Abstract
Cervical cancer is one of the most common tumors in women and is a major problem in gynecological health. Studies have shown that Yinjia pills (YJP), a traditional Chinese medicine, can effectively slow the progression of cervical cancer. Therefore, this study mainly explored the molecular mechanism by which YJP delays the progression of cervical cancer. The expression level of PKM2 in cervical cancer was evaluated by the gene expression profiling interactive analysis (GEPIA) database, and the prognostic value of the PKM2 gene was evaluated by the Kaplan‒Meier plotter database. HeLa cervical cancer cells were treated with different concentrations of YJP (2.5, 5, 10, and 20 mg/mL). The levels of the inflammatory factors were detected by ELISA. Cell proliferation activity, migration and invasion were detected by CCK-8 assay, Transwell assays and cell scratch experiment. Apoptosis was detected by flow cytometry. Western blotting was used to detect the expression of proteins. In this study, PKM2 was upregulated in both cervical squamous cell carcinoma (CESC) and endometrial adenocarcinoma tissues, and a Kaplan‒Meier analysis showed that higher PKM2 expression was associated with lower patient survival. YJP inhibited the proliferation, migration and invasion of HeLa cells in a dose-dependent manner, promoted the apoptosis of HeLa cells, and inhibited the expression of inflammatory factors. In addition, YJP inhibited the activation of the JAK/STAT3 pathway and the occurrence of EMT. Knockdown of PKM2 also inhibited the malignant biological behavior of HeLa cells, but overexpression of PKM2 weakened the inhibitory effect of YJP on the malignant biological behavior of HeLa cells. Angoline, a JAK/STAT3 pathway inhibitor, attenuated the effect of PKM2 overexpression on the efficacy of YJP. In conclusion, YJP can inhibit the activation of the JAK/STAT3 pathway by regulating PKM2, thereby inhibiting the malignant biological behavior of HeLa cells.
Keywords: Cervical cancer, YJP, PKM2, JAK/STAT3 pathway
Introduction
Cervical cancer is a common gynecological disease that mostly affects middle-aged and elderly women. However, due to the unhealthy lifestyles and habits of modern people, the age of cervical cancer patients is showing a downward trend. It is worth noting that in addition to a large number of patients with cervical cancer, it also has a strong lethal ability. According to the relevant information, a woman dies of cervical cancer every two minutes in the world (Koh et al. 2019). Due to the large population base in China, the number of patients with cervical cancer is also relatively large. Once a woman suffers from the disease, it will bring huge economic pressure to the patient’s family, leading countless happy families into the abyss of pain. The stable development of society will have a certain impact. Although modern medical methods are abundant and medical development is relatively fast, no substantial breakthrough has been made in the treatment of cancer. The current treatment methods for cervical cancer are relatively scarce, and the therapeutic effect is also limited (Xia et al. 2019). However, researchers continue to search for more effective treatments for cervical cancer, and traditional Chinese medicine (TCM), including Chinese herbal medicine, has been used in cancer patients for decades (Qian et al. 2019). According to the analysis of the existing evidence, Chinese medicine can induce apoptosis and inhibit invasion or metastasis in vitro or in animal models (Hsiao et al. 2019).
Yinjia pill (YJP) is a prescription composed of 15 kinds of traditional Chinese medicine. After many clinical trials, YJP has become a stable and reliable medicine, and it is often used in the clinical treatment of pelvic inflammatory disease (Chen and Guan 2016; Wu et al. 2019). Cervical cancer and pelvic inflammatory disease are both diseases that occur within the female reproductive organs, and a recent study reported that YJP is also effective in slowing cervical cancer progression (Du and Yang 2020). However, since YJP contains a variety of pharmaceutical components, multiple metabolic pathways are affected when the body ingests YJP. Since different components function in different metabolic pathways and each component has a specific target, they constitute a complex network of YJP mechanisms of action on cervical cancer. Because of its complexity, the current understanding of the molecular mechanism of YJP in the treatment of cervical cancer is relatively low. When a patient develops cervical cancer, cytokines, including IL-6, IL-8, and IL-17, are released in large amounts in the diseased tissue, indicating that some cytokines are associated with tumor formation and growth (Li et al. 2019; Liu et al. 2020b; Zhang et al. 2015). Previous studies have found that YJP reduces the levels of inflammatory factors, including IL-6, IL-2, IL-1β, and TNF-α, in pelvic inflammatory disease (Li 2018; Zhang and Zhao 2020). Therefore, we hypothesized that YJP has potential functional value in the active treatment of cervical cancer.
At present, cytokines have multiple family classifications, and various cytokines have different physiological regulatory functions. The level of cytokines in collective tissues can also intuitively reflect the physiological and metabolic conditions of the collective. The GP130 cytokine family has been reported in many articles, and IL-6 has been identified as one of them. Similar to some cytokines, IL-6 also has immunomodulatory effects and is involved in suppressing tumor progression (Hong et al. 2007; Hunter and Jones 2015). It has been previously reported that after IL-6 specifically binds to IL-6Rα, Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3) signaling is activated, leading to chronic inflammation in cervical cancer. Abnormal JAK/STAT3 signaling promotes tumor cell growth, invasion, metastasis, and inflammation (Taniguchi and Karin 2014). Accumulating evidence suggests that TCM ameliorates inflammatory and anticancer effects (Chen et al. 2020; Yan et al. 2017), including cervical cancer (Lin et al. 2017). For example, Kaempferia parviflora, a TCM, inhibits IL-6 secretion and STAT3 activation in HeLa cervical cancer cells (Suradej et al. 2019). Previous studies have shown that TCM can inhibit cell proliferation, invasion, and migration (Su et al. 2019). Interestingly, Xijiao Dihuang decoction, a TCM similar to YJP, reduces pyruvate kinase 2 (PKM2) expression (Lu et al. 2020; Zhao et al. 2019). It is well known that ATP can be produced under the catalysis of PKM2; however, researchers found that the development of cervical cancer upregulates the expression of PKM2 (Tyszka-Czochara et al. 2018) and that the levels of proinflammatory cytokines and cell proliferation in colorectal cancer were increased with the upregulation of PKM2 (Yang et al. 2015). However, there is currently no explanation of how YJP inhibits the physiological activities of tumor cells, and the regulatory mechanism between YJP, PKM2 and cervical cancer remains unclear.
