% of the initial value for the fast concentric and to 87% for the slow concentric contractions. In isometric protocols evoked by the same stimulation patterns, the force x time area rose to 110% for brief-burst contractions and dropped to 95% for the long-burst contractions. 3. Following the physiological procedures, the experimental muscle and its contralateral control were removed and prepared for histochemical analysis. Serial sections were stained for glycogen (periodic acid-Schiff (PAS) method) and myofibrillar ATPase (mATPase), the latter reactions being used for classifying the fibres as types I, IIA, IIBd and IIBm. 4. For deep 'red' regions of non-stimulated contralateral control muscles the optical density of PAS staining was ranked between fibre types such that I < IIA < IIBd < IIBm. In superficial 'white' regions of the same muscles, no significant difference in PAS staining density was found between IIBd and IIBm fibres (types I and IIA not present). 5. All contractile protocols produced a significant glycogen degradation in IIBm fibres, and the fast concentric activation procedure was associated with a significant decline of PAS staining in all fibre types. For all activation protocols, the relative degree of glycogen degradation within a given region was ranked such that IIBm > IIBd > IIA > I. For IIBm vs. IIBd fibres, the differences in relative degradation were greater and more consistently significant for superficial white regions than in the deeper red muscle portions. 6. The results are discussed in relation to glycogen degradation measurements in studies of motor unit recruitment. Furthermore, the results from red vs. white muscle regions underline that fibres of seemingly the same mATPase type may differ considerably in other properties.
Full text
PDF


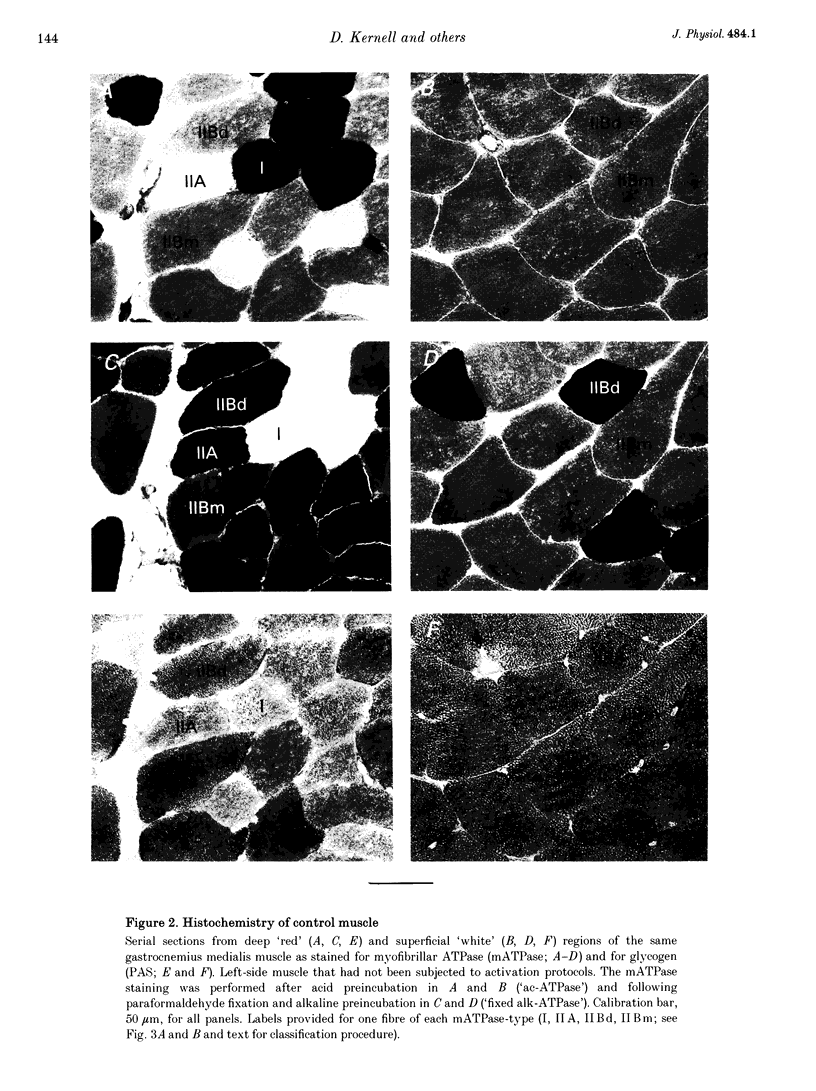
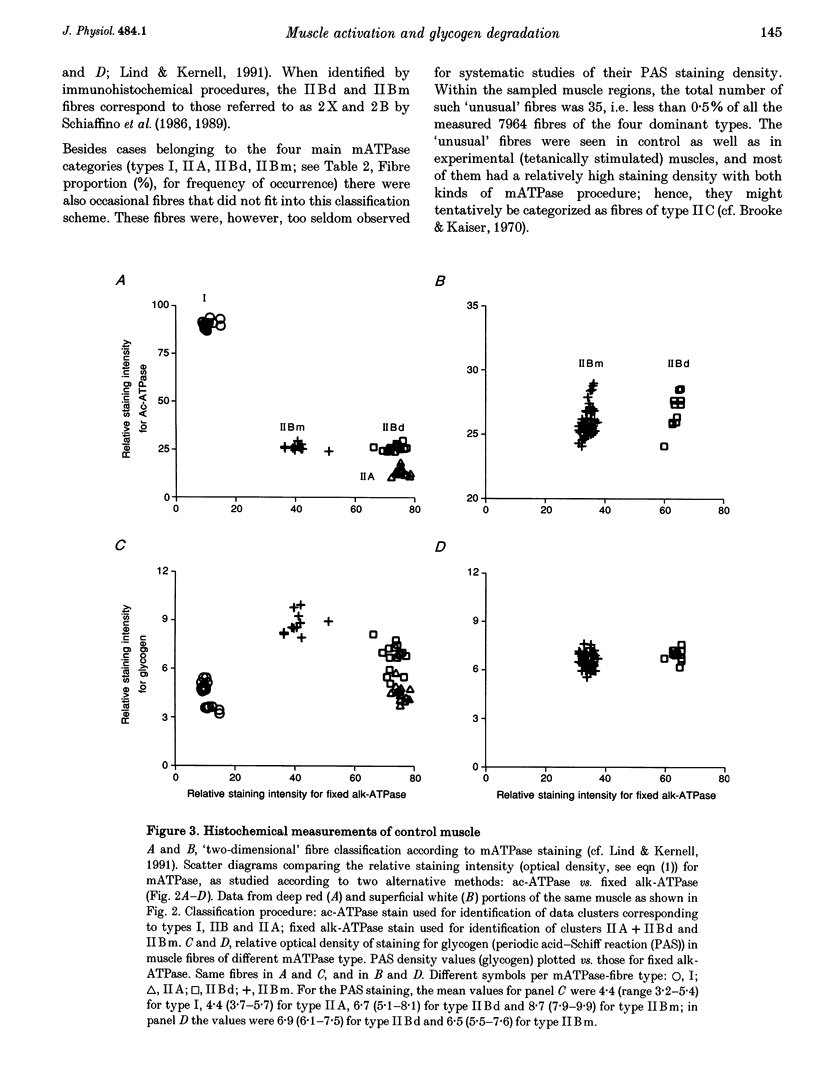





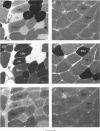
Images in this article
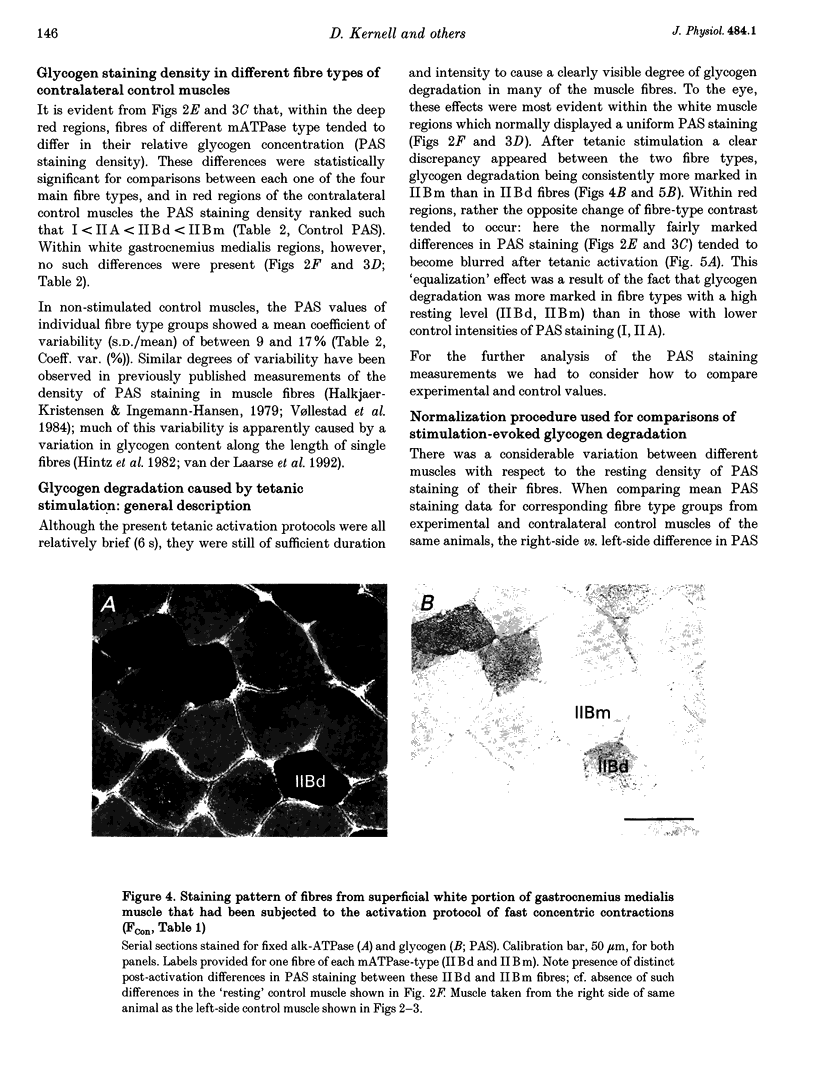
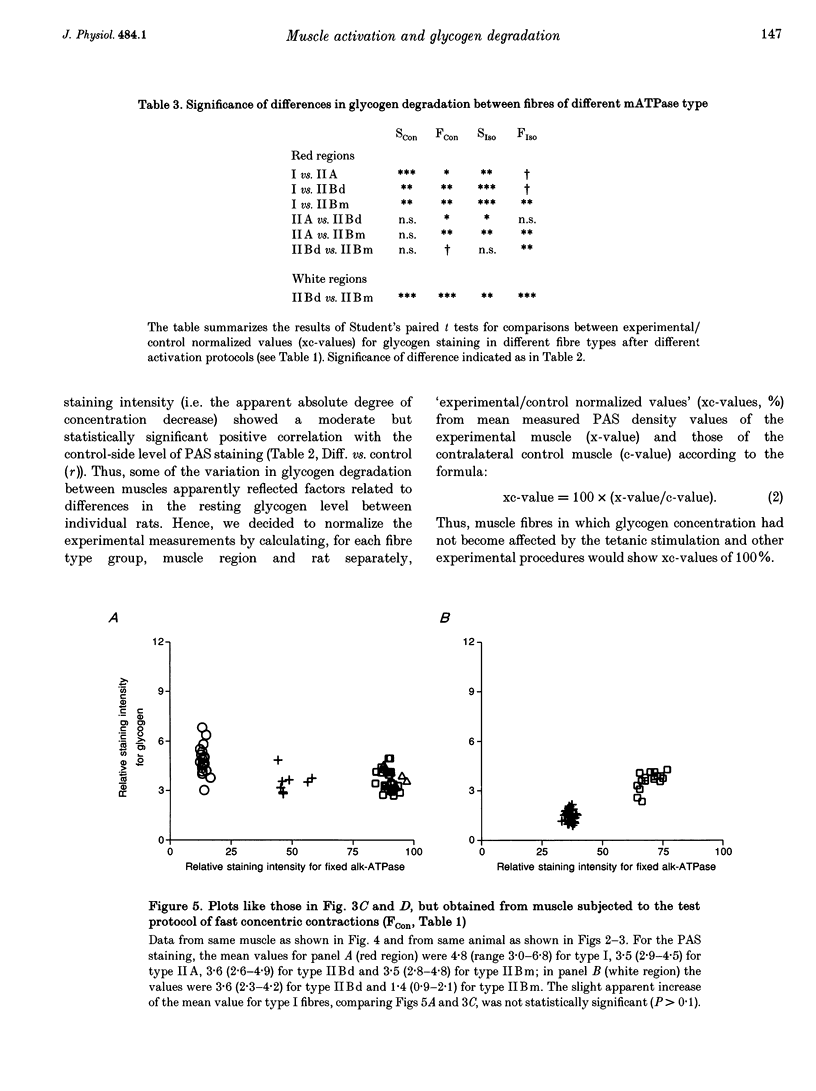
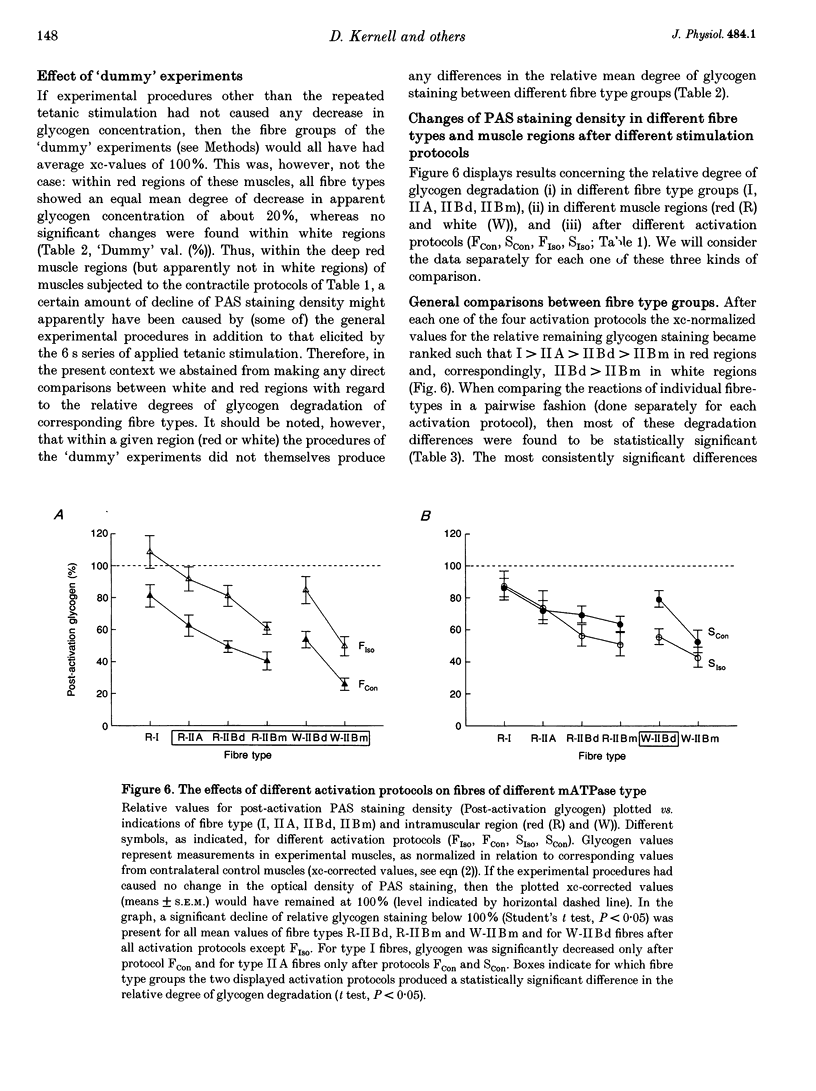
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. B., Saubert C. W., 4th, Sembrowich W. L., Shepherd R. E., Gollnick P. D. Glycogen depletion in rat skeletal muscle fibers at different intensities and durations of exercise. Pflugers Arch. 1974;352(3):243–256. doi: 10.1007/BF00590489. [DOI] [PubMed] [Google Scholar]
- Bangsbo J., Graham T. E., Kiens B., Saltin B. Elevated muscle glycogen and anaerobic energy production during exhaustive exercise in man. J Physiol. 1992;451:205–227. doi: 10.1113/jphysiol.1992.sp019161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen A., McDermott J. C., Hutber C. A. Carbohydrate metabolism in skeletal muscle: an update of current concepts. Int J Sports Med. 1989 Dec;10(6):385–401. doi: 10.1055/s-2007-1024932. [DOI] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Muscle fiber types: how many and what kind? Arch Neurol. 