Abstract
Air quality management commonly aims to mitigate nitrogen oxide (NOx) emissions from combustion, reducing ozone (O3) and particulate matter (PM) pollution. Despite such ongoing efforts, regulations have recently proven ineffective in rural areas like the Salton Sea Air Basin of Southern California, which routinely violates O3 and PM air quality standards. With over $2 billion in annual agricultural sales and low population density, air quality in the region is likely influenced by the year-round farming activity. We conducted a source apportionment of NOx (an important precursor to both O3 and PM) using nitrogen stable isotopes of ambient NO2, which revealed a significant contribution from soil-emitted NOx to the regional budget. The soil source strength was estimated based on the mean δ15N-NOx from each emission category in the California Air Resources Board’s NOx inventory. Our annual average soil emission estimate for the air basin was 11.4 ± 4 tons/d, representing ~ 30% of the extant NOx inventory, 10× larger than the state’s inventory for soil emissions. Unconstrained environmental factors such as nutrient availability, soil moisture, and temperature have a first-order impact on soil NOx production in this agriculturally intensive region, with fertilization and irrigation practices likely driving most of the emissions variability. Without spatially and temporally accurate data on fertilizer application rates and irrigation schedules, it is difficult to determine the direct impacts that these variations have on our observations. Nevertheless, comparative analysis with previous studies indicates that soil NOx emissions in the Imperial Valley are likely underrepresented in current inventories, highlighting the need for more detailed and localized observational data to constrain the sizeable and variable emissions from these arid, agricultural soils.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-78361-y.
Subject terms: Biogeochemistry, Environmental sciences, Chemistry
Introduction
Nitrogen oxides (NOx = NO + NO2) are a precursor to ozone (O3) and particulate matter (PM), both of which are federally regulated criteria pollutants known to negatively impact human health and the environment1. NOx is produced through natural, biogenic, and anthropogenic processes, although a critical distinction is necessary. Natural sources of NOx occur without influence from human activity, such as lightning or weather-induced wildfires. Biogenic NOx is naturally occurring and is produced by living organisms through microbial nitrification/denitrification cycles in soils. Further, anthropogenic NOx results from human activities such as fossil fuel combustion in vehicles and power plants. However, these natural processes can be affected by anthropogenic activity, such as biomass burning in agricultural systems, human-ignited wildfires in poorly managed landscapes, and microbial activity in soils of fertilized croplands.
Anthropogenic combustion sources have traditionally been the primary focus of air quality management practices, as they have historically been the dominant source of NOx emissions, with biogenic processes accounting for ~ 20% of the global NOx budget, on average2–8. These practices have largely reduced precursor emissions, yet several of the United States’ worst-air quality districts remain in rural regions9,10, including the Imperial and Coachella Valleys of Southern California. Together these valleys constitute the Salton Sea Air Basin (SSAB, Fig. 1), which is currently nonattainment for the O3, PM2.5, and PM10 National Ambient Air Quality Standards (NAAQS). Interregional transport from larger metropolitan areas is often blamed11–16, hewing to the traditional paradigm of combustion dominant sources. This issue is a serious matter of environmental injustice because residents of Imperial County are 86% Latino and 21% of residents live below the poverty line17. Additional burdens these communities face include high rates of asthma, insufficient housing availability and poor infrastructure, language barriers, and impaired water quality18,19. These factors put residents at risk of respiratory damage and diseases like asthma and COVID-1920. In fact, Imperial County held both the highest infection and mortality rates per capita in California from COVID-19 throughout the height of the pandemic21,22. Members of the rural Eastern Coachella Valley also suffer from similar socioeconomic and environmental injustices19.
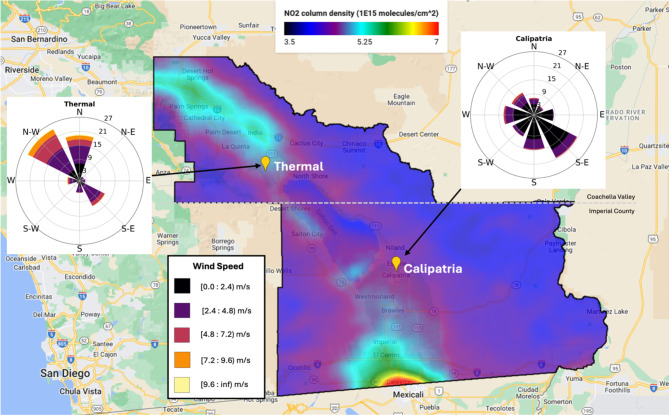
Fig. 1.
NO2 column density was averaged from June 1, 2022, to May 31, 2023, from TROPOMI satellite data for the Salton Sea Air Basin to show the average spatial variation of NO2. Calipatria (SE) and Thermal (NW), our two sampling sites, are indicated by a yellow marker. Wind roses for Calipatria and Thermal are overlaid on the map and were created using CARB’s Meteorological Data Query Tool https://www.arb.ca.gov/aqmis2/metselect.php. The map was created in Google Earth Engine using the Sentinel-5P NRTI NO2: Near Real-Time Nitrogen Dioxide dataset; the script to access this data was modified but the original script can be found at this link: https://developers.google.com/earth-engine/datasets/catalog/COPERNICUS_S5P_NRTI_L3_NO2.
The SSAB is an agriculturally active desert responsible for over $2 billion in annual agricultural sales due to mild temperatures and abundant sunshine in the winter, allowing for year-round production23. However, growing crops in sandy soil is challenging and can require anthropogenic land modifications such as carbon-based amendments, heavy application of inorganic fertilizers, and intensive irrigation. This is primarily due to the soil’s texture, with larger particle diameters and lower organic carbon composition, resulting in poor retainment of water and nutrients necessary for plant growth24. Tillage is often required for agricultural production in sandy soils, which can result in structural damage to the soil, leading to compacted soils that are difficult to cultivate when dry, as well as entrainment of particulate matter24–26. Hard-setting soils have high soil strength that is not suitable for root growth or shoot development. Consequently, this requires frequent soil-wetting through irrigation practices, especially in summer months when temperatures regularly exceed 35 °C; these dry, hot conditions and changes in volumetric soil moisture (VSM) may lead to large, transient soil NOx emissions (Fig. 1 & S1)10,24,27.
In addition, inorganic fertilizer amendments are not regulated, leading to over-application and nutrient leaching into the surrounding environment, such as the groundwater, local water sources, atmosphere, and soils24,28. The objective of this work is to understand the implications of agricultural practices in arid agroecosystems of the SSAB on regional air quality. Observations and modeling studies show a massive expansion of arid lands globally due to climate change, with estimates ranging from 2.5 to 6.5 million km2 by the end of the century, especially in large croplands in the northern hemisphere29. Consequently, agricultural production in arid ecosystems will become more prominent30, therefore the implications of these practices need to be better understood. Microbes in soils naturally produce nitric oxide (NO), nitrous oxide (N2O), and nitrous acid (HONO) gases during nitrification and denitrification processes, but their relative amounts are highly uncertain. These processes are dependent on many variables, but most importantly on the availability of reactive nitrogen and molecular oxygen, nitrifying and denitrifying bacteria, soil moisture and pH, and temperature31–35. Nitrification is a multi-step microbial process (shown below separated into 2 steps) that converts available ammonia (NH3) into nitrate (NO3−) and involves ammonia-oxidizing and nitrite-oxidizing bacteria36. NO can be a byproduct in Step 1 under high ammonia concentrations or low oxygen conditions. Irrigation and precipitation events temporarily suppress bacterial activity until water is redistributed or evaporated, restoring oxygen and nutrient availability and resulting in NO pulsing events.
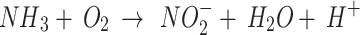 |
Step 1 |
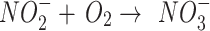 |
Step 2 |
Denitrification (Step 3) is a sequence of biochemical reactions that reduce nitrate to dinitrogen (N2) and other gaseous products (e.g., HONO, NO, N2O), typically occurring under anaerobic conditions when bacteria use nitrate as an oxidizing agent in the absence of oxygen37. In this process, NO is an intermediate and can be emitted when there is an imbalance in electron donors and acceptors (e.g., not enough organic carbon) or under acidic soil conditions where denitrifying bacterial activity is inhibited. However, in this step N2O emissions are believed to dominate over NO particularly in wetter soils.
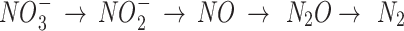 |
Step 3 |
In agricultural soils the availability of reactive N in the form of NH4+ and NO3-, which fuels these nitrification/denitrification reactions, is directly dependent on N fertilizer inputs, meaning overapplication of N fertilizers results in emissive nitrogen gases. Applying excessive ammonia-based fertilizer with insufficient oxygen availability (e.g., when fields are flooded) can result in NO production from nitrification because nitrite is not able to fully oxidize to nitrate. In addition, soil texture influences the relative proportions of N gases released from nitrification and denitrification; denitrification is favored in clay soils while nitrification is favored in freely draining sandy soils, like the ones in the SSAB38,39. Soil NO emissions are observed to increase exponentially with temperature due to enzymatic activity and microbial turnover rates, then plateau between 30 and 40 °C, as long as these other soil components are not limiting factors34,40,41. The exact temperature that these emissions plateau is still debated, however, we believe that the plateau point may be higher in hot agroecosystems like the SSAB, where microbes have likely adapted to the extreme heat42.