Based on the above studies, we hypothesized that YJP could regulate the JAK/STAT3 signaling pathway through PKM2, thereby inhibiting the proliferation, invasion and migration of cervical cancer cells. This study aimed to elucidate the mechanism of action of YJP in cervical cancer at the cellular level. Our study reveals for the first time that YJP can influence cervical cancer progression through the PKM2/JAK/STAT3 molecular axis, providing new potential therapeutic targets for cervical cancer.
Materials and methods
GEPIA
The GEPIA database (http://gepia.cancer-pku.cn/) (Tang et al. 2017) was used to assess the expression level of PKM2 in cervical cancer. Genes with |log2FC| > 1 and p value < 0.05 were counted according to the alignment.
Survival analysis
The prognostic value of genes in cervical cancer was assessed by the Kaplan‒Meier plotter database (https://kmplot.com/analysis/). Hazard ratios and log-rank p values with 95% confidence intervals were recorded. Log-rank p values < 0.05 were counted according to the alignment.
Preparation of YJP
YJP preparations were from Chengdu University of Traditional Chinese Medicine and were prepared from the 15 traditional Chinese medicines shown in Table 1. For the specific preparation method, refer to the report of Xu et al. (2019). Briefly, aqueous extracts of YJP were diluted 10 times in double-distilled water and heated at 100 °C for 3 h under continuous stirring conditions. The steps were repeated twice following which the extracts were subjected to centrifugation at 1500×g. The supernatants were collected and kept under low pressure at 70 °C for evaporation until the semisolid state of YJP (lyophilized powder) is formed. The pH value of the mixture was regulated between 6 and 8 by using triethanolamine as a neutralizer. YJP was diluted in 0.9% saline to maintain a concentration of 1 g/ml. Small aliquots of YJP solution were made and stored at − 20 °C until use.
Table 1.
Constituents of Yinjia pills
| Chinese name | Botanical name | Weight (g) |
|---|---|---|
| Wuyao | Lindera aggregate (Sims) Kosterm | 15 |
| Jinyinhua | Lonicera japonica Thunb | 30 |
| Lianqiao | Forsythia mira M. C. Chang | 30 |
| Xiakucao | Prunella vulgaris L | 15 |
| Chuanlianzi | Melia toosendan Sieb. et Zucc | 9 |
| Xiangfu | Cyperus rotundus L | 12 |
| Caohongteng | Sargentodoxa cuneata (Oliv.) Rehd. et Wils | 30 |
| Aojia | Trionyx sinensis Wiegmann | 30 |
| Shaoyao | Paeonia lactiflora Pall | 15 |
| Pugongying | Taraxacum mongolicum Hand.-Mazz | 30 |
| Zihuadiding | Viola philippica Cav | 30 |
| Danggui | Angelica sinensis (Oliv.) Diels | 12 |
| Hupo | Amber | 9 |
| Chuanxiong | Ligusticum chuanxiong Hort | 6 |
| Sigualuo | Luffa cylindrica (L.) Roem | 15 |
The names, including Chinese and common names, and the weight of each included herb
Cell cultures
HeLa cervical cancer cells were obtained from Beijing Beina Chuanglian Institute of Biotechnology (Beijing, China) and were grown in RPMI-1640 medium (ThermoFisher Scientific, California, USA) containing 10% fetal bovine serum (FBS, Gibco).
Cell transfections and treatments
Small interfering RNA (siRNA) that directly targets PKM2 (si-PKM2) and a PKM2 overexpression plasmid (oe-PKM2; GenePharma, Shanghai, China) were transfected into HeLa cells. The transfection kit used was a Lipofectamine 2000 kit (Invitrogen, USA). Two days after transfection, transfection efficiency was assessed. For the YJP treatments, cells were incubated with different concentrations of YJP (0, 2.5, 5, 10, and 20 mg/ml) for 48 h.
CCK-8
According to previous studies (Liu et al. 2020a), HeLa cells of each group were seeded into 96-well plates and cultured for 24 h. Then, 10 μL CCK-8 solution was added to each well. After 4 h of culture, the absorbance was measured at 450 nm wavelength utilizing a microplate reader (BioTek Instruments Inc), and the percentages of surviving cells were calculated.
Detection of cell apoptosis
As described by Rafat et al. (2022), HeLa cell apoptosis was detected with an Annexin V-FITC/PI kit (Beyotime, Shanghai, China). HeLa cells were collected from each group, washed twice with PBS and centrifuged. The cells were re-suspended with 1× binding buffer. Then, the cells were added with 5 μL Annexin V-FITC, mixed and incubated for 15 min away from light, and then added with 10 μL PI. After mixing, apoptosis was detected by flow cytometry (BD Biosciences, USA).
Enzyme-linked immunosorbent assay (ELISA)
Refer to previous research methods (Farahzadi et al. 2023), the cells were collected and centrifuged, and the supernatant was collected. The expression levels of the inflammatory factors IL-2, IL-6 and TNF-α in the supernatant were detected by ELISA kits (Abcam, Cambridge, UK). In brief, 96-well plates were coated with capture antibodies and incubated at 4 °C overnight. Then 200 μL sealing buffer was added to each well, and after washing with PBS, the cell supernatant was added to the 96-well plate. Finally, 100 μL diluted detector antibody was added to each well for detection via sandwich ELISA technology.
Transwell assay
As described in previous studies (Liu et al. 2020a), HeLa cells suspension was taken to count the cell number and then diluted to 1 × 105 cells/mL. Transwell chambers were put into 24-well plates, and the cell suspension was inoculated into the apical chambers; medium (0.5 mL) containing 10% FBS was added into the basolateral chambers for incubation in a 5% CO2 incubator at 37 °C for 24 h. Migrated cells on the basal side of the membranes were treated with paraformaldehyde (4%) and crystal violet (0.1%) were used to stain cells for 5 min, followed by PBS washing. Each chamber was photographed using a light microscope, and the number of cells in each field was an average number obtained. For cell invasion assay, the transwell chambers were pre-coated with Matrigel (BD Biosciences, NJ, USA) following the similar approach.
Wound healing assay
The wound healing was performed as previously described (You et al. 2021), HeLa cells in logarithmic growth phase were digested, subcultured and seeded into 6-well plates. When the cell density reached 90%, the cells were scratched with a 200 μL pipette tip. After 48 h of cell culture, the migration of the cells was observed under a light microscope, and the migration rate was analyzed by ImageJ software.