1970 Oct;23(4):369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Edgerton V. R., Barnard R. J., Peter J. B., Simpson D. R., Gillespie C. A. Response of muscle glycogen and phosphorylase to electrical stimulation in trained and nontrained guinea pigs. Exp Neurol. 1970 Apr;27(1):46–56. doi: 10.1016/0014-4886(70)90200-1. [DOI] [PubMed] [Google Scholar]
- Edström L., Kugelberg E. Histochemical composition, distribution of fibres and fatiguability of single motor units. Anterior tibial muscle of the rat. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):424–433. doi: 10.1136/jnnp.31.5.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick P. D., Piehl K., Saltin B. Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rates. J Physiol. 1974 Aug;241(1):45–57. doi: 10.1113/jphysiol.1974.sp010639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H. J. How important is endogenous muscle glycogen to fatigue in prolonged exercise? Can J Physiol Pharmacol. 1991 Feb;69(2):290–297. doi: 10.1139/y91-045. [DOI] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Procedure for the histochemical demonstration of actomyosin ATPase. Exp Neurol. 1970 Aug;28(2):365–367. [PubMed] [Google Scholar]
- Halkjaer-Kristensen J., Ingemann-Hansen T. Microphotometric determination of glycogen in single fibres of human quadriceps muscle. Histochem J. 1979 Nov;11(6):629–638. doi: 10.1007/BF01004727. [DOI] [PubMed] [Google Scholar]
- Hintz C. S., Chi M. M., Fell R. D., Ivy J. L., Kaiser K. K., Lowry C. V., Lowry O. H. Metabolite changes in individual rat muscle fibers during stimulation. Am J Physiol. 1982 Mar;242(3):C218–C228. doi: 10.1152/ajpcell.1982.242.3.C218. [DOI] [PubMed] [Google Scholar]