Further, high pH soils are generally believed to favor microbial ecosystems that promote nitrification and NO production, while acidic soils produce HONO through chemical equilibrium with nitrite in water34. However, Oswald et al. (2013) found comparable quantities of HONO and NO emitted from nonacidic soils, suggesting that HONO may be an unaccounted, yet significant amount of reactive nitrogen released from soil worldwide, particularly dominant in arid and arable environments like the SSAB. This is crucial as the lifetime of HONO with respect to photolysis yielding NO + OH is around 15 min during the daytime, causing soil emissions to rapidly cycle into the photostationary state of O3-NO-NO2, becoming an additional source of NOx to the atmosphere43.
As NOx emissions from combustion sources decline under ongoing air quality regulations, it becomes crucial to comprehend the impact of anthropogenically induced biogenic NOx production, particularly from agricultural soils. The nitrogen stable isotope composition (δ15N) is proposed as a valuable tool for NOx source apportionment due to differences in mean δ15N-NOx values between anthropogenic and biogenic emission sources44–46. Ambient δ15N-NO2 is recognized as an effective means of source apportionment, benefiting from well-quantified isotope fractionation factors and offsetting isotope effects resulting from the interplay between isotope equilibrium and photochemical fractionation47 Previous ambient δ15N-NO2 studies have successfully tracked the influence of highly variable biogenic soil NO emissions impacting the NOx budget in a small Midwestern city during the summer48, suggesting it could be a powerful tool for constraining biogenic soil emissions in the SSAB.
In this study, δ15N was quantified from NO2 (and total nitrate, tNO3) actively collected on a Thermo Scientific™ ChemComb® Speciation Cartridge (CCSC). Ambient NO2 samples were collected in two locations in the SSAB for 10 months and δ15N was analyzed to quantify the soil source contributions to the region’s NOx inventories. Using our field measured δ15N-NO2 values and accounting for nitrogen isotopic fractionation to adjust these values to δ15N-NOx, we used a mixing model approach to estimate the soil source strength of the field observations based on the mean δ15N-NOx from each emission source and from the a priori source apportionment reported in CARB’s California Emissions Projection Analysis Model (CEPAM) inventory. Because nitrate is influenced by long-range transport, we do not use tNO3 as a marker for source apportionment. This study aims to improve our understanding of the sources of persistent air pollution in the SSAB by assessing the current NOx inventory’s estimation of soil NOx emissions.
Methods
Sample collection, extraction, and isotopic analysis
Ambient NO2 and total particulate nitrate (tNO3) were collected and quantitatively speciated using a denuder-filter pack active sampling device known as a ChemComb Speciation Cartridge (CCSC), as previously described49. Briefly, the body of the CCSC contains an inlet with an impactor plate for the removal of coarse particles, a glass transition piece to induce laminar flow, two honeycomb denuders to collect ambient NO2 gas, and a downstream filter pack50. Denuders were soaked in 10% (v/v) hydrochloric acid (HCl) for 24 h, then rinsed in triplicate with 18.2 MΩ Millipore water and air-dried prior to field preparation. For the collection of NO2, denuders were coated with 10-mL of 2.5 M potassium hydroxide (KOH) and 25% by weight of guaiacol (C7H8O2) using methanol as a solvent, which was allowed to air dry, then capped until assembly for field analysis51–53. This mixture selectively binds NO2 as nitrite (NO2−). Following the denuders is a single Nylon filter (Measurement Technologies Laboratory NY47P, 47 mm and 1 μm pore size) for the collection of tNO3. Ambient air was sampled at a flow rate of 10 LPM using a mass-flow controller (MKS IE50A008304SBVP20) and vacuum pump. Details about chemicals and materials used can be found in Supplementary Table S1. Field sampling was performed from June 2022 – April 2023 at two sampling sites in the SSAB (Fig. 1), one in the Imperial Valley (Calipatria High School in Calipatria, CA) and one in the Coachella Valley (Torres-Martinez air station in Thermal, CA). This project incorporated community engagement and citizen science; Comité Cívico del Valle (CCV), a grassroots community organization based in Imperial County that was founded on the principle that “Informed People Build Healthy Communities”, served as our community partners. Trained air monitoring technicians from CCV oversaw field set-up and break-down. Sampling was performed for 3–7 days, approximately monthly over a period of ten months.
The extraction of NO2 and tNO3 was performed with 30-mL and 20-mL of MQ water for the front denuder and nylon filter, respectively, then vacuum filtered through a Whatman grade 1 filter. Back denuders were used to check for breakthrough and were not used for isotopic analysis. Field blanks were collected for method validation, however, blanks contained negligible concentrations of NO2 and tNO3. Nitrate (NO3−) and nitrite concentrations in aqueous solution were determined on a UV-Vis spectrophotometer using a colorimetric method developed by Doane and Horwath (2003) (extraction methods in Text S2)54. NO2 denuder extracts were neutralized with 0.1 M HCl, then isotopic analysis was performed at the UC Davis Stable Isotope Facility by the denitrifier method on a GasBench-PreCon-Isotopic Ratio Mass Spectrometer to determine δ15N-NO255,56. The pooled standard deviation for the15N reference was 0.11‰.
The lifetime of aerosols in the atmosphere is typically 5–10 days, meaning the N in particulate nitrate is derived from a large integrated area that spans several air basins across California. Although there was fair correlation between NO2 and particulate NO3 δ15N values across the experiment (Pearson correlation coefficient of ~ 0.45), the average for the NO3 samples was ~ 10‰ heavier than for NO2, indicating that the aerosol nitrate was likely more strongly influenced by distant, traditional fossil-fuel combustion sources of NOx. Additionally, quantifying isotope effects associated with tNO3 formation is more difficult57. Uncertainty in knowing exactly where the tNO3 was produced along its long-range trajectory makes it a much more challenging marker for source apportionment studies, therefore we do not use the tNO3 data (but it is reported in Table 1).
Table 1.
Field sampling results for δ15N-NO2 and estimated δ15N-NOx based on isotopic fractionation calculations, as well as the results for δ15N-NO3 for Calipatria and Thermal. All reported units are per mil, ‰.
| Calipatria | Thermal | |||||
|---|---|---|---|---|---|---|
| δ15N-NO2 | δ15N-NOx | δ15N-tNO3 | δ15N-NO2 | δ15N-NOx | δ15N-tNO3 | |
| Jun 2022 | − 13.2 | − 13.6 | − 0.02 | No measurements | ||
| Jul 2022 | − 13.4 | − 14.1 | − 2.6 | − 14.3 | − 16.1 | − 4.5 |
| Aug 2022 | − 14.4 | − 14.9 | − 9.8 | − 4.6 | − 8.0 | − 4.8 |
| Sep 2022 | − 15.6 | − 16.6 | − 3.4 | − 5.9 | − 8.0 | N/A |
| Oct 2022 | − 5.3 | − 6.1 | − 1.1 | − 9.7 | − 12.0 | − 8.2 |
| Nov 2022 | − 12.4 | − 13.4 | − 2.9 | 1.8 | 0.2 | 2.8 |
| Dec 2022 | − 13.3 | − 14.9 | − 2.9 | − 7.5 | − 9.1 | 3.4 |
| Jan 2023 | − 9.6 | − 11.6 | − 0.7 | − 1.5 | − 3.2 | 3.9 |
| Feb 2023 | − 14.8 | − 15.5 | 0.1 | − 11.3 | − 12.0 | 2.6 |
| Apr 2023 | − 16.0 | − 16.0 | − 3.4 | − 6.8 | − 7.6 | 1.8 |
| Average | − 12.8 | − 13.7 | − 2.7 | − 6.6 | − 8.4 | − 0.38 |
Evaluation of δ15N references
The isotopic signature of nitrogen is expressed in terms of its δ15N (per mil, ‰), where:
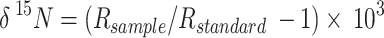 |
In this equation, R represents the 15N/14N ratio. The standard is atmospheric N2, which is considered to have a globally uniform isotopic composition58. Studies of the natural variations in 15N/14N ratios from different sources are used to apportion sources of NOx pollution.
A literature review was conducted to evaluate published stable15N isotope ratios for various sources of NOx, and relevant δ15N-NOx values are summarized in Table 2. On average, biogenic soil sources have been observed to have a significantly larger ratio of light14N isotopes than combustion sources. This phenomenon can be elucidated through the “leaky pipe” analogy, a concept derived from the hole-in-the-pipe model proposed by Davidson and Firestone32. In the “leaky pipe” scenario, soil NO-producing processes, such as nitrification, denitrification, and chemodenitrification, act as microbial conduits where microbes preferentially utilize the lighter14N isotope. This preference arises from kinetic factors that favor the incorporation of the lighter N isotope, leading to a more depleted δ15N signature51,58,59. The “leaky pipe” effect in soils contributes to the observed larger variations reflected in the standard deviations reported in the literature (Table 2 and Supplementary Fig. S4).
Table 2.