Western blot assay
Western blot tests were performed as previously described (Bagheri et al. 2021). In brief, total proteins were extracted from HeLa cells using RIPA buffers containing 1% protease inhibitors and phosphatase inhibitors. The protein concentration was determined according to the instructions of the BCA protein detection kit (Solarbio, Beijing, China). The proteins were then separated by SDS-PAGE and transferred to PVDF membranes. Then the membranes were blocked with 5% skim milk at room temperature for 2 h. Next, the membranes were incubated with primary antibodies at 4 °C overnight, the primary antibodies used were as follow: anti-E-cadherin (1:1000; no. ab40772, Abcam, Cambridge, UK), anti-N- cadherin (1:5000; no. ab200661, Abcam), anti-vimentin (1:2000; no. ab92547, Abcam), anti-Fibronectin (1:1000; no. ab2413, Abcam), anti-PKM2 (1:1000; no. ab150377, Abcam), anti-JAK1 (1:2000; no. ab138005, Abcam), anti-p-JAK1 (1:1000; no. ab138005, Abcam), anti-JAK2 (1:2000; no. ab108596, Abcam), anti-p-JAK2 (1:1000; no. ab32101, Abcam), anti-STAT3 (1:1000; no. ab68153, Abcam), anti-p-STAT3(Tyrosine residue phosphorylation) (1:1000; no. ab76315, Abcam), and β-actin (1:5000; no. ab8226, Abcam) antibodies. Then, the membranes and HRP labeled secondary antibodies were incubated at room temperature for 2 h. After incubation, the proteins were semiquantitatively assessed for expression by enhanced chemiluminescence (ECL) chromogenic and ImageJ software.
Statistical analysis
The data are shown as the mean ± standard deviation. The comparison of two groups or between multiple groups was performed by t test or one-way ANOVA, respectively. The data in this study were normally distributed and were analyzed by variance analysis and a post hoc test (Bonferroni or Tukey’s). GraphPad Prism 7 plotted the experimental data. P < 0.05 indicates a significant difference.
Results
PKM2 showed an upregulated trend in cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC) tissues
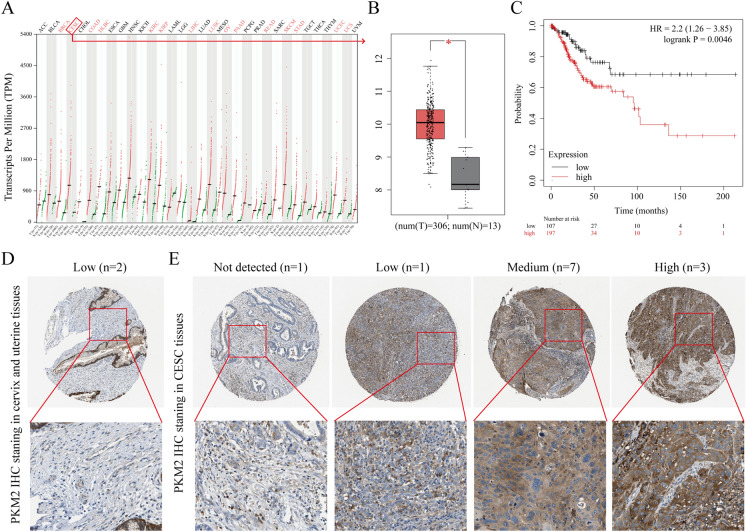
GEPIA was used to test the expression of PKM2 at the mRNA level in tissues. PKM2 mRNA expression was remarkably upregulated from healthy cervical tissue (n = 13) to CESC tissue (n = 306) (Fig. 1A–B). Kaplan‒Meier analysis demonstrated that higher PKM2 expression was associated with lower patient survival (Fig. 1C). Additionally, PKM2 protein levels were assessed by searching the CESC staining data in the Human Protein Atlas (www.proteinatlas.org/) database. While PKM2 staining in healthy cervical and uterine tissues displayed low PKM2 staining (Fig. 1D), moderate (7/12) and high (3/12) PKM2 staining was often observed in severe CESC tissues (Fig. 1E). From these data, it was found that the mRNA and protein levels of PKM2 were remarkably upregulated in CESC tissues.
Fig. 1.
Expression analysis of PKM2 in CESC tissues. A PKM2 mRNA expression in different normal human tissues and cancer tissues. B Comparison of PKM2 mRNA expression in CESC tissues (n = 306) and normal cervical tissues (n = 13). C Prognostic value of PKM2 in CESC. D Representative IHC images of PKM2 in normal cervical and uterine tissues. E Representative IHC images of PKM2 in CESC tissues
YJP depends on dose to inhibit proliferation, migration, invasion, and inflammatory cytokines and accelerates the apoptosis of HeLa cells
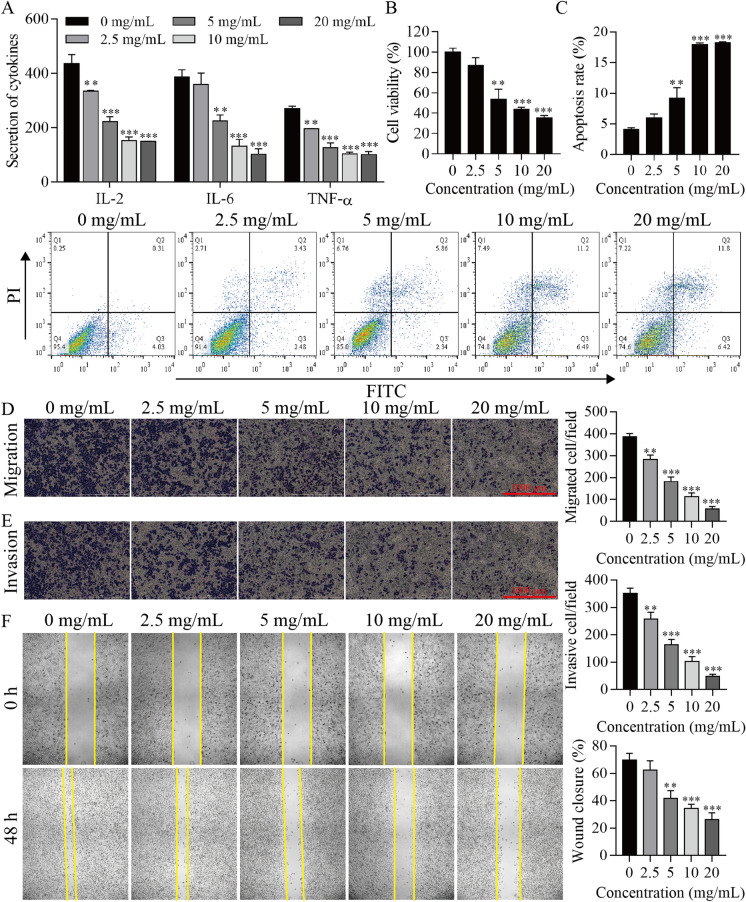
HeLa cells were stimulated with YJP (0, 2.5, 5, 10, 20 mg/mL) for 2 days, and some physiological parameters of the cells were determined. It was found that YJP at concentrations of 2.5, 5, 10 and 20 mg/mL inhibited cell inflammatory cytokines IL-2, IL-6 and TNF-α, cell proliferation, migration and invasion and promoted apoptosis in HeLa cells, and these effects increased with an increasing YJP concentration (Fig. 2). These results suggest that YJP may depend on dose to inhibit proliferation, migration, invasion, and inflammatory cytokines and accelerate the apoptosis of HeLa cells.