- Kernell D. Organized variability in the neuromuscular system: a survey of task-related adaptations. Arch Ital Biol. 1992 Jan;130(1):19–66. [PubMed] [Google Scholar]
- Kugelberg E., Edström L. Differential histochemical effects of muscle contractions on phosphorylase and glycogen in various types of fibres: relation to fatigue. J Neurol Neurosurg Psychiatry. 1968 Oct;31(5):415–423. doi: 10.1136/jnnp.31.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugelberg E. Histochemical composition, contraction speed and fatiguability of rat soleus motor units. J Neurol Sci. 1973 Oct;20(2):177–198. doi: 10.1016/0022-510x(73)90029-4. [DOI] [PubMed] [Google Scholar]
- Larsson L., Ansved T., Edström L., Gorza L., Schiaffino S. Effects of age on physiological, immunohistochemical and biochemical properties of fast-twitch single motor units in the rat. J Physiol. 1991 Nov;443:257–275. doi: 10.1113/jphysiol.1991.sp018833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L., Edström L., Lindegren B., Gorza L., Schiaffino S. MHC composition and enzyme-histochemical and physiological properties of a novel fast-twitch motor unit type. Am J Physiol. 1991 Jul;261(1 Pt 1):C93–101. doi: 10.1152/ajpcell.1991.261.1.C93. [DOI] [PubMed] [Google Scholar]
- Larsson L. Is the motor unit uniform? Acta Physiol Scand. 1992 Feb;144(2):143–154. doi: 10.1111/j.1748-1716.1992.tb09279.x. [DOI] [PubMed] [Google Scholar]