Summary of previously reported δ15N-NOx values sorted by emission source type.
| Source type | δ15N-NOx | Mean (‰) | Standard deviation (‰) | References |
|---|---|---|---|---|
| Mobile source | − 8.1‰ to + 9.8‰ | − 2.5 | 2.7 | Walters, Goodwin, et al.66a, Miller et al.65 |
| Biomass burning | − 7‰ to + 12‰ | 1.0 | 4.1 | Fibiger & Hastings45 |
| Stationary source | − 19.7‰ to − 13.9‰ | − 16.5 | 1.7 | Walters, Tharp, et al. (2015) |
| Biogenic soil emission | − 59.8‰ to − 14.2‰ | − 33.2 | 9.6 | Li & Wang51, Yu & Elliot59, Miller et al. (2018) |
aOnly a portion of the measurements from this paper were used since they were measured with a cold, neutral engine, which wouldn’t be relevant for ambient temperatures.
It is important to consider the uncertainties of the isotopic signatures for each source due to variations in measurement techniques found in the literature. For this study, passive sampling techniques60–62 were excluded because the measurement periods were much longer, could allow for a mixture of NOx sources, and are more difficult to reproduce, ultimately introducing a bias in the δ15N-NOx63. Additionally, studies on vehicles without catalytic converters were excluded64 since catalytic converters are mandatory and regulated through California smog checks. For these reasons, only two publications were used to represent mobile source emissions: Miller et al. (2017) and Walters, Goodwin et al. (2015)65,66. Not all measured values reported in Walters, Goodwin et al. (2015) were used because some measurements were taken from engines that were cold and/or in neutral. Catalytic converters take a few minutes to warm up and work efficiently, and efficient catalytic converters preferentially remove lighter14N isotopes65,66, leading to heavier15N emissions. Therefore, cold engines are biased with more negative isotopic signatures and likely do not represent background mobile source emissions measured in the Salton Sea Air Basin. Additionally, both studies were performed in the northeastern US in large metropolitan areas where biogenic sources are believed to be negligible. The reported mean from Miller et al. (2017) was then averaged with the mean of warm and/or driven vehicles from Walters, Goodwin et al. (2015) to represent mobile source δ15N-NOx (Table 3). Further, coal burning is not a method of energy production in the Salton Sea Air Basin or surrounding areas, therefore, studies that measured the isotopic signature of NOx produced through coal combustion have been excluded from our stationary source measurements64,67.
Table 3.
The 2022 California emissions Projection Analysis Model (CEPAM) NOx emission inventory and our estimated soil NOx adjustments for Imperial County and the Coachella Valley, as well as for the combined SSAB, are shown. The CEPAM inventory is based on an annual average, so we averaged our soil source estimates for our sampling duration. Also shown are the adjustments to the NOx inventory based on the average results from our field sampling campaign. The EPA’s National emissions Inventory (NEI) for 2020 is also shown for comparison for Imperial County only.
| Source type | NEI (2020) | CEPAM (2022) | This Study | ||||
|---|---|---|---|---|---|---|---|
| Imperial County (tons/d) | Imperial County (tons/d) | Coachella valley (tons/d) | SSAB total (tons/d) | Imperial County (tons/d) | Coachella Valley (tons/d) | SSAB total (tons/d) | |
| Mobile | 11.9 (72.7%) | 12.4 (81.8%) | 15.9 (88.2%) | 28.3 (85.2%) | 12.4 (56.7%) | 14.7 (69.9%) | 28.2 (62.1%) |
| Biomass burning | 0.5 (3.1%) | 0.1 (0.9%) | 0.7 (4.0%) | 0.8 (2.4%) | 0.1 (0.6%) | 0.6 (3.2%) | 0.9 (1.9%) |
| Stationary | 1.3 (8.0%) | 1.7 (11.4%) | 1.3 (7.4%) | 3.0 (9.0%) | 1.7 (7.9%) | 1.4 (5.9%) | 3.1 (6.7%) |
| Biogenic Soil | 2.7 (16.2%) | 0.9 (5.9%) | 0.1 (0.4%) | 1.0 (3.0%) | 6.7 (34.7%) | 4.7 (21.0%) | 11.4 (29.2%) |
| Total NOx | 16.3 | 15.2 | 18.0 | 33.2 | 21.8 | 22.7 | 45.5 |
Soil source strength mixing model estimation and propagation of uncertainty
A best estimate of the sources of NOx in the SSAB is compiled in the California Air Resources Board’s California Emissions Projection Analysis Model (CEPAM) shown in Table 3. CEPAM was used over the EPA’s National Emissions Inventory (NEI) because CEPAM is broken into county and air basin, and due to its California focus, should in principle be more accurate. Because chemical and physical processing can induce isotopic fractionation such that δ15N may not be conserved, isotopic fractionation was calculated to determine δ15N-NOx (see Text S3 and Eq. S1-3)57,68. These δ15N-NOx values were then used to calculate the soil source emission strength (Es) in Eq. 4 based on the average δ15N-NOx of each emission source and based on the a priori source apportionment reported in the CEPAM NOx inventory, which is used for regulatory modeling in California. Here, δobs represents the observed δ15N-NOx, i represents the main source types accounted for in the CEPAM NOx inventory (where a, b, c, and s represent mobile, biomass burning, stationary, and soil sources, respectively), αi represents the proportion each source contributes to the overall CEPAM NOx inventory, δi represents the literature derived δ15N-NOx for each source (Table 3), and Einv represents the total NOx budgeted in the CEPAM inventory (in tons/d).
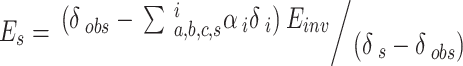 |
4 |
Equation 1 assumes that the current CEPAM inventory is correct aside from the soil source because the observed δ15N was (usually) lighter than expected; without adjusting the soil source signature, the CEPAM inventory would indicate a mean δ15N-NOx of -5.04‰ and − 3.11‰ for Imperial County and the Coachella Valley, respectively. Therefore, we solve for the magnitude of the soil source such that the observed δ15N signature can be explained by the sum of the inventory sources plus the revised soil emissions. This procedure further assumes that the sampled NO2 is entirely from NOx (or HONO) emitted from sources within the air basin. Figure 1 shows annual NO2 column data from the European Space Agency’s TROPOspheric Monitoring Instrument (TROPOMI) for the year of our sampling.
We propagate the various uncertainties associated with these measurements of sources from the literature to estimate the overall error associated with our calculated soil emissions. Equation 5 comes from the propagation of error for the final soil source strength (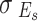 ). The analytical uncertainty δobs (on average 0.11‰) was ignored because it is an order of magnitude smaller than the standard deviations of the δ15N-NOx measurements for each source (Table 2).
). The analytical uncertainty δobs (on average 0.11‰) was ignored because it is an order of magnitude smaller than the standard deviations of the δ15N-NOx measurements for each source (Table 2).
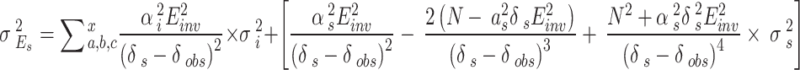 |
5 |
where 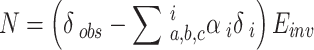
Results and discussion
Field sampling results
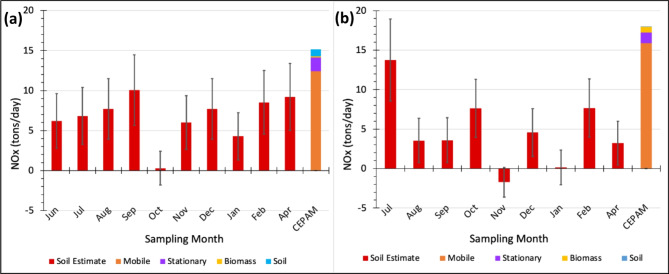
Results of collected δ15N-NO2 signatures are shown in Table 1. Figure 2 displays the soil emission estimates for this field campaign at both sites, comparing the monthly magnitudes to the overall annual CEPAM NOx inventory. It is apparent that the CEPAM NOx budget underestimates the soil contribution to ambient NOx (and consequently to PM2.5 and O3) in both Calipatria (Imperial Valley) and Thermal (Coachella Valley). Soil NOx contributed 0.3–10.1 (mean: 6.7 ± 3) tons/d and 0–13.8 (mean: 4.7 ± 4) tons/d throughout the duration of the field sampling for the Calipatria and Thermal sites, respectively. These soil emissions amount to on average 11.4 ± 4 tons/d throughout the entire SSAB, an order of magnitude larger than what is represented in the inventory (1.0 ton/d).
Fig. 2.
The NOx budget (in tons/day) based on CEPAM2019v1.03 for the year 2022 is shown, which is split into mobile, stationary, biomass burning, and soil emission sources for (a) Calipatria and (b) Thermal. Soil emission estimates from this study are shown in red, and estimated uncertainties are denoted by the error bars. The annual total CEPAM NOx inventory for Imperial County (a) and the Coachella Valley (b) is presented on the right, where mobile, stationary, biomass, and soil sources are shown in orange, purple, yellow, and blue. Note that in Thermal for the November sampling, there was a negative estimated soil contribution, but we assume that the soil source was zero for this month and justified this assumption due to the error bar range.