Fig. 2.
Analysis of the effect of YJP on different indices of cells. A ELISA for inflammatory cytokine levels. B CCK-8 analysis of cell proliferation viability. C Flow cytometry for apoptosis. Transwell assay for cell migration (D) and invasion (E). F Wound healing assay. **P < 0.01, ***P < 0.001 vs. 0 mg/mL
YJP antitumor activation might be mediated through the PKM2/JAK/STAT3 signaling pathway
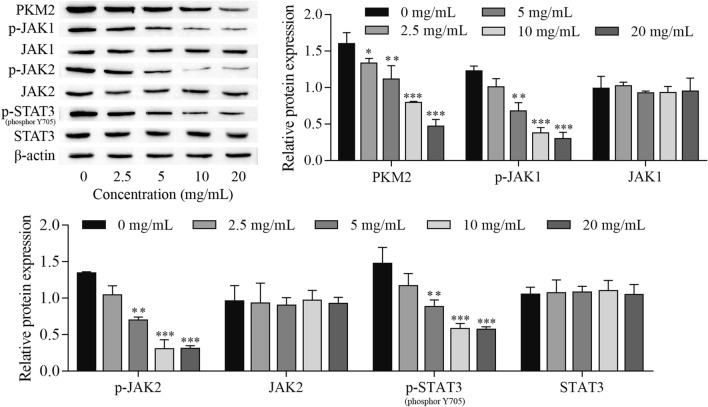
To demonstrate the role of signaling pathways in YJP-mediated antitumor activity, HeLa cells treated with different concentrations of YJP were subjected to protein extraction and used for Western blot detection. PKM2 and JAK/STAT3 signaling pathway-related proteins were examined. The results showed that the expression of PKM2, p-JAK1, p-JAK2 and p-STAT3 was inhibited in cells treated with 2.5, 5, 10 and 20 mg/mL YJP (Fig. 3). These results indicated that YJP antitumor activation might be mediated through the PKM2/JAK/STAT3 signaling pathway. In subsequent experiments, 10 mg/mL YJP was selected to treat HeLa cells.
Fig. 3.
YJP inhibits PKM2 expression and the JAK/STAT3 signaling pathway. Western blot for the expression of PKM2 and JAK/STAT3 signaling pathway-related proteins. *P < 0.05 and **P < 0.01, ***P < 0.001 vs. 0 mg/mL
YJP inhibits proliferation, migration, invasion, inflammatory cytokine production, and promotes apoptosis of HeLa cells by regulating PKM2
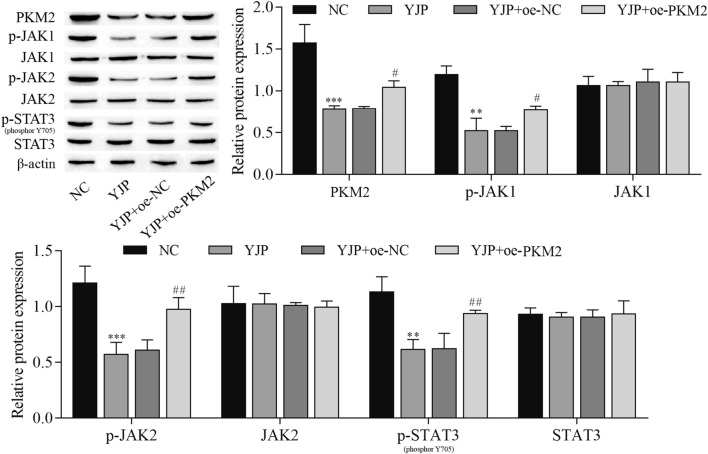
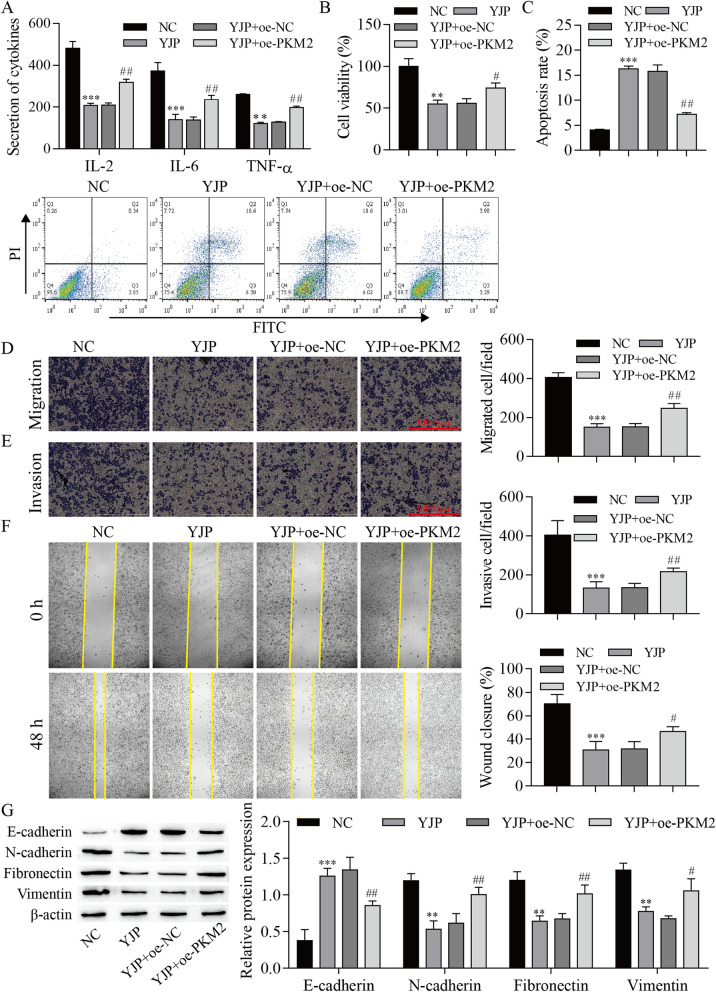
To further explore the effect of PKM2 on YJP antitumor activation, oe-PKM2 and oe-NC were transfected into HeLa cells. The experimental data indicated that YJP decreased the expression of PKM2, p-JAK1, p-JAK2, and p-STAT3, which was reversed by PKM2 overexpression (Fig. 4). Next, the data, including cell inflammatory cytokines and viability, were measured. The results showed that cell inflammatory cytokines, proliferation, migration, and invasion were inhibited by YJP, but these effects were reversed by PKM2 overexpression. In contrast, cell apoptosis was promoted by YJP, which was reversed by PKM2 overexpression. In addition, Western blotting was used to analyze the expression of EMT-related proteins. The expression of E-cadherin was elevated with YJP treatment, but overexpression of PKM2 inhibited E-cadherin expression. In contrast, the expression of N-cadherin, Fibronectin, and Vimentin was decreased after YJP treatment, and these effects were reversed by PKM2 overexpression (Fig. 5). From these results, it can be concluded that the effects of YJP on cell proliferation, migration, invasion, inflammatory cytokines, apoptosis, and EMT were weakened by PKM2 overexpression in HeLa cells.
