Abstract
Surgical site infection (SSI) continues to be a common complication of surgery. The real benefit of using topical antibiotics for the prevention of SSI in abdominal hernia repair surgery is still unknown. This study aimed to evaluate the usefulness of topical gentamicin in SSI prophylaxis in incisional hernia repair with mesh. A randomized controlled trial was conducted in patients undergoing open incisional hernia repair. Patients were randomly assigned to one of two groups: in the gentamicin group, each layer of the abdominal wall was irrigated with gentamicin solution before wound closure, and in the saline solution group (placebo), each layer of the abdominal wall was irrigated with normal saline solution. The incidence of SSI and other surgical site complications was compared between both groups, and the presence of adverse effects with the use of topical gentamicin. Data from 146 patients were included for analysis: 74 in the gentamicin group and 72 in the saline solution group. SSI was observed in six patients (8.1%) in the gentamicin group and eight patients (11.1%) in the saline solution group, with no significant differences (p = 0.538) between both groups. No statistically significant differences were observed in the presentation of seroma, hematoma, and surgical wound dehiscence between both groups. No adverse effects were reported from topical application of gentamicin. In this clinical trial, the use of topical gentamicin in incisional hernia repair with mesh did not significantly reduce the incidence of SSI. EU Clinical Trials Register: EudraCT 2018-001860-45 (04/07/2019).
Keywords: Surgical site infection, Topical antibiotics, Gentamicin, Prevention, Incisional hernia repair
Subject terms: Diseases, Medical research
Introduction
Surgical site infection (SSI) continues to be one of the most common complications of surgery despite advances in surgical technology and prevention measures, accounting for up to 20% of all healthcare-associated infections1. SSI is the second leading cause of hospital-acquired infection and remains one of the most common preventable infections today2,3.
The estimated incidence of SSI is around 5%4–6. However, this may be an underestimated rate, given that approximately 50% of SSIs is diagnosed outside the hospital after the patient has been discharged1,7. Additionally, the incidence of SSI varies greatly depending on several factors, such as the type of surgical procedure performed. In colorectal surgery, the incidence of SSI can be up to 32%8. After abdominal wall surgery, inguinal hernia infection rates range from 2.4 to 4.9%9,10, while those of incisional hernia can reach 33% 11.
SSI causes increased morbidity and mortality7,12, impairs the wound healing process, resulting in a higher probability of having an incisional hernia13, prolongs hospital stay14, and causes higher healthcare costs15. The incidence of infectious complications is one of the most important outcome measures in surgery, so everything possible must be done to design effective methods to reduce them.
Among measures to prevent SSI, topical antibiotics have been used for decades in surgical practice16,17 however, there is still controversy about their use, and there is a lack of consistent evidence on the effectiveness of this measure. The World Health Organization (WHO) guidelines do not recommend topical antibiotics before wound closure18. The Centers for Disease Control and Prevention (CDC) guidelines also advise against applying ointments, solutions, or powders to the incision site but indicate that irrigation with antibiotic agents of intra-abdominal, deep tissues, or subcutaneous remains an unresolved question2. Evidence evaluated by some meta-analyses also demonstrated that topical antibiotics before wound closure could not be recommended19,20. On the other hand, two recent meta-analyses concluded that the use of topical antibiotics probably prevents SSI compared to placebo and antiseptics, but that these results should be taken with caution due to the low number of studies used for comparison and the low statistical power from some studies21,22 Overall, high-quality data supporting the use of topical antibiotics for SSI prevention is lacking; however, there may be benefits in specific procedures and populations such as joint arthroplasty, cataract surgery, spine surgery, and obese patients undergoing abdominal surgery and, specifically, colorectal resections2,23. In summary, the use of topical antibiotics cannot currently be recommended, but clinical trials evaluating their effectiveness are still necessary19.
SSI represents a major concern in mesh ventral hernia repair because of the risk of a devastating complication such as mesh infection that can occur in up to 7-10% of patients24. Additionally, SSI is associated with increased morbidity, poorer quality of life, increased costs, and hernia recurrence25–28. Clinical studies analyzing topical antibiotics in abdominal wall surgery are scarce, carried out mainly in inguinal hernia repair29,30, and experimental studies with good results after using prosthetic material with gentamicin31,32. Considering this, we believe conducting clinical trials on this topic is necessary.
The main objective of the present trial was to evaluate the usefulness of topical gentamicin as a prophylaxis for surgical site infection in incisional hernia repair with mesh. Secondary objectives were to evaluate the incidence of other surgical site occurrences and adverse effects or complications associated with the use of topical gentamicin.
Materials and methods
Study design
A randomized controlled trial was conducted in patients undergoing open incisional hernia repair from March 2017 to March 2021. The study was carried out at the Hospital Plató, a basic general hospital with a high workload in abdominal wall surgery. Since 2021, it has been part of the Hospital Clínic Barcelona, a tertiary reference center. The trial was registered in the European database of clinical trials with medicines EudraCT (www.clinicaltrialsregister.eu) with the identifier 2018-001860-45 (04/07/2019). The manuscript was prepared following the guidelines of the CONSORT (Consolidated Standards of Reporting Trials) statement33.
Eligibility criteria
The inclusion criteria were adults 18 years or older and elective open ventral incisional hernia repair surgery. The incisional hernia was diagnosed by clinical examination or imaging findings with ultrasound or abdominal tomography.
The exclusion criteria were known allergic reaction to aminoglycosides, parastomal hernia, repair by minimally invasive surgery, performance of another concomitant surgical procedure, infection at the time of surgery, antibiotic treatment until less than 48 h before surgery, and patients who did not wish to participate in the study.
Preoperative and intraoperative data collection
For each patient, demographic data (age, sex, body mass index), comorbidities (diabetes mellitus, heart disease, chronic lung disease, kidney disease, liver disease, obesity), use of immunosuppressive medication or steroids, tobacco consumption, anesthetic risk using the American Society of Anesthesiologists (ASA) classification34, and presence of recurrent incisional hernia.
The intraoperative data recorded were operative time, hernia size, hernia size classification according to the European Hernia Society (EHS)35, location of the hernia according to the EHS classification35, size of the mesh used, abdominal wall plane where the mesh was placed, associated intestinal resection, and drain placement. Surgical wound classification (clean, clean-contaminated, contaminated, dirty-infected) was reported according to the CDC definition7.
Random sequence generation and blinding
Randomization was done after the preoperative visit, and the patient’s consent was obtained to participate in the trial. Randomization was performed with the Spanish version of the free and open-source OxMaR system software available online36. Restricted randomization was performed through minimization to assign patients to groups. Criteria for minimization were sex (male, female), age, diabetes mellitus, and body mass index (BMI).
Patients were randomly assigned to one of two groups: gentamicin group and saline solution (placebo) group.
The study was double-blind, where the patients did not know the group to which they were assigned, and the outcome assessors were also unaware of the treatment groups. The operating surgeons and operating room nurses were aware of the nature of the study and the assignment of patients to each group at the time of surgery.
Surgical procedure
All patients followed the recommendations of the PREVINQ-CAT program, a regional initiative of the Department of Health of Catalonia to prevent SSI37. These preventive measures or bundles include six general recommendations used in all patients in this study. A dose of 2 g of intravenous cefazolin was administered 30 min before surgery, and 600 mg of intravenous clindamycin was administered to patients with beta-lactam allergy. Before starting surgery, the skin was prepared by washing with chlorhexidine soap and painting the surgical field with 2% alcoholic chlorhexidine. Skin hair removal was avoided, and an electric razor was used for shaving if necessary. Maintenance of perioperative normoglycemia and normothermia was ensured.
Procedures were performed under general or spinal anesthesia with patients lying supine. All procedures were performed using an open approach by two surgeons with experience in abdominal wall surgery.
A spindle incision of the skin was made with the excision of the scar from the previous surgery, the subcutaneous cellular tissue opening, identification, and dissection of the hernial sac. To repair the hernia defect, a 60 g/m2 monofilament polypropylene mesh with a 1.15 mm pore was used, placed according to the characteristics of the hernia at the preperitoneal, retromuscular or supra aponeurotic level. The meshes were fixed with a poly-4-hydroxybutyrate monofilament absorbable synthetic suture. The aponeurosis closure was performed with a continuous suture according to the small bite technique using absorbable monofilament synthetic suture of poly-4-hydroxybutyrate, the closure of the subcutaneous cellular tissue with absorbable multifilament synthetic suture of polyglactin 910 and the skin with a continuous intradermal suture with absorbable monofilament synthetic of polyester Glycomer 631. Depending on the characteristics of the hernia and the surgeon’s choice, a closed system drain was placed in the retromuscular or subcutaneous space, inserted through an incision in the skin far from the surgical wound.
Study intervention
After placing the mesh in the gentamicin group, each layer of the abdominal wall was irrigated with 160 mg of gentamicin diluted in 500 ml of normal saline solution (0.9% NaCl). In the saline solution (placebo) group, each layer of the abdominal wall was irrigated with 500 ml of normal saline solution. After the intervention, the surgical wound was closed.
Postoperative care
In the hospitalization room, vital signs and drainage (if present) were evaluated. Analgesia during admission was achieved with paracetamol 1 g every eight hours and dexketoprofen every eight hours intravenously. Systemic or topical antibiotics were not routinely administered postoperatively. The wounds were examined every day by a surgeon until discharge. Drains were removed before discharge if the discharge was scant and had non-haematic characteristics. The surgical wound dressings were removed on the second postoperative day, and showering was permitted. Oral analgesia at discharge was with paracetamol and dexketoprofen.
Follow-up
Patient follow-up was carried out in outpatient surgery consultations at seven days, 30 days, six months, and 12 months after the intervention. The surgical outcome was evaluated by a surgeon who was not involved in the surgery and was unaware of the group assignment. At each visit, wound healing was assessed, and complications, including SSI, were recorded. If SSI with discharge was detected, wound cultures were taken.
Primary outcome
The study’s primary outcome was the incidence of SSI (superficial, deep, and organ/space), which was defined according to the criteria developed by the CDC7. These criteria define superficial SSI as occurring up to 30 days after surgery and affecting only the skin or subcutaneous tissue. Deep SSI was defined as one that involves deep soft tissues such as fascia and muscle that occur up to one year after surgery if a prosthesis is placed. Organ/space infection was defined as infection deeper than the fascia or muscular wall, which has been opened or manipulated during the surgical procedure.
Secondary outcomes
The incidence of other surgical site occurrences such as seroma (bag of sterile, clear serous fluid at the incision site), hematoma (accumulation of blood or clots in the surgical wound), wound dehiscence, or enterocutaneous fistula was analyzed26.
The isolation of microorganisms in surgical wound samples in patients with SSI and the treatment performed in patients with SSI, such as systemic antibiotics, drainage of abscesses, or need for reintervention to remove the mesh, were analyzed.
Other outcomes analyzed were postoperative complications outside the surgical site, postoperative complications according to the Clavien-Dindo classification38, hospital stay, mortality 30 days after surgery, and hernia recurrence after one year of follow-up.
Adverse events that were potentially attributable to the study antibiotic were also reported. An independent data and safety monitoring committee monitored the trial continuously.
Sample size calculation
The primary endpoint for calculating the required sample size was the incidence of surgical wound infection. Based on the results of two clinical trials39,40, we assumed an SSI rate of 23% in the group without topical gentamicin and 6% in the group with topical gentamicin. The sample size calculated was 146 patients to identify this difference with an alpha error of 0.05 and a power of 0.8. The dropout rate was expected to be 5%; therefore, we aimed for a total sample size of 154 patients. Due to the low dropout rate, we ended recruitment after 150 patients. The sample size was calculated with Gpower 3.1 software.
Statistic analysis
Categorical variables were analyzed using Pearson’s Chi-square or Fisher’s exact tests. For continuous variables, the normality of the data distribution was tested using the Kolmogorov-Smirnov test. The student t-test was used to analyze differences in continuous variables with a normal distribution, and the non-parametric Mann-Whitney U test was used to analyze continuous variables with a non-normal distribution.
A level of p < 0.05 was considered to establish statistical significance. A per-protocol approach was used for data analysis. Statistical analyses were performed using IBM SPSS 27.0 software (Armonk, NY: IBM Corp).
Ethics
The protocol of this trial was approved by the drug research ethics committee of the Catalan Union of Hospitals Foundation, and its implementation was authorized by the Spanish Agency for Medicines and Health Products. All eligible patients gave written informed consent. This study was performed in accordance with the Declaration of Helsinki.
Results
Recruited patients