The quantitative basis of our estimates relies on the assumption that the ambient NO2 at the sampling site comes from sources entirely within the SSAB. Figure 1 (and Supplementary Fig. S1) show the overall pattern of column NO2 observed by TROPOMI during its overpass time near 13:30 local time. The satellite data illustrates two important aspects of the regional NOx. First, although there is a flow connection between the Coachella Valley and upwind urban sources in the LA basin (Supplementary Fig. S2), the valley plume is distinct and does not appear to simply represent a decaying tail (especially noticeable in the Fall and Winter maps of Supplementary Fig. S1). The Imperial Valley, on the other hand, is only downwind of Mexicali during July/August (Supplementary Fig. S3) and yet it exhibits a broad amorphous shape that deviates from the circular symmetry of a concentration field falling off with distance from an urban core. Second, the basin-wide concentrations are greatest during spring/summer when the photochemical lifetime of NOx is shortest, supporting our finding that agricultural soil sources are significant, especially during the warmest part of the growing season. Regardless of the details of source apportionment for the sampling, Eq. 4 shows that any influence from urban sources upwind (with their heavier δ15N fractions) would effectively increase our soil emission estimates, meaning that the values reported here represent lower limits. Further details of the regional flow and NOx advection are discussed in Text S1.
Environmental influences on soil NOx production
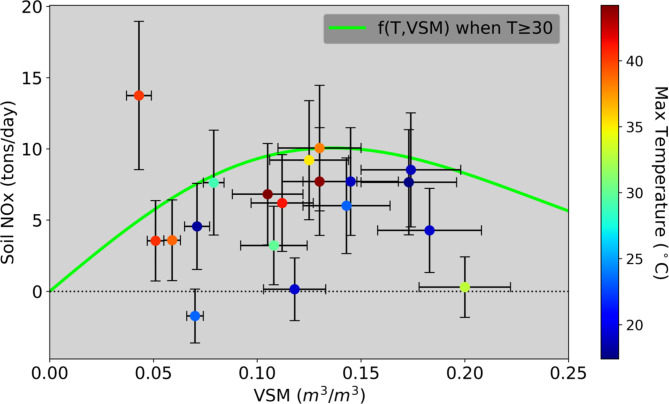
To better understand the environmental influences that control the production of soil NOx, we look at the leading parameters used in modeling estimates; namely nutrient availability, temperature, and soil moisture41,69. Temperature is known to strongly influence soil NOx production only when soil moisture and nutrient availability are not the limiting factors, although the exact dependence at higher temperatures is still debated40, and has been observed by satellite over croplands27. Nevertheless, no clear temperature dependence was observed in this study (Figs. 2 and 3) despite observations ranging from 17 to 44 °C (Supplementary Tables S3, S4, and Fig. S6). As an agriculturally active desert, NOx production is likely limited not principally by temperature as in unmanaged landscapes but by nutrient abundance and soil moisture determined by agricultural activity. In addition, the soil microbes in this high-temperature agroecosystem may have acclimated to the extreme desert heat, so their behavior may vary compared to agricultural ecosystems in more temperate regions42.
Fig. 3.
Soil NOx estimates from our field campaign compared to the average VSM and maximum temperature during the measurement period. Maximum temperature was determined for the sampling period from the CARB meteorology station at Calipatria and Thermal. VSM was determined for the Imperial Valley for Calipatria measurements and the Coachella Valley for Thermal measurements using SMAP satellite data, filtered for 4:30 pm (closest time to when Tmax occurs).
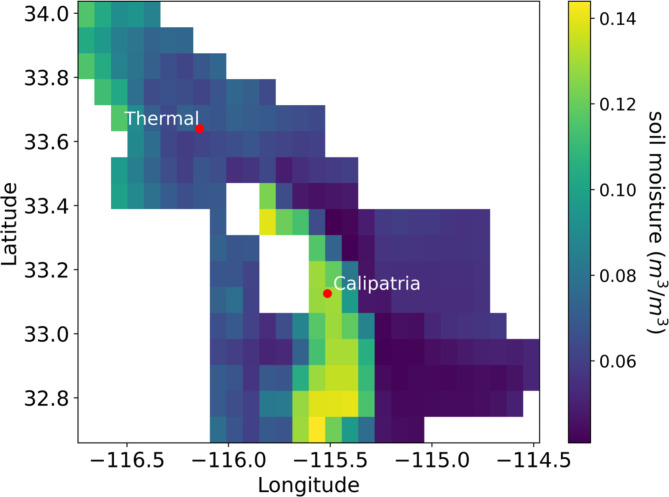
The role of soil moisture on the production of NOx is related to both gas diffusivity and microbial activity, with increasing soil water content stimulating anaerobic conditions and denitrification more. Nitrification, on the other hand, peaks 2–3 days after wetting when the amount of excess water and the rate of downward movement have decreased42. In addition, extensive dry periods limit substrate diffusion and cause water-stressed nitrifying bacteria to remain dormant, which allows N substrate to accumulate while temporarily suppressing NO emissions69–71. Irrigation reactivates these microbes and redistributes substrate, resulting in NO pulsing events that typically last 1–2 days and can be 10–100 times background emission rates41,69. Moreover, soil NO emissions have been shown to spike after all rewetting events, even if fertilizer had not been applied within the last 30 days42. To further understand the influence of these parameters, we looked at volumetric soil moisture content using NASA’s Soil Moisture Active Passive (SMAP) satellite data. Soil moisture tends to be higher on average in the Imperial Valley agricultural lands than in any other region in the air basin (Fig. 4). This is because of the region’s appropriation of approximately 3 billion m3 of water from the Colorado River, used primarily for frequent irrigation of its ~ 200,000–275,000 ha of arable soil. This is equivalent to ~ 150 cm of water over the croplands whereas the climatological rainfall observed usually only amounts to < 5–10 cm (Supplementary Tables S5 and S6). Due to the amount of agricultural land in the SSAB and uncertainty of irrigation per parcel of land and crop type, it is difficult to accurately estimate the contribution of soil re-wetting on our measurements, however this factor likely contributes to our soil NOx estimates, but it is unlikely that our ~ 10 annual samples captured irrigation variability completely. Furthermore, because the regional distribution of soil NOx fluxes are highly positively skewed due to these pulsing activities, it is most likely that our ~ 40 days of sampling (Table S2) represents an underestimate to the overall annual sum of emissions, and the mean NOx concentrations in Calipatria during sampling days was observed to be significantly lower than the annual average (Table S7) further supporting that our estimate of annual emissions in the Imperial Valley is likely a lower limit.
Fig. 4.
Average volumetric soil moisture (VSM) for the SSAB from June 2022 – May 2023, obtained from Soil Moisture Active-Passive (SMAP) satellite data.
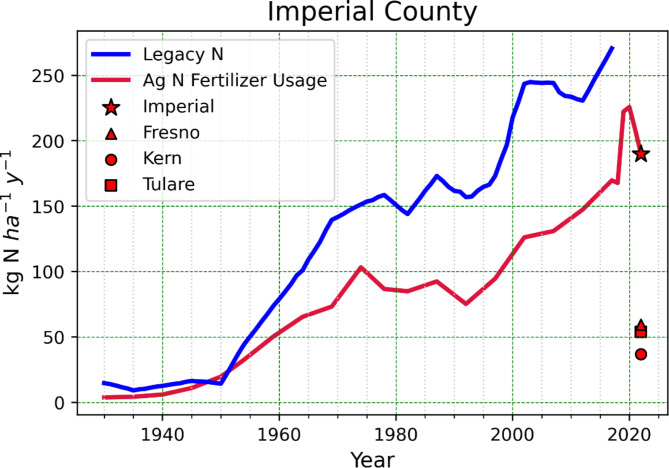
Although Fig. 3 could be interpreted as exhibiting a crude correspondence between soil NOx emissions and VSM, temperature only appears to be weakly related, which likely indicates that nutrient availability is the dominant factor controlling soil NOx production in this heavily agricultural region. According to the California Department of Food and Agriculture (CDFA), N fertilizer sales in Imperial County have increased by 137% since 199123,72, and an estimated 18-fold since 193073, much greater than the national average sevenfold increase. In addition, despite being ranked as the 9th highest in county agricultural sales in the state, Imperial County used more N fertilizer than any other county during 2022 (8% of California total), including the top three crop producing counties (Fig. 5), likely a result of the insufficient nutrient retainment in sandy soils. When N fertilizer is applied in excess, this increases the likelihood that N gases are emitted from the soils. A previous study showed that doubling the fertilization amount from 50 kg N ha− 1 to 100 kg N ha− 1 increased the integrated NOx fluxes by a factor of 542.
Fig. 5.
Imperial County N fertilizer usage (red) and legacy N history (blue) was prepared using the Trajectories Nutrient Dataset for Nitrogen (TREND-nitrogen) from Byrnes et al., 2020. The agricultural fertilizer use was extended after 2017 using the CDFA agricultural statistics for N fertilizer sales. Fresno, Kern, and Tulare Counties are ranked 1st, 2nd, and 3rd in state agricultural sales, while Imperial County is ranked 9th. Fertilizer usages are reported as kg of N used per hectare of agricultural land.