Fig. 4.
YJP inhibits the JAK/STAT3 signaling pathway by downregulating PKM2 expression. Western blot for the expression of PKM2 and JAK/STAT3 signaling pathway-related proteins. **P < 0.01, ***P < 0.001 vs. NC, #P < 0.05 and ##P < 0.01 vs. YJP; NC, negative control; oe, overexpression
Fig. 5.
YJP inhibits proliferation, migration, invasion, inflammatory cytokine production, and accelerates the apoptosis of HeLa cells by regulating PKM2. A ELISA for inflammatory cytokine levels. B CCK-8 analysis of cell proliferation viability. C Flow cytometry for apoptosis. Transwell assay for cell migration (D) and invasion (E). F Wound healing assay. G Western blot showing the expression of EMT-related proteins. **P < 0.01, ***P < 0.001 vs. NC; #P < 0.05 and ##P < 0.01 vs. YJP; NC, negative control; oe, overexpression
YJP inhibits proliferation, migration, invasion, inflammatory cytokine production, and promotes apoptosis of HeLa cells by regulating PKM2/JAK/STAT3
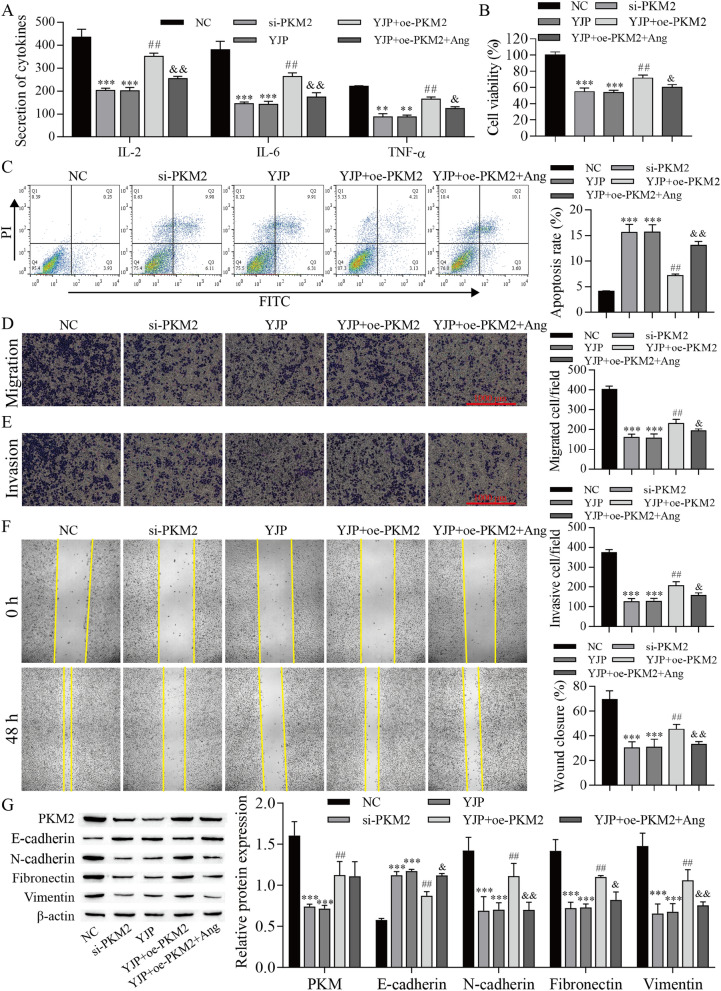
To further investigate the PKM2/JAK/STAT3 mechanism, STAT3 signaling was inhibited by using the STAT3 inhibitor angoline in this study. Next, the data, including cell inflammatory cytokines and viability, were measured. The results showed that the cell inflammatory cytokines, proliferation, migration, and invasion were reduced by PKM2 knockdown and YJP treatments, but these inhibitory effects of YJP treatment were reversed by PKM2 overexpression and the effect of PKM2 overexpression were finally repressed with angoline treatments. In contrast, cell apoptosis was promoted by PKM2 knockdown and YJP treatment, but the promotion effect of YJP treatment was weakened by PKM2 overexpression, and the effect of PKM2 overexpression was finally weakened by angoline treatment. Next, Western blotting was used to analyze the expression of EMT-related proteins. YJP and PKM2 knockdown decreased PKM2 expression, and the inhibitory effect of YJP was reversed by PKM2 overexpression; angoline did not change PKM2 expression. Additionally, PKM2 knockdown and YJP treatments promoted the expression of E-cadherin, inhibited the expression of N-cadherin, Fibronectin, and Vimentin, but the inhibitory effect of YJP treatment was weakened by PKM2 overexpression, and angoline ultimately repressed EMT process (Fig. 6). These results indicated that YJP affects cell proliferation, migration, invasion, inflammatory cytokines, and apoptosis by downregulating PKM2 expression and inhibit the JAK/STAT3 signaling pathway in HeLa cells.
