Abstract
1. Isolated jejunal loops of rat small intestine were perfused by a single pass of bicarbonate Krebs-Ringer solution containing either D- or L-phenylalanine or one of eight dipeptides formed from D- or L-alanine plus D- or L-phenylalanine. 2. At 0.5 mM L-phenylalanyl-L-alanine increased serosal phenylalanine appearance to forty times the control rate giving a value similar to that found with 0.5 mM free L-phenylalanine. No serosal dipeptide could be detected. 3. Perfusions with the two mixed dipeptides with N-terminal D-amino acids (D-alanyl-L-phenylalanine and D-phenylalanyl-L-alanine) gave rise to the appearance of intact dipeptides in the serosal secretions although there were substantial differences in their rates of absorption and subsequent hydrolysis. 4. L-Alanyl-D-phenylalanine was absorbed from the lumen three to five times as fast as L-phenylalanyl-D-alanine. At 1 mM L-alanyl-D-phenylalanine transferred D-phenylalanine across the epithelial layer at more than seven times the rate found with the same concentration of the free D-amino acid. 5. Perfusions with D-alanyl-D-phenylalanine or D-phenylalanyl-D-alanine showed that these two dipeptides are poor substrates for both transport and hydrolysis by the rat small intestine. 6. Analysis of mucosal tissue extracts after perfusion with the two mixed dipeptides with N-terminal D-amino acids revealed that both dipeptides were accumulated within the mucosa