Spatially and temporally relevant irrigation and fertilization data are not currently publicly available, therefore it is difficult to assess the direct influence of these leading parameters on the soil NOx emission variance observed in this study. In Fig. 5, we used data from the Trajectories Nutrient Dataset for Nitrogen (TREND-nitrogen) to look at historical N fertilizer applications and legacy N concentrations in Imperial County. N surplus was calculated using TREND-nitrogen, where the county-scale surplus or “legacy” nitrogen (kg N ha− 1 y− 1) is calculated by mass balance from the inputs of atmospheric deposition, fertilizer application, biological N fixation, and human N waste, minus the crop and pastureland N uptake73. It is apparent that N fertilizer usage and surplus N in Imperial County has been steadily increasing from 1920 to the present day (Fig. 5). Our estimates are based on a small fraction of days in the Salton Sea Air Basin; in our study, we sampled ~ 39 days in Calipatria and ~ 43 days in Thermal, accounting for about 11% of the year. Inspecting the conditions of our 19 sampling intervals with respect to the annual values for VSM and air temperature (Supplementary Fig. S5 and Table S7), they compare well. Nevertheless, without knowing the exact timing of N amendments to all the cropped area, it is possible that while our estimates of annual average VSM and temperature are not statistically different from the average, the soil emissions, predominately influenced by the fertilizer inputs, may be much higher than our estimates having missed many periods of high fertilization in our sporadic sampling.
To understand the overall influence of fertilizer inputs on atmospheric N, we calculated the NOx flux as a proportion of fertilizer consumption using the net fertilizer usage recorded23. Imperial County purchased 57,630 tons of all nitrogen fertilizer, minerals, and compost in 202223. Our annual average soil emission of 6.7 tons NOx/d in Imperial County implies an average flux of 1.3% of the applied N being released as NO or HONO from agricultural fertilizer inputs. This is squarely within the 0.3–2.5% range reported by Jaeglé et al. (2005). Note that we were unable to quantify this for the Coachella Valley because it is part of the much larger Riverside County and fertilizer sales are only reported county-wide.
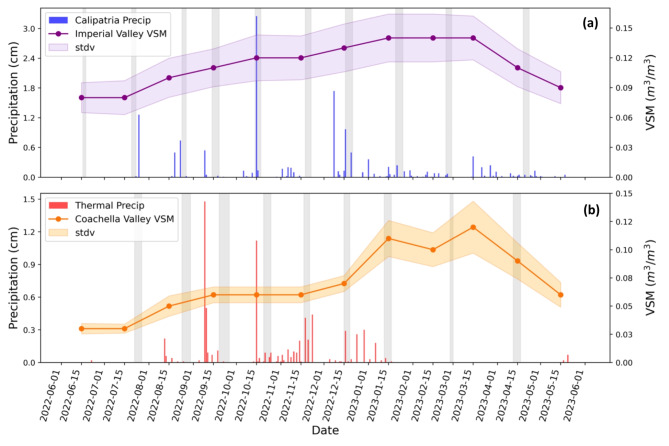
Furthermore, δ15N outliers were observed for October in Calipatria and for November and January in Thermal, yielding estimated soil emissions that were effectively zero within the errors of our measurement technique (Fig. 2). The outliers measured in October (Calipatria) and November (Thermal) correspond to precipitation events during our measurement periods that likely suppressed NOx emissions during the short intervals of sampling by wetting the soil to excess temporarily (Fig. 6)41. During our sampling interval in January (Thermal), precipitation was negligible, but examining HYSPLIT back trajectories for the interval, it appeared that there was a high-pressure system over the region that may have forced subsidence of cleaner tropospheric air into the Coachella Valley, which is consistent with the sampled NOx being lower than the monthly average by 5.5 ppb (Supplementary Tables S4 and S6). However, this circulation did not persist throughout the entire 5-day sampling period, and the February sample was also collected when NOx was 5 ppb lower than average, so ultimately, we are uncertain as to the exact cause of this near zero soil emissions measured at Thermal in January.
Fig. 6.
Monthly average Volumetric Soil Moisture, VSM (2022–2023, shown as a line plot), and the standard deviation (shown with shading) in (a) Imperial Valley and (b) Coachella Valley compared to the total daily precipitation (2022–2023, shown as bars) at our field sampling sites. Precipitation data was obtained from AQMIS and CIMIS and VSM data was obtained from NASA’s SMAP satellite. The grey boxes indicate our sampling dates at each site.
Comparison between studies
To further assess the validity of our results for Imperial Valley, we compared our measurements to Oikawa et al. (2015), which found some of the highest soil NOx emissions on record from chamber studies across the growing season. They further tested their findings by using WRF-Chem (with soil NOx emissions calculated using the Model of Emissions of Gases and Aerosols from Nature, MEGAN v2.0) to compare against ambient NOx measurements from the air quality network. The default soil emissions in the model needed to be enhanced by an order of magnitude to match their chamber measurements more closely, and the enhancement was found to minimize the model’s root-mean-square error relative to ambient measurements for one week in September 2012. However, this enhancement still underpredicted tropospheric column densities of NO2, indicating that there is no single emission factor that can be used to accurately simulate both tropospheric NO2 columns and surface NO2 concentrations in the MEGAN parameterization they used.
To compare our integrated valley emissions to other modeled surface fluxes, we used the area of agricultural land in the Imperial Valley to be 270,500 ha based on MODIS satellite land surface characterization27. The integrated emissions for their one week in September (the month of our highest estimate of 10.1 ± 4 tons/d) modeled by Oikawa et al. (2015) amounts to 17.2 tons/d (Table 4). That study also attempted to match agreement with satellite NO2 measurements and found that the emission rate that corresponded best was more like 111.2 tons/day. Their study also found that such soil NOx emission increases led to concomitant rises in surface level ozone of 2–9 ppb, highlighting the importance of this underestimated source to air quality and the observed disappearance of ozone abatement in the region9.
Table 4.
Comparing our annual average soil NOx flux estimate for Imperial County to CARB and other studies.
| NOx flux | This study | CARB CEPAMa | Jaeglé et al. (2005)4b | Hudman et al. (2012)69b | Vinken et al. (2014)75,b | Oikawa et al. (2015)42,c]c | Almaraz et al. (2018)10 | Guo et al. (2020)78 | Wang et al. (2021)40,d] | Luo et al. (2022)77 |
|---|---|---|---|---|---|---|---|---|---|---|
| tons NOx day− 1 | 6.7 | 0.9 | 4.3 | 8.5 | 8.5 | 17.2–111.2 | 140.0 | 1.6 | 5.2–10.4 | 13.5 |
| kg N ha− 1 yr− 1 | 2.5 | 0.3 | 6.2 | 3.1 | 3.1 | 6.3–40.6 | 51.2 | 0.6 | 1.9–3.8 | 4.9 |
aCEPAM2019v1.03 CARB’s standard emissions tool for the year 2022 (5.9% of total county NOx emissions).
bCorrected from county-wide map estimate to agricultural area assuming majority of emissions arise from cropped soils (county to cropland area ratio of 3.9).
cModel estimates for September of 2012 to best match satellite and surface network NO2 observations.
dModel run for June/July/August 2005–2019; range represents default BDSNP parameterization to proposed higher temperature parameterization.
We further complied annual average modeled soil NOx for the Imperial Valley from several other published studies40,69,70,74,75 that use some version of the common Berkeley Dalhousie Soil NOx Parameterization (BDSNP) and report them in Table 4. When reading these numbers from large maps of overall emissions, we corrected the fluxes by the factor of 3.9 representing the ratio of the total county area (~ 1 million ha) to the arable land assuming that the majority of emissions originate from agricultural soils in the region. The study by Wang et al. (2021) proposed a higher emissions parameterization with a temperature plateau that is not reached until 40 °C and compared this to the default parameterization, finding that the new one approximately doubled the emissions for the summer months. Our estimate, 2.5 kg N h− 1 y− 1, falls in between their two results of 1.9–3.8 kg N ha− 1 y− 1. Because the climate in the Imperial Valley is so different from other agricultural regions, comparisons must consider that the bulk of the planting occurs between September to April76. However, given that our observed estimates for June, July, and August closely match our annual averages, it seems reasonable to compare summer emissions to annual averages. This could occur because nitrogen applications might be at their lowest while soil temperatures are at their highest during these months, with these factors largely balancing each other out.