Fig. 6.
YJP inhibits proliferation, migration, invasion, inflammatory cytokine production, and accelerates the apoptosis of HeLa cells by regulating PKM2/JAK/STAT3. A ELISA for inflammatory cytokine levels. B CCK-8 analysis of cell proliferation viability. C Flow cytometry for apoptosis. Transwell assay for cell migration (D) and invasion (E). F Wound healing assay. G Western blot for the expression of EMT-related proteins. **P < 0.01, ***P < 0.001 vs. NC; ##P < 0.01 vs. YJP; &P < 0.05 and &&P < 0.01 vs. YJP + oe-PKM2; NC negative control, oe overexpression, si small interfering RNA
Discussion
There are many enzymes involved in the catalytic synthesis of ATP. Pyruvate kinase has been widely reported as one of the rate-limiting enzymes. Under its catalysis, ADP is consumed, and pyruvate is generated. In addition to its function in catalyzing ATP, the level of pyruvate kinase is also used as a reliable indicator for the diagnosis of myocardial infarction, cervical cancer and other diseases (Zahra et al. 2020). Each enzyme may have one or several bodies; pyruvate kinase is no exception, and PKM2 is an isoform of this enzyme. In a previous report, researchers found that PKM2 has a striking regulatory function in the metabolic budget system of tumor cells (Mazurek 2011). Among them, many studies have shown that the upregulation of PKM2 can be found in various tumor tissues, including pancreatic ductal adenocarcinoma and bladder cancer, which further proves that PKM2 is a physiologically active factor worthy of attention by medical personnel (Xie et al. 2019; Yang et al. 2015; Zhou et al. 2019). However, the details of PKM2 in cervical cancer progression remain largely unknown. Through database analysis, we found that the mRNA and protein levels of PKM2 were remarkably upregulated in CESC tissues. These findings suggest that PKM2 is critical for cervical cancer progression and prognosis.
Currently, medical research is constantly progressing, and traditional Chinese medicine has been proven to have broad prospects in the treatment of cancer (Jiao et al. 2018). Related reports have pointed out that the TCM Xiao ji can inhibit the growth of lung cancer cells by regulating the AMPK signaling pathway (Zhao et al. 2016). In addition, matrine plays a significant role in inhibiting the metastasis of human cervical cancer cells, and the mechanism of action is related to the regulation of the p38 signaling pathway (Wu et al. 2017). Ze–Qi–Tang has a significant function in restricting the growth of non-small cell lung cancer cells, and the mechanism of action is related to the regulation of the p53 pathway (Xu et al. 2019). We consulted the literature and learned that YJP attenuates the progression of cervical cancer (Du and Yang 2020). Here, we found that YJP depends on dose to inhibit proliferation, migration, invasion, and inflammatory cytokines and accelerates the apoptosis of HeLa cells. Notably, YJP also inhibits PKM2 expression and JAK/STAT3 signaling activation. Accumulating evidence shows that PKM2 accelerates inflammatory cytokine secretion and cell proliferation in cancer (Xie et al. 2016; Yang et al. 2015). IL-6 activates JAK/STAT3 signaling (Taniguchi and Karin 2014). Aberrant activation of JAK/STAT3 has been implicated in cervical cancer and other chronic cervical diseases (Cheng et al. 2017; Floberg et al. 2021; Wang et al. 2019). In this study, we found that PKM2 overexpression reduced the effect of YJP on HeLa cells, while the addition of STAT3 signaling pathway inhibitor Angoline weakened the effect of PKM2 overexpression, thereby alleviating the malignant biological behavior of HeLa cells. This suggests that YJP may inhibit HeLa cell proliferation, migration, invasion, inflammatory cytokine production and accelerate HeLa cell apoptosis through PKM2 regulation of JAK/STAT3 signaling pathway. Our findings provide a new potential therapeutic target for YJP in the treatment of cervical cancer.
As an evolutionarily conserved developmental program, EMT can alter some of the physiological functions of cells and confer metastatic properties to cancer cells. In other words, EMT can have a special impact on the formation and development of cancer (Mittal 2018). A recent study reported that PKM2 regulates EMT programs in hepatocellular carcinoma (Tan et al. 2019). Accumulating evidence shows that the JAK/STAT3 signaling pathway regulates metastasis by EMT programs (Jin 2020; Liu et al. 2014; Yang et al. 2020). Consistently, in our study, PKM2 and JAK/STAT3 pathways were found to be related to EMT of HeLa cells. Interestingly, in China, traditional Chinese medicine has a powerful function in the comprehensive treatment of tumors. Chemotherapy and radiotherapy can be combined to achieve a better effect (Tang et al. 2020). A large number of clinical trials and biomedical research reports have shown that traditional Chinese medicine can reduce the side effects of chemotherapy or radiotherapy in cancer patients (Zhang et al. 2018). In this study, YJP inhibited EMT in HeLa cells, suggesting that YJP may have an auxiliary function in the treatment of cancer. However, overexpression of PKM2 weakened the effect of YJP and promoted EMT in HeLa cells. Further addition of STAT3 signaling pathway inhibitor Angoline partially reversed the effect of overexpression of PKM2 and inhibited EMT in HeLa cells. This suggests that YJP may play a unique role in regulating the EMT process of cervical cancer cells through the PKM2/JAK/STAT3 signaling pathway. This is also a new discovery linking the PKM2/JAK/STAT3 molecular axis with EMT, which provides a valuable theoretical reference for cancer treatment.