and suggested that exit across the basolateral membrane was rate limiting for transepithelial dipeptide transport.
Full text
PDF

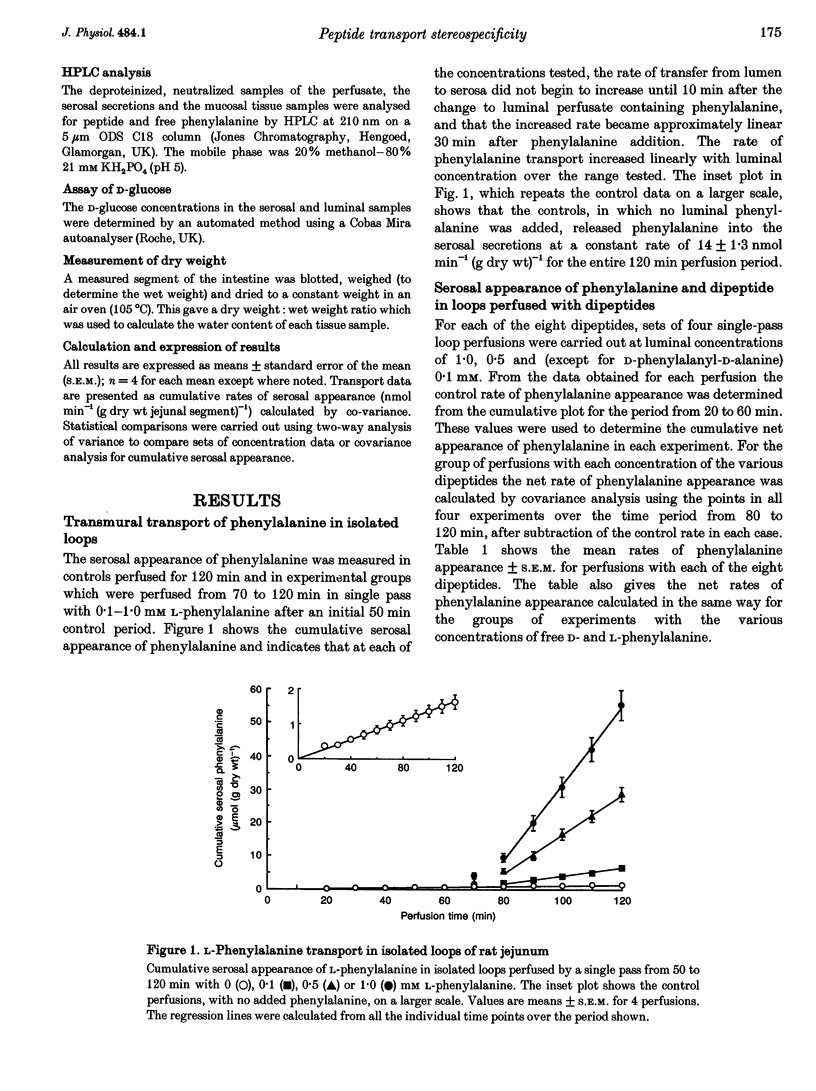
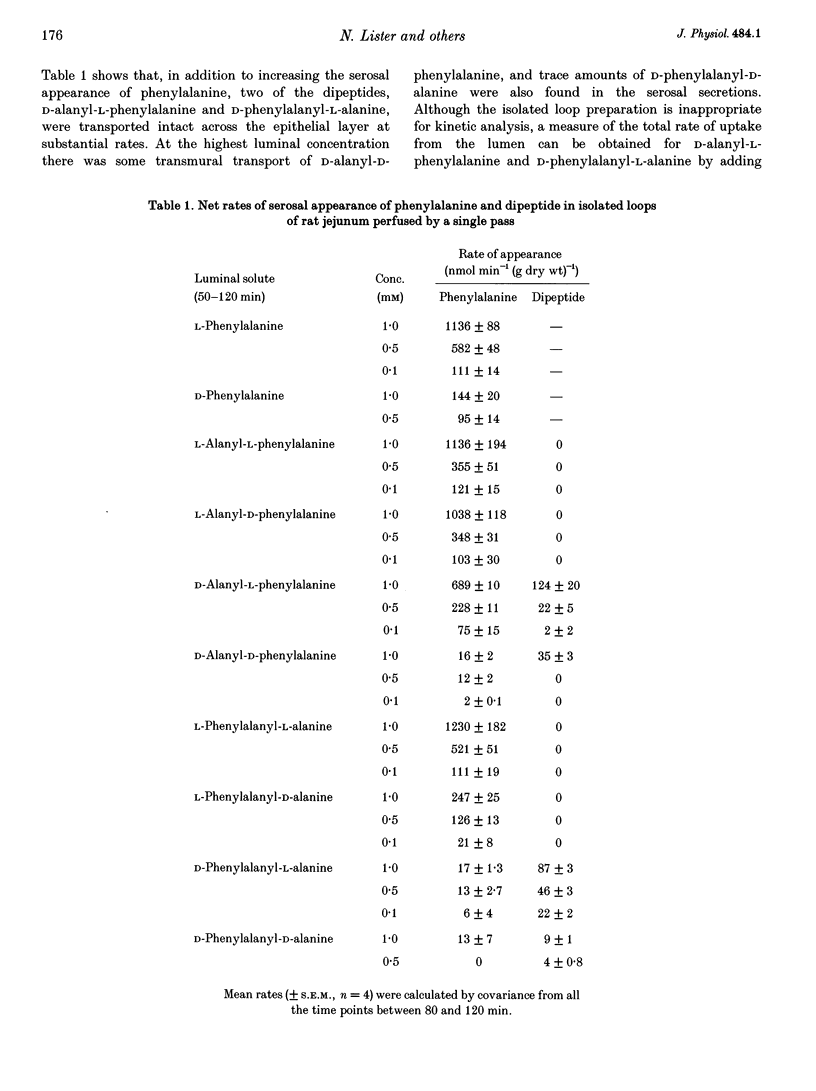

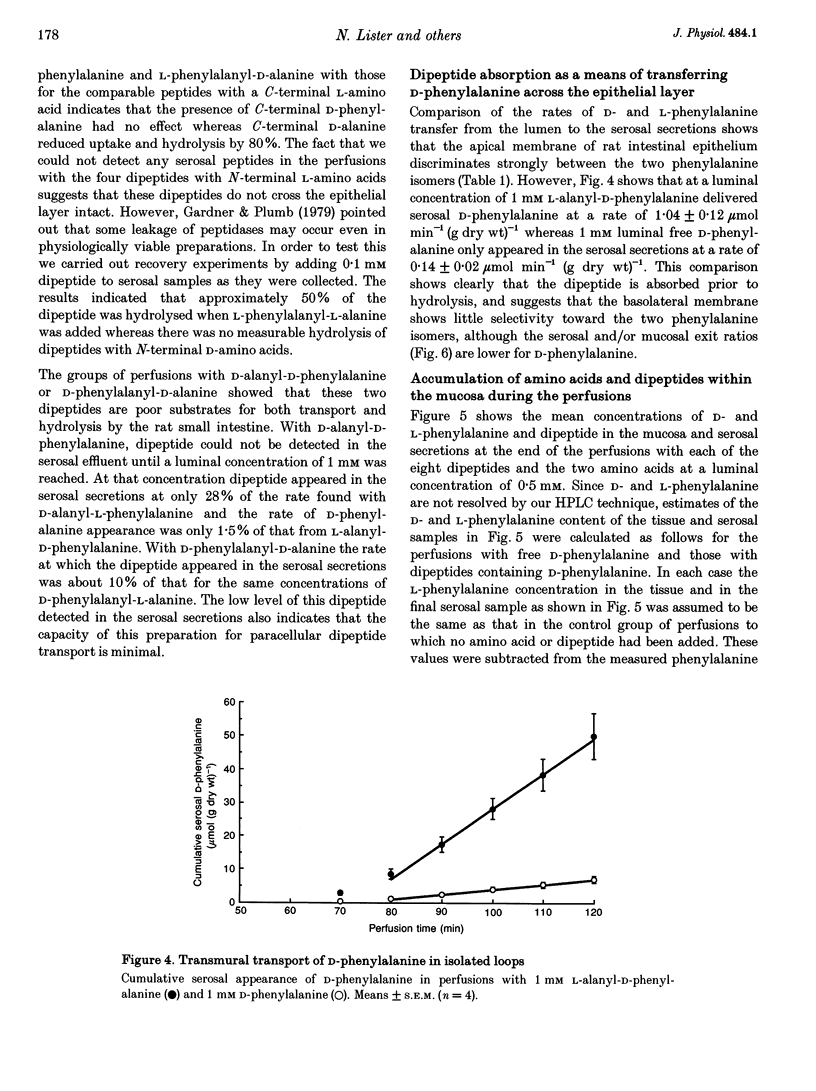
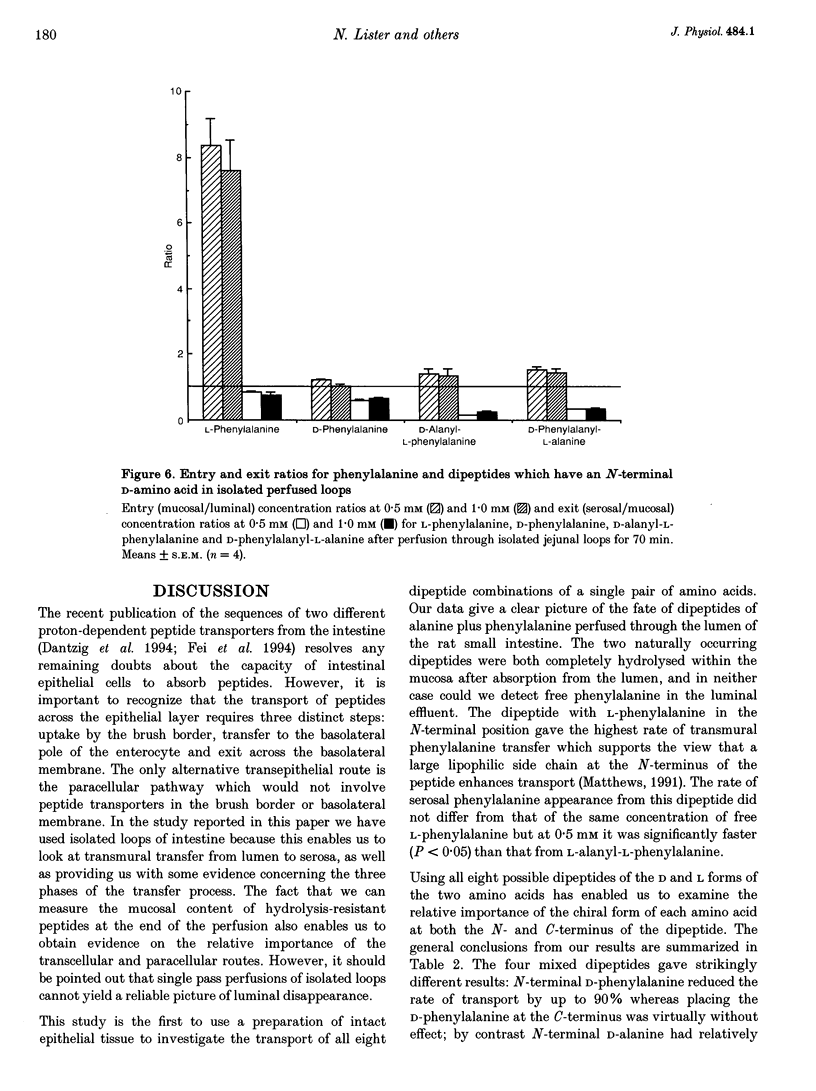


Images in this article
Selected References
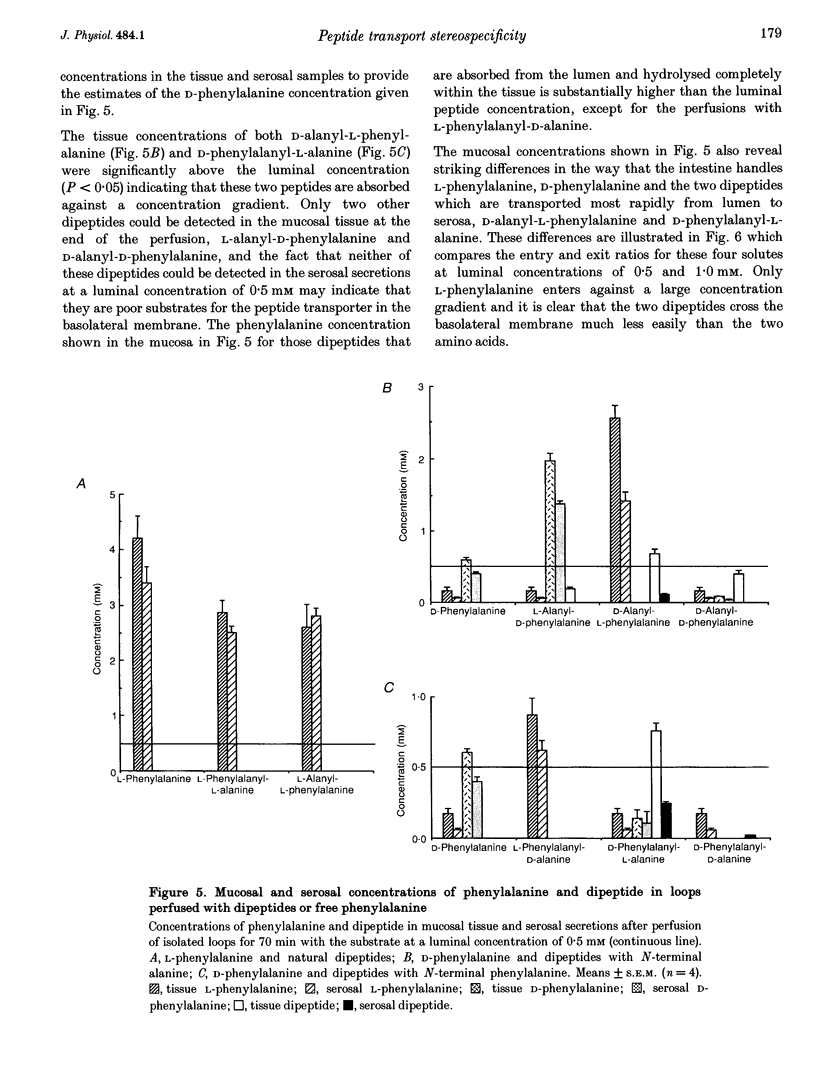
These references are in PubMed. This may not be the complete list of references from this article.