Almaraz et al. (2018) observed the highest modeled soil NOx emissions published to date for Imperial Valley10. Their method modeled the spatial distribution of soil NOx emissions using an N isotope model in natural areas and an Integrated Model for the Assessment of the Global Environment (IMAGE) in cropland areas to estimate N losses from soils based on surplus N. Their average NOx flux estimated for agricultural lands in Imperial County was ~ 140 tons/day (51.2 kg N ha− 1 yr− 1). Our results were also compared to a modeling study performed using an enhanced version of Fertilizer Emission Scenario Tool for the CMAQ (FEST-C) agroecosystem model paired with the Air Pollution Emission Experiments and Policy Analysis (APEEP), further abbreviated as FEST-C*.77 Data from their 2017 model for Imperial County indicates an annual average soil emission rate of 13.5 tons/day (4.9 kg N ha− 1 yr− 1) about twice as large as our estimate. Finally, Guo et al. (2020) modeled soil NOx emissions using the DeNitrification-DeComposition (DNDC) model paired with CMAQ, finding the total contribution to the state NOx inventory was 1.1%.78 However, they did point out a region of anomalously high emissions in the Imperial Valley, with an annual flux of 0.58 kg N ha− 1 yr− 1. Finally, recent research also suggests the possibility that NO and HONO emissions from drylands79 and biocrusts80 could influence air quality to some extent, albeit not as much as the intensive agricultural soils. These studies suggest that such microbial sources could emit as much as 0.1–0.25 kg N ha− 1 y− 1, but from potentially much larger areas across the desert landscape, and thus should be considered in future projections of ambient NOx (and O3 and PM2.5).
While there is considerable variability between the various estimates presented (Table 4), undoubtedly in part due to the highly episodic and transient nature of soil NOx emissions, the CEPAM inventory estimate is consistently much lower than most of the other models and observations. We expect that the dominant control of the instantaneous emission rates is the N amendments made to the agricultural soils daily, so the wide range presented in Table 4 is likely consistent with such inherent variability. Therefore, to obtain an accurate annual average emission rate from soils will take much more comprehensive understanding of N availability in the soils across these highly variable croplands. More work is needed to better constrain the impact of N availability on soil NOx emissions, as well as the precise soil conditions that control microbial activity and gas-exchange. As of 2022, the Sustainable Groundwater Management Act (SGMA) was modified to require farmers in California to report their daily N inputs for consequences of excess N leaching into groundwater. This emerging data set will be crucial for future studies to investigate soil NOx emissions in arid agricultural environments and accurately assess its impacts on air quality in rural communities.
Suggestions for future research
While our study does indicate the need for the reassessment of agricultural soil influences on NOx inventories, especially in high-temperature agroecosystems, there are some aspects of future research that we believe should be prioritized. To better understand the nonlinear relationship of nutrient availability and soil moisture for our higher-than-average NOx fluxes, having access to publicly available databases that contain spatially and temporally relevant fertilization and irrigation information is crucial. As of 2022, the California Water Resources Control Board now requires farmers to report their fertilizer usage per crop type and acreage as a part of SGMA. However, this information is not currently available to the public. In addition, while fertilizer usage and irrigation data are reported on an annual basis, it is hard to describe the seasonal variability of these factors. We suggest that the state mandate reporting a shorter time interval (weekly to monthly) and make it publicly accessible to support the ongoing study of air pollution from agricultural practices. Understanding the biogeochemical processes that contribute to the production of NOx (and other nitrogen gases like NH3, N2O and HONO) will allow for a better assessment for long-term air quality and climate change, as well as provide insights on agricultural best practices that could be promulgated to improve regional air quality.
It is evident by previous modeling studies that the lack of observational data is significantly hindering the effectiveness of models to produce accurate soil NOx emissions. Moving forward, there should be more seasonal field analysis of NOx, HONO, and N2O emissions from different land/crop types, especially in agricultural systems, since the environmental factors that contribute to N emissions and diffusivity change seasonally and with irrigation/fertilization cycles. These biogeochemical processes are highly complex and influenced by various factors, including fertilizer type, which models often oversimplify or inaccurately detail. More research needs to be supported in understanding all components of this mechanism, especially fertilizer amount, type, application frequency, irrigation timing, organic carbon availability, soil composition, and pH, in descending order of importance. These studies will improve modeling efforts, hopefully increasing the urgency in addressing these air quality issues, which can be improved with proper management and regulatory efforts.
Moreover, utilizing more complex forms of nitrogen like urea-based fertilizer have been shown to reduce the rate of N diffusivity as NO42, however, the influence on other air pollutants such as N2O and other environmental implications need to be better understood. While urea-based fertilizer is typically cheaper, less hazardous, and has a higher nitrogen content (requiring less input), if applied inefficiently it can result in higher N2O emissions, volatilization of ammonia, and increased acidity in the soil. Oikawa et al. (2015) showed that injecting urea-based fertilizer into the soil rather than applying fertilizer to the top resulted in lower NO emissions compared to dissolved ammonium nitrate, however another study by Thornton et al. (1996) showed high rates of N2O loss42,81. A comprehensive investigation on fertilizer type, application methods, and their environmental implications is crucial to avoid trading NOx emissions for other, potentially more hazardous environmental issues. Furthermore, the impact of soil NOx on the production of fine particulate matter, specifically nitrate, is uncertain and is dependent also on ammonia fluxes. To better understand the contribution of agricultural nitrate sources, there must be more research on the impacts of agriculture and controlled animal feeding operations (CAFOs) on the production of both NOx and NH3, specifically focused on the impacts of fertilizer usage and waste management. This information is crucial, as most agricultural regions in the state are nonattainment for the PM2.5 NAAQS due to the recent lowering of the standard from 12 to 9 µg/m3.82
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was made possible by support from the University of California, Davis (UC Davis) Environmental Health Sciences Center (EHSC) under award number P30ES023513 of the National Institute of Environmental Health Sciences, National Institute of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the UC Davis EHSC, nor the National Institutes of Health. Co-funding was also provided by the Western Center of Agricultural Health and Safety via NIOSH grant #U54OH007550. I. C. Faloona’s effort was supported by the USDA National Institute of Food and Agriculture, [Hatch project CA-D-LAW-2481-H, “Understanding Background Atmospheric Composition, Regional Emissions, and Transport Patterns Across California”]. We would like to acknowledge our community collaborators. First, this work would not have been possible without the support team at Comité Cívico del Valle (CCV). Additionally, our site in Thermal was located on Torres-Martinez tribal land so we would like to recognize them for their generous use of their air monitoring facility. A special thanks to Joel Craig, William “Jedi” Jeidi, and Diana Navarro for coordinating logistics and helping with set-up. The comments of two anonymous reviewers were helpful in clarifying, extending, and improving this work and we are consequently grateful for their efforts. Early conversations with Cort Anastasio also helped this study immensely.
Author contributions
Conceptualization of the research was done by I.F. and H.L. Methodology was developed by W.W., H.L., and I.F. Data was collected by H.L., I.F., M.M., E.R., C.T., and L.O. H.L. wrote the manuscript with significant input from I.F. and W.W. Funding was acquired by I.F. and H.L.
Data availability
The isotope data presented in this study are available from the corresponding author upon request. Other data such as meteorology and satellite data is available publicly at the following sites. NO2 column data for Fig. 1 and S1 was obtained from Google Earth Engine using Sentinel-5P NRTI NO2: Near Real-Time Nitrogen Dioxide. The script can be found at: https://developers.google.com/earth-engine/datasets/catalog/COPERNICUS_S5P_NRTI_L3_NO2, and the data was analyzed for June 2022- May 2023 at the Salton Sea Air Basin. The air basin was selected using a https://ww2.arb.ca.gov/geographical-information-system-gis-library. Air quality and meteorological data was obtained from AQMIS (https://www.arb.ca.gov/aqmis2/aqdselect.php and https://www.arb.ca.gov/aqmis2/metselect.php) and CIMIS (https://cimis.water.ca.gov/Default.aspx). The 2022 annual average CEPAM NOx inventory can be found https://ww2.arb.ca.gov/applications/cepam2019v103-standard-emission-tool. Soil Moisture Active Passive (SMAP) data was obtained from NASA’s Earth Data Inventory; specifically, we used https://nsidc.org/data/spl4smgp/versions/7. The TREND-nitrogen data set is available 10.1594/PANGAEA.917583.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Caplin, A., Ghandehari, M., Lim, C., Glimcher, P. & Thurston, G. Advancing environmental exposure assessment science to benefit society. Nat. Commun.10, 1236 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowler, D. et al. The global nitrogen cycle in the twenty-first century. Philos. Trans. R Soc. B Biol. Sci.368, 20130164 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu, L. et al. Global budget of tropospheric ozone: evaluating recent model advances with satellite (OMI), aircraft (IAGOS), and ozonesonde observations. Atmos. Environ.167, 323–334 (2017). [Google Scholar]
- 4.Jaeglé, L., Steinberger, L., Martin, R. V. & Chance, K. Global partitioning of NOx sources using satellite observations: relative roles of fossil fuel combustion, biomass burning and soil emissions. Faraday Discuss.130, 407 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Miyazaki, K. & Eskes, H. Constraints on surface NO x emissions by assimilating satellite observations of multiple species: MULTIPLE CONSTRAINTS ON NO X EMISSIONS. Geophys. Res. Lett.40, 4745–4750 (2013). [Google Scholar]
- 6.Song, W., Liu, X. Y., Houlton, B. Z. & Liu, C. Q. Isotopic constraints confirm the significant role of microbial nitrogen oxides emissions from the land and ocean environment. Natl. Sci. Rev.9, nwac106 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevenson, D. S. et al. Multimodel ensemble simulations of present-day and near-future tropospheric ozone. J. Geophys. Res.111, D08301 (2006). [Google Scholar]
- 8.van Vuuren, D. P., Bouwman, L. F., Smith, S. J. & Dentener, F. Global projections for anthropogenic reactive nitrogen emissions to the atmosphere: an assessment of scenarios in the scientific literature. Curr. Opin. Environ. Sustain.3, 359–369 (2011). [Google Scholar]
- 9.Parrish, D. D., Young, L. M., Newman, M. H., Aikin, K. C. & Ryerson, T. B. Ozone Design Values in Southern California’s Air basins: temporal evolution and U.S. background contribution: southern California ozone design values. J. Geophys. Res. Atmos.122, (2017). 11,166 – 11,182.