In summary, our study revealed for the first time that YJP could inhibit the proliferation, migration, invasion, inflammatory cytokine production and EMT of HeLa cells, and promote cell apoptosis by inhibiting the PKM2/JAK/STAT3 molecular axis. This study elucidates the potential molecular pathway of the anti-tumor effect of YJP in cervical cancer, which may provide theoretical basis for further understanding of the functional mechanism of YJP in the malignant development of cervical cancer. However, there are some limitations to this study. Firstly, the highest concentration of YJP used in this study was 20 mg/mL, and it still needs a large number of experiments to verify whether increasing the concentration of YJP has a higher inhibitory effect on HeLa cell viability or a reduced inhibitory effect. Secondly, this study only examined the effect of YJP at the cellular level. Due to the complexity of the in vivo environment, it is necessary to verify this regulatory mechanism of YJP in animal models.
Acknowledgements
Not applicable.
Author contributions
Conceptualization, Ying Shi and Xiaoli Min; methodology, Ying Shi, Xiaoli Min and Yi Li; software, Yi Li and Lihua Guo; validation, Ying Shi, Lihua Guo and Zheng Cai; formal analysis, Ying Shi, Xiaoli Min, Lihua Guo and Xiaoxia Yang; investigation, Lihua Guo and Zheng Cai; resources, Ying Shi, data curation, Ying Shi and Xiaoli Min; writing—original draft preparation, Xiaoli Min, Yi Li and Lihua Guo; writing—review and editing, Ying Shi, Xiaoli Min and Yi Li; visualization, Dongge Li, Xueying Jiang, Ni Feng and Xiaolin Li; supervision, Ying Shi and Xiaoli Min; funding acquisition, Ying Shi. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Associated Project of Yunnan Province Science & Technology Department and Yunnan University of Chinese Medicine Basic Research for Application (Grant Nos. 2018FF001 (-055) and 2018FF001 (-013)).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
Not applicable.
Consent for publication
All the authors agreed on the publication of this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ying Shi and Xiaoli Min are co-first authors.
References
- Bagheri Y, Barati A, Nouraei S et al (2021) Comparative study of gavage and intraperitoneal administration of gamma-oryzanol in alleviation/attenuation in a rat animal model of renal ischemia/reperfusion-induced injury. Iran J Basic Med Sci 24:175–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Guan HX (2016) Modified yinjia pills in treatment of infertility with damp heat and blood stasis due to chronic pelvic inflammation after laparoscopy. Chin J Exp Tradit Med Form 22:195–198 [Google Scholar]
- Chen C, Li M, Liu X et al (2020) Traditional Chinese medicine Da–Cheng–Qi–Tang ameliorates impaired gastrointestinal motility and intestinal inflammatory response in a mouse model of postoperative ileus. Evid Based Complementary Altern Medi Ecam 2020:9074069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Rosario G, Cohen TV et al (2017) Tissue-specific ablation of the LIF receptor in the murine uterine epithelium results in implantation failure. Endocrinology 158:1916–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du FF, Yang MT (2020) One case report of low grade cervical intraepithelial neoplasia treated with integrated traditional Chinese and Western Medicine. Hunan J Tradit Chin Med 36:84–85 [Google Scholar]
- Farahzadi R, Fathi E, Mesbah-Namin SA et al (2023) Granulocyte differentiation of rat bone marrow resident C-kit(+) hematopoietic stem cells induced by mesenchymal stem cells could be considered as new option in cell-based therapy. Regener Ther 23:94–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floberg JM, Zhang J, Muhammad N et al (2021) Standardized uptake value for 18F-Fluorodeoxyglucose is a marker of inflammatory state and immune infiltrate in cervical cancer. Clin Cancer Res [DOI] [PMC free article] [PubMed]
- Hong D, Angelo L, Kurzrock R (2007) Interleukin-6 and its receptor in cancer: implications for translational therapeutics. Cancer 110:1911–1928 [DOI] [PubMed] [Google Scholar]
- Hsiao Y, Lin C, Wang P et al (2019) The potential of Chinese Herbal medicines in the treatment of cervical cancer. Integr Cancer Ther 18:1534735419861693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter C, Jones S (2015) IL-6 as a keystone cytokine in health and disease. Nat Immunol 16:448–457 [DOI] [PubMed] [Google Scholar]
- Jiao L, Bi L, Lu Y et al (2018) Cancer chemoprevention and therapy using chinese herbal medicine. Biol Proced Online 20:1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W (2020) Role of JAK/STAT3 signaling in the regulation of metastasis, the transition of cancer stem cells, and chemoresistance of cancer by epithelial-mesenchymal transition. Cells 9 [DOI] [PMC free article] [PubMed]
- Koh WJ, Abu-Rustum NR, Bean S et al (2019) Cervical cancer, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw JNCCN 17:64–84 [DOI] [PubMed] [Google Scholar]
- Li CH (2018) Study on the mechanism of Yin jia wan for treating chronic pelvic inflammatory disease with dampness and heat stasis. Hubei University for Nationalities
- Li H, Chi X, Li R et al (2019) HIV-1-infected cell-derived exosomes promote the growth and progression of cervical cancer. Int J Biol Sci 15:2438–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Chen L, Qiu X et al (2017) Traditional Chinese medicine for human papillomavirus (HPV) infections: a systematic review. Biosci Trends 11:267–273 [DOI] [PubMed] [Google Scholar]
- Liu RY, Zeng Y, Lei Z et al (2014) JAK/STAT3 signaling is required for TGF-β-induced epithelial-mesenchymal transition in lung cancer cells. Int J Oncol 44:1643–1651 [DOI] [PubMed] [Google Scholar]
- Liu D, Huang K, Wang T et al (2020a) NR2F2-AS1 accelerates cell proliferation through regulating miR-4429/MBD1 axis in cervical cancer. Biosci Rep 40 [DOI] [PMC free article] [PubMed]
- Liu Y, Li L, Li Y et al (2020b) Research progress on tumor-associated macrophages and inflammation in cervical cancer. Biomed Res Int 2020:6842963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhang L, Cheng L et al (2020) Xijiao Dihuang decoction improves prognosis of sepsis via inhibition of aerobic glycolysis. Biomed Pharmacother 129:110501 [DOI] [PubMed] [Google Scholar]