- Asatoor A. M., Chadha A., Milne M. D., Prosser D. I. Intestinal absorption of stereoisomers of dipeptides in the rat. Clin Sci Mol Med. 1973 Aug;45(2):199–212. doi: 10.1042/cs0450199. [DOI] [PubMed] [Google Scholar]
- Bai J. P., Amidon G. L. Structural specificity of mucosal-cell transport and metabolism of peptide drugs: implication for oral peptide drug delivery. Pharm Res. 1992 Aug;9(8):969–978. doi: 10.1023/a:1015885823793. [DOI] [PubMed] [Google Scholar]
- Boyd C. A., Ward M. R. A micro-electrode study of oligopeptide absorption by the small intestinal epithelium of Necturus maculosus. J Physiol. 1982 Mar;324:411–428. doi: 10.1113/jphysiol.1982.sp014121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronk J. R., Hastewell J. G. The transport and metabolism of naturally occurring pyrimidine nucleosides by isolated rat jejunum. J Physiol. 1988 Jan;395:349–361. doi: 10.1113/jphysiol.1988.sp016923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman C. I., Johnston G. Glycyl-L-leucine transport in the rat small intestine. Can J Physiol Pharmacol. 1982 Sep;60(9):1177–1184. doi: 10.1139/y82-171. [DOI] [PubMed] [Google Scholar]
- Dantzig A. H., Hoskins J. A., Tabas L. B., Bright S., Shepard R. L., Jenkins I. L., Duckworth D. C., Sportsman J. R., Mackensen D., Rosteck P. R., Jr Association of intestinal peptide transport with a protein related to the cadherin superfamily. Science. 1994 Apr 15;264(5157):430–433. doi: 10.1126/science.8153632. [DOI] [PubMed] [Google Scholar]
- Fei Y. J., Kanai Y., Nussberger S., Ganapathy V., Leibach F. H., Romero M. F., Singh S. K., Boron W. F., Hediger M. A. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature. 1994 Apr 7;368(6471):563–566. doi: 10.1038/368563a0. [DOI] [PubMed] [Google Scholar]
- Fisher R. B., Gardner M. L. A kinetic approach to the study of absorption of solutes by isolated perfused small intestine. J Physiol. 1974 Aug;241(1):211–234. doi: 10.1113/jphysiol.1974.sp010650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker G., Bruns C., Munzer J., Briner U., Albert R., Kissel T., Vonderscher J. Intestinal absorption of the octapeptide SMS 201-995 visualized by fluorescence derivatization. Gastroenterology. 1991 Jun;100(6):1544–1552. doi: 10.1016/0016-5085(91)90651-z. [DOI] [PubMed] [Google Scholar]
- Ganapathy, Leibach F. H. Is intestinal peptide transport energized by a proton gradient? Am J Physiol. 1985 Aug;249(2 Pt 1):G153–G160. doi: 10.1152/ajpgi.1985.249.2.G153. [DOI] [PubMed] [Google Scholar]
- Gardner M. L. Gastrointestinal absorption of intact proteins. Annu Rev Nutr. 1988;8:329–350. doi: 10.1146/annurev.nu.08.070188.001553. [DOI] [PubMed] [Google Scholar]
- Gardner M. L., Plumb J. A. Release of dipeptide hydrolase activities from rat small intestine perfused in vitro and in vivo. Clin Sci (Lond) 1979 Dec;57(6):529–534. doi: 10.1042/cs0570529. [DOI] [PubMed] [Google Scholar]
- Hanson P. J., Parsons D. S. The utilization of glucose and production of lactate by in vitro preparations of rat small intestine: effects of vascular perfusion. J Physiol. 1976 Mar;255(3):775–795. doi: 10.1113/jphysiol.1976.sp011307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer J. R., Dahl C. E., Karnovsky M. L., Maggio J. E. Intestinal absorption and excretion of octapeptides composed of D amino acids. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1942–1945. doi: 10.1073/pnas.91.5.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]