- 10.Almaraz, M. et al. Agriculture is a major source of NOx pollution in California. Sci. Adv.4, eaao3477 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.California Air Resources Board (CARB). Appendix C Coachella Valley Ozone Weight of Evidence Analysis. (2022).
- 12.Environmental Protection Agency (EPA) & Clean Air, P. 8-Hour Ozone Nonattainment Area Requirements; Determination of Attainment by the Attainment Date; Imperial County, California. Federal Register (2008). https://www.federalregister.gov/documents/2020/02/27/2020-03152/clean-air-plans-2008-8-hour-ozone-nonattainment-area-requirements-determination-of-attainment-by-the (2020).
- 13.Environmental Protection Agency (EPA). PM10 Maintenance Plan and Redesignation Request; Imperial Valley Planning Area; California. Federal Register (2020). https://www.federalregister.gov/documents/2020/09/18/2020-18427/pm10-maintenance-plan-and-redesignation-request-imperial-valley-planning-area-california
- 14.Environmental Protection Agency (EPA). Determination of Attainment by the Attainment Date But for International Emissions for the 2015 Ozone National Ambient Air Quality Standard; Imperial County, California. Federal Register (2022). https://www.federalregister.gov/documents/2022/10/20/2022-22276/determination-of-attainment-by-the-attainment-date-but-for-international-emissions-for-the-2015
- 15.Environmental Protection Agency (EPA). Determination of Attainment by the Attainment Date, Clean Data Determination, and Approval of Base Year Emissions Inventory for the Imperial County, California Nonattainment Area for the 2012 Annual Fine Particulate Matter NAAQS. Federal Register (2023). https://www.federalregister.gov/documents/2023/01/11/2022-28278/determination-of-attainment-by-the-attainment-date-clean-data-determination-and-approval-of-base
- 16.Mendoza, A., Pardo, E. I. & Gutierrez, A. A. Chemical characterization and preliminary source contribution of fine particulate matter in the Mexicali/Imperial Valley Border Area. J. Air Waste Manag Assoc.60, 258–270 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Census Bureau, U. S. U.S. Census Bureau QuickFacts: Imperial County, California. (2023). https://www.census.gov/quickfacts/fact/table/imperialcountycalifornia/PST045223
- 18.Miao, Y., Porter, W. C., Schwabe, K. & LeComte-Hinely, J. Evaluating health outcome metrics and their connections to air pollution and vulnerability in Southern California’s Coachella Valley. Sci. Total Environ.821, 153255 (2022). [DOI] [PubMed] [Google Scholar]
- 19.OEHHA. CalEnviroScreen 4.0. (2023). https://oehha.ca.gov/calenviroscreen/report/calenviroscreen-40
- 20.Comunian, S., Dongo, D., Milani, C. & Palestini, P. Air Pollution and COVID-19: the role of Particulate Matter in the spread and increase of COVID-19’s morbidity and mortality. Int. J. Environ. Res. Public. Health. 17, 4487 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Los Angeles Times. California coronavirus cases: tracking the outbreak. Los Angeles Times (2023). https://www.latimes.com/projects/california-coronavirus-cases-tracking-outbreak/
- 22.The New York Times. California Coronavirus Map and Case Count - The New York Times. (2023). https://www.nytimes.com/interactive/2021/us/california-covid-cases.html
- 23.California Department of Food and Agriculture (CDFA). California Agricultural Statistics Review 2021–2022. (2022).
- 24.Huang, J. & Hartemink, A. E. Soil and environmental issues in sandy soils. Earth-Sci. Rev.208, 103295 (2020). [Google Scholar]
- 25.Li, H., Tatarko, J., Kucharski, M. & Dong, Z. PM2.5 and PM10 emissions from agricultural soils by wind erosion. Aeolian Res.19, 171–182 (2015). [Google Scholar]
- 26.Moore, K. D. et al. Particulate-matter emission estimates from agricultural spring-tillage operations using LIDAR and inverse modeling. J. Appl. Remote Sens.9, 096066 (2015). [Google Scholar]
- 27.Wang, Y., Faloona, I. C. & Houlton, B. Z. Satellite NO 2 trends reveal pervasive impacts of wildfire and soil emissions across California landscapes. Environ. Res. Lett.18, 094032 (2023). [Google Scholar]
- 28.Ribaudo, M. et al. Nitrogen in Agricultural systems: implications for Conservation Policy. SSRN Electron. J.10.2139/ssrn.2115532 (2011). [Google Scholar]
- 29.Spinoni, J. et al. How will the progressive global increase of arid areas affect population and land-use in the 21st century? Glob Planet. Change. 205, 103597 (2021). [Google Scholar]
- 30.Intergovernmental Panel on Climate Change. Climate Change and Land: IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems (Cambridge University Press, 2022). 10.1017/9781009157988
- 31.Williams, E. J. & Fehsenfeld, F. C. Measurement of soil nitrogen oxide emissions at three north American ecosystems. J. Geophys. Res. Atmos.96, 1033–1042 (1991). [Google Scholar]
- 32.Firestone, M. K. & Davidson, E. A. Microbial basis of NO and N2O production and consumption in soil. in Exchange of trace gases between terrestrial ecosystems and the atmosphere 7–21 (John Wiley & Sons Ltd., Chichester, UK, (1989).
- 33.Hall, S. J., Matson, P. A., Roth, P. M. & NOx EMISSIONS FROM SOIL: implications for Air Quality modeling in Agricultural regions. Annu. Rev. Energy Environ.21, 311–346 (1996). [Google Scholar]
- 34.Pilegaard, K. Processes regulating nitric oxide emissions from soils. Philos. Trans. R Soc. B Biol. Sci.368, 20130126 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oswald, R. et al. HONO emissions from Soil Bacteria as a Major Source of Atmospheric Reactive Nitrogen. Science. 341, 1233–1235 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Ward, B. B. & Nitrification (2008).
- 37.Prosser, J. I. The Ecology of nitrifying Bacteria. in Biology of the Nitrogen Cycle 223–243 (Elsevier, doi:10.1016/B978-044452857-5.50016-3. (2007).
- 38.Barton, L., McLay, C. D. A., Schipper, L. A. & Smith, C. T. Annual denitrification rates in agricultural and forest soils: a review. Soil. Res.37, 1073 (1999). [Google Scholar]
- 39.Parton, W. J. et al. Generalized model for NO x and N 2 O emissions from soils. J. Geophys. Res. Atmos.106, 17403–17419 (2001). [Google Scholar]
- 40.Wang, Y. et al. Improved modelling of soil NOx emissions in a high temperature agricultural region: role of background emissions on NO2 trend over the US. Environ. Res. Lett.16, 084061 (2021). [Google Scholar]
- 41.Yienger, J. J. & Levy, H. Empirical model of global soil-biogenic NO χ emissions. J. Geophys. Res. Atmos.100, 11447–11464 (1995). [Google Scholar]
- 42.Oikawa, P. Y. et al. Unusually high soil nitrogen oxide emissions influence air quality in a high-temperature agricultural region. Nat. Commun.6, 8753 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xue, C. et al. Evidence for strong HONO Emission from Fertilized Agricultural fields and its remarkable impact on Regional O3 Pollution in the Summer North China Plain. ACS Earth Space Chem.5, 340–347 (2021). [Google Scholar]
- 44.Chang, Y. et al. Nitrogen isotope fractionation during gas-to-particle conversion of NOx to NO3- in the atmosphere – implications for isotope-based NOx source apportionment. Atmospheric Chem. Phys.18, 11647–11661 (2018). [Google Scholar]
- 45.Fibiger, D. L. & Hastings, M. G. First Measurements of the Nitrogen Isotopic Composition of NO x from Biomass Burning. Environ. Sci. Technol.50, 11569–11574 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Hastings, M. G., Casciotti, K. L. & Elliott, E. M. Stable Isotopes as Tracers of Anthropogenic Nitrogen Sources, Deposition, and Impacts. Elements 9, 339–344 (2013).