- Mazurek S (2011) Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol 43:969–980 [DOI] [PubMed] [Google Scholar]
- Mittal V (2018) Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol 13:395–412 [DOI] [PubMed] [Google Scholar]
- Qian Q, Chen W, Cao Y et al (2019) Targeting reactive oxygen species in cancer via chinese herbal medicine. Oxid Med Cell Longev 2019:9240426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafat A, Dizaji Asl K, Mazloumi Z et al (2022) Telomerase inhibition on acute myeloid leukemia stem cell induced apoptosis with both intrinsic and extrinsic pathways. Life Sci 295:120402 [DOI] [PubMed] [Google Scholar]
- Su M, Gong XJ, and Zhou X (2019) Research progress in mechanism of traditional Chinese medicine active ingredients against cervical cancer. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J Chin Materia Medica 44:675–684 [DOI] [PubMed]
- Suradej B, Sookkhee S, Panyakaew J et al (2019) Kaempferia parviflora Extract Inhibits STAT3 Activation and Interleukin-6 Production in HeLa cervical cancer cells. Int J Mol Sci 20 [DOI] [PMC free article] [PubMed]
- Tan HW, Leung CO, Chan KK et al (2019) Deregulated GATA6 modulates stem cell-like properties and metabolic phenotype in hepatocellular carcinoma. Int J Cancer 145:1860–1873 [DOI] [PubMed] [Google Scholar]
- Tang Z, Li C, Kang B et al (2017) GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 45:W98-w102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Shu P, Liu S et al (2020) Traditional Chinese medicine in oncotherapy: the research status. Nutr Cancer 72:992–998 [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Karin M (2014) IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol 26:54–74 [DOI] [PubMed] [Google Scholar]
- Tyszka-Czochara M, Bukowska-Strakova K, Kocemba-Pilarczyk KA et al (2018) Caffeic acid targets AMPK signaling and regulates tricarboxylic acid cycle anaplerosis while metformin downregulates HIF-1α-induced glycolytic enzymes in human cervical squamous cell carcinoma lines. Nutrients 10 [DOI] [PMC free article] [PubMed]
- Wang DW, You D, Dong J et al (2019) Knockdown of long non-coding RNA LINC00518 inhibits cervical cancer proliferation and metastasis by modulating JAK/STAT3 signaling. Eur Rev Med Pharmacol Sci 23:496–506 [DOI] [PubMed] [Google Scholar]
- Wu X, Zhou J, Cai D et al (2017) Matrine inhibits the metastatic properties of human cervical cancer cells via downregulating the p38 signaling pathway. Oncol Rep 38:1312–1320 [DOI] [PubMed] [Google Scholar]
- Wu JL, Wei SB, Wang YH (2019) Clinical effect of Yinjia pills combined with levofloxacin lactate in the treatment of pelvic inflammation. Clin Res Pract 4:141–143 [Google Scholar]
- Xia C, Hu S, Xu X et al (2019) Projections up to 2100 and a budget optimisation strategy towards cervical cancer elimination in China: a modelling study. Lancet Public Health 4:e462–e472 [DOI] [PubMed] [Google Scholar]
- Xie M, Yu Y, Kang R et al (2016) PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat Commun 7:13280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie R, Chen X, Chen Z et al (2019) Polypyrimidine tract binding protein 1 promotes lymphatic metastasis and proliferation of bladder cancer via alternative splicing of MEIS2 and PKM. Cancer Lett 449:31–44 [DOI] [PubMed] [Google Scholar]
- Xu Z, Zhang F, Zhu Y et al (2019) Traditional Chinese medicine Ze–Qi–Tang formula inhibit growth of non-small-cell lung cancer cells through the p53 pathway. J Ethnopharmacol 234:180–188 [DOI] [PubMed] [Google Scholar]
- Yan Z, Lai Z, Lin J (2017) Anticancer properties of traditional chinese medicine. Comb Chem High Throughput Screen 20:423–429 [DOI] [PubMed] [Google Scholar]
- Yang P, Li Z, Li H et al (2015) Pyruvate kinase M2 accelerates pro-inflammatory cytokine secretion and cell proliferation induced by lipopolysaccharide in colorectal cancer. Cell Signal 27:1525–1532 [DOI] [PubMed] [Google Scholar]
- Yang X, Gao Y, Liu Q et al (2020) Zoledronic acid re-sensitises gefitinib-resistant lung cancer cells by inhibiting the JAK/STAT3 signalling pathway and reversing epithelial-mesenchymal transition. Oncol Rep 45:459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You X, Wang Y, Meng J et al (2021) Exosomal miR‑663b exposed to TGF‑β1 promotes cervical cancer metastasis and epithelial‑mesenchymal transition by targeting MGAT3. Oncol Rep 45 [DOI] [PMC free article] [PubMed]
- Zahra K, Dey T, Ashish et al (2020) Pyruvate kinase M2 and cancer: the role of PKM2 in promoting tumorigenesis. Front Oncol 10:159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang AH, Zhao JM (2020) Clinical efficacy of modified Yinjiapill combined western medicine in the treatment of chronic pelvic inflammation with dampness and heat stasis. J Heze Med Coll 32:42–44 [Google Scholar]
- Zhang W, Tian X, Mumtahana F et al (2015) The existence of Th22, pure Th17 and Th1 cells in CIN and cervical cancer along with their frequency variation in different stages of cervical cancer. BMC Cancer 15:717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HW, Lin ZX, Cheung F et al (2018) Moxibustion for alleviating side effects of chemotherapy or radiotherapy in people with cancer. Cochrane Database Syst Rev 11:Cd010559 [DOI] [PMC free article] [PubMed]
- Zhao S, Wu J, Tang Q et al (2016) Chinese herbal medicine Xiaoji decoction inhibited growth of lung cancer cells through AMPKα-mediated inhibition of Sp1 and DNA methyltransferase 1. J Ethnopharmacol 181:172–181 [DOI] [PubMed] [Google Scholar]
- Zhao P, Han SN, Arumugam S et al (2019) Digoxin improves steatohepatitis with differential involvement of liver cell subsets in mice through inhibition of PKM2 transactivation. Am J Physiol Gastrointest Liver Physiol 317:G387-g397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Hui X, Mao Y et al (2019) Identification of novel genes associated with a poor prognosis in pancreatic ductal adenocarcinoma via a bioinformatics analysis. Biosci Rep 39 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.