- Lind A., Kernell D. Myofibrillar ATPase histochemistry of rat skeletal muscles: a "two-dimensional" quantitative approach. J Histochem Cytochem. 1991 May;39(5):589–597. doi: 10.1177/39.5.1826695. [DOI] [PubMed] [Google Scholar]
- Ren J. M., Broberg S., Sahlin K., Hultman E. Influence of reduced glycogen level on glycogenolysis during short-term stimulation in man. Acta Physiol Scand. 1990 Jul;139(3):467–474. doi: 10.1111/j.1748-1716.1990.tb08948.x. [DOI] [PubMed] [Google Scholar]
- Richter E. A., Galbo H. High glycogen levels enhance glycogen breakdown in isolated contracting skeletal muscle. J Appl Physiol (1985) 1986 Sep;61(3):827–831. doi: 10.1152/jappl.1986.61.3.827. [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Gorza L., Sartore S., Saggin L., Ausoni S., Vianello M., Gundersen K., Lømo T. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989 Jun;10(3):197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- Spriet L. L., Berardinucci L., Marsh D. R., Campbell C. B., Graham T. E. Glycogen content has no effect on skeletal muscle glycogenolysis during short-term tetanic stimulation. J Appl Physiol (1985) 1990 May;68(5):1883–1888. doi: 10.1152/jappl.1990.68.5.1883. [DOI] [PubMed] [Google Scholar]
- Söderlund K., Greenhaff P. L., Hultman E. Energy metabolism in type I and type II human muscle fibres during short term electrical stimulation at different frequencies. Acta Physiol Scand. 1992 Jan;144(1):15–22. doi: 10.1111/j.1748-1716.1992.tb09262.x. [DOI] [PubMed] [Google Scholar]
- Tötösy de Zepetnek J. E., Zung H. V., Erdebil S., Gordon T. Motor-unit categorization based on contractile and histochemical properties: a glycogen depletion analysis of normal and reinnervated rat tibialis anterior muscle. J Neurophysiol. 1992 May;67(5):1404–1415. doi: 10.1152/jn.1992.67.5.1404. [DOI] [PubMed] [Google Scholar]
- Vøllestad N. K., Tabata I., Medbø J. I. Glycogen breakdown in different human muscle fibre types during exhaustive exercise of short duration. Acta Physiol Scand. 1992 Feb;144(2):135–141. doi: 10.1111/j.1748-1716.1992.tb09278.x. [DOI] [PubMed] [Google Scholar]
- Vøllestad N. K., Vaage O., Hermansen L. Muscle glycogen depletion patterns in type I and subgroups of type II fibres during prolonged severe exercise in man. Acta Physiol Scand. 1984 Dec;122(4):433–441. doi: 10.1111/j.1748-1716.1984.tb07531.x. [DOI] [PubMed] [Google Scholar]
- de Haan A. High-energy phosphates and fatigue during repeated dynamic contractions of rat muscle. Exp Physiol. 1990 Nov;75(6):851–854. doi: 10.1113/expphysiol.1990.sp003468. [DOI] [PubMed] [Google Scholar]
- de Haan A., Jones D. A., Sargeant A. J. Changes in velocity of shortening, power output and relaxation rate during fatigue of rat medial gastrocnemius muscle. Pflugers Arch. 1989 Feb;413(4):422–428. doi: 10.1007/BF00584493. [DOI] [PubMed] [Google Scholar]
- van der Laarse W. J., van Noort P., Diegenbach P. C. Calibration of quantitative histochemical methods: estimation of glycogen content of muscle fibers using the PAS reaction. Biotech Histochem. 1992 Sep;67(5):303–308. doi: 10.3109/10520299209110039. [DOI] [PubMed] [Google Scholar]