- 47.Li, J., Zhang, X., Orlando, J., Tyndall, G. & Michalsi, G. Quantifying the nitrogen isotope effects during photochemical equilibrium between NO and NO2: implications for δ15N in tropospheric reactive nitrogen. Atmospheric Chem. Phys.20, 9805–9819 (2020). [Google Scholar]
- 48.Walters, W. W., Fang, H. & Michalski, G. Summertime diurnal variations in the isotopic composition of atmospheric nitrogen dioxide at a small midwestern United States city. Atmos. Environ.179, 1–11 (2018). [Google Scholar]
- 49.Blum, D. E., Walters, W. W. & Hastings, M. G. Speciated Collection of nitric acid and fine particulate nitrate for Nitrogen and Oxygen stable isotope determination. Anal. Chem.92, 16079–16088 (2020). [DOI] [PubMed] [Google Scholar]
- 50.Koutrakis, P. et al. Development and evaluation of a glass honeycomb denuder/filter pack system to collect atmospheric gases and particles. Environ. Sci. Technol.27, 2497–2501 (1993). [Google Scholar]
- 51.Li, D. & Wang, X. Nitrogen isotopic signature of soil-released nitric oxide (NO) after fertilizer application. Atmos. Environ.42, 4747–4754 (2008). [Google Scholar]
- 52.Walters, W. W., Simonini, D. S. & Michalski, G. Nitrogen isotope exchange between NO and NO 2 and its implications for δ 15 N variations in tropospheric NO x and atmospheric nitrate. Geophys. Res. Lett.43, 440–448 (2016). [Google Scholar]
- 53.Williams, E. L. & Grosjean, D. Removal of atmospheric oxidants with annular denuders. Environ. Sci. Technol.24, 811–814 (1990). [Google Scholar]
- 54.Doane, T. A. & Horwáth, W. R. Spectrophotometric Determination of Nitrate with a single reagent. Anal. Lett.36, 2713–2722 (2003). [Google Scholar]
- 55.Casciotti, K. L., Sigman, D. M., Hastings, M. G., Böhlke, J. K. & Hilkert, A. Measurement of the Oxygen Isotopic Composition of Nitrate in seawater and freshwater using the Denitrifier Method. Anal. Chem.74, 4905–4912 (2002). [DOI] [PubMed] [Google Scholar]
- 56.Sigman, D. M. et al. A bacterial method for the Nitrogen Isotopic Analysis of Nitrate in seawater and freshwater. Anal. Chem.73, 4145–4153 (2001). [DOI] [PubMed] [Google Scholar]
- 57.Bekker, C., Walters, W. W., Murray, L. T. & Hastings, M. G. Nitrate chemistry in the northeast US – Part 1: Nitrogen isotope seasonality tracks nitrate formation chemistry. Atmospheric Chem. Phys.23, 4185–4201 (2023). [Google Scholar]
- 58.Högberg, P. Tansley Review 95 15 N natural abundance in soil-plant systems. New. Phytol. 137, 179–203 (1997). [DOI] [PubMed] [Google Scholar]
- 59.Yu, Z. & Elliott, E. M. Novel Method for Nitrogen Isotopic Analysis of Soil-Emitted Nitric Oxide. Environ. Sci. Technol.51, 6268–6278 (2017). [DOI] [PubMed] [Google Scholar]
- 60.Ammann, M. et al. Estimating the uptake of traffic-derived NO2 from 15 N abundance in Norway spruce needles. Oecologia. 118, 124–131 (1999). [DOI] [PubMed] [Google Scholar]
- 61.Felix, J. D. & Elliott, E. M. Isotopic composition of passively collected nitrogen dioxide emissions: vehicle, soil and livestock source signatures. Atmos. Environ.92, 359–366 (2014). [Google Scholar]
- 62.Redling, K., Elliott, E., Bain, D. & Sherwell, J. Highway contributions to reactive nitrogen deposition: tracing the fate of vehicular NOx using stable isotopes and plant biomonitors. Biogeochemistry. 116, 261–274 (2013). [Google Scholar]
- 63.Dahal, B. & Hastings, M. G. Technical considerations for the use of passive samplers to quantify the isotopic composition of NOx and NO2 using the denitrifier method. Atmos. Environ.143, 60–66 (2016). [Google Scholar]
- 64.Heaton, T. H. E. 15 N/14 N ratios of NOx from vehicle engines and coal-fired power stations. Tellus. 42, 304–307 (1990). [Google Scholar]
- 65.Miller, D. J., Wojtal, P. K., Clark, S. C. & Hastings, M. G. Vehicle NOx emission plume isotopic signatures: spatial variability across the eastern United States. J. Geophys. Res. Atmos.122, 4698–4717 (2017). [Google Scholar]
- 66.Walters, W. W., Goodwin, S. R. & Michalski, G. Nitrogen stable isotope composition (δ15N) of vehicle-emitted NOx. Environ. Sci. Technol.49, 2278–2285 (2015). [DOI] [PubMed] [Google Scholar]
- 67.Felix, J. D., Elliott, E. M. & Shaw, S. L. Nitrogen Isotopic composition of coal-fired power plant NOx: influence of Emission controls and implications for Global Emission inventories. Environ. Sci. Technol.46, 3528–3535 (2012). [DOI] [PubMed] [Google Scholar]
- 68.Walters, W. W. & Michalski, G. Theoretical calculation of nitrogen isotope equilibrium exchange fractionation factors for various NO y molecules. Geochim. Cosmochim. Acta. 164, 284–297 (2015). [Google Scholar]
- 69.Hudman, R. C. et al. Steps towards a mechanistic model of global soil nitric oxide emissions: implementation and space based-constraints. Atmospheric Chem. Phys.12, 7779–7795 (2012). [Google Scholar]
- 70.Jaeglé, L. et al. Satellite mapping of rain-induced nitric oxide emissions from soils. J. Geophys. Res. Atmos.109, 2004JD004787 (2004). [Google Scholar]
- 71.Davidson, E. A. Pulses of nitric oxide and Nitrous Oxide Flux following Wetting of Dry Soil: an Assessment of probable sources and importance relative to Annual fluxes. Ecol. Bull.42, 149–155 (1992). [Google Scholar]
- 72.California Department of Food and Agriculture (CDFA). California Agricultural Statistics Review 1991–1992. (1991).
- 73.Byrnes, D. K., Van Meter, K. J. & Basu, N. B. Long-term shifts in U.S. Nitrogen sources and sinks revealed by the New TREND‐Nitrogen Data Set (1930–2017). Glob Biogeochem. Cycles34, (2020).
- 74.Geddes, J. A., Pusede, S. E. & Wong, A. Y. H. Changes in the Relative Importance of Biogenic Isoprene and Soil NOx Emissions on Ozone Concentrations in Nonattainment Areas of the United States. J. Geophys. Res. Atmospheres 127, eJD036361 (2022). (2021).
- 75.Atmospheric Chem. Phys. 14, 10363–10381 (2014).
- 76.UC Davis Agricultural and Resource Economics. Cost & Return Studies. (2004). https://coststudies.ucdavis.edu/
- 77.Luo, L., Ran, L., Rasool, Q. Z. & Cohan, D. S. Integrated Modeling of U.S. Agricultural Soil Emissions of Reactive Nitrogen and Associated impacts on Air Pollution, Health, and Climate. Environ. Sci. Technol.56, 9265–9276 (2022). [DOI] [PubMed] [Google Scholar]
- 78.Guo, L. et al. Assessment of Nitrogen Oxide emissions and San Joaquin Valley PM2.5 impacts from soils in California. J. Geophys. Res. Atmos.125, e2020JD033304 (2020). [Google Scholar]
- 79.Eberwein, J. R., Homyak, P. M., Carey, C. J., Aronson, E. L. & Jenerette, G. D. large nitrogen oxide emission pulses from desert soils and associated microbiomes. Biogeochemistry. 149, 239–250 (2020). [Google Scholar]
- 80.Weber, B. et al. Biological soil crusts accelerate the nitrogen cycle through large NO and HONO emissions in drylands. Proc. Natl. Acad. Sci.112, 15384–15389 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thornton, F. C., Bock, B. R. & Tyler, D. D. Soil emissions of nitric oxide and Nitrous Oxide from Injected Anhydrous ammonium and urea. J. Environ. Qual.25, 1378–1384 (1996). [Google Scholar]
- 82.US EPA. Final Reconsideration of the National Ambient Air Quality Standards for Particulate Matter (PM). (2024). https://www.epa.gov/pm-pollution/final-reconsideration-national-ambient-air-quality-standards-particulate-matter-pm
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The isotope data presented in this study are available from the corresponding author upon request. Other data such as meteorology and satellite data is available publicly at the following sites. NO2 column data for Fig. 1 and S1 was obtained from Google Earth Engine using Sentinel-5P NRTI NO2: Near Real-Time Nitrogen Dioxide. The script can be found at: https://developers.google.com/earth-engine/datasets/catalog/COPERNICUS_S5P_NRTI_L3_NO2, and the data was analyzed for June 2022- May 2023 at the Salton Sea Air Basin. The air basin was selected using a https://ww2.arb.ca.gov/geographical-information-system-gis-library. Air quality and meteorological data was obtained from AQMIS (https://www.arb.ca.gov/aqmis2/aqdselect.php and https://www.arb.ca.gov/aqmis2/metselect.php) and CIMIS (https://cimis.water.ca.gov/Default.aspx). The 2022 annual average CEPAM NOx inventory can be found https://ww2.arb.ca.gov/applications/cepam2019v103-standard-emission-tool. Soil Moisture Active Passive (SMAP) data was obtained from NASA’s Earth Data Inventory; specifically, we used https://nsidc.org/data/spl4smgp/versions/7. The TREND-nitrogen data set is available 10.1594/PANGAEA.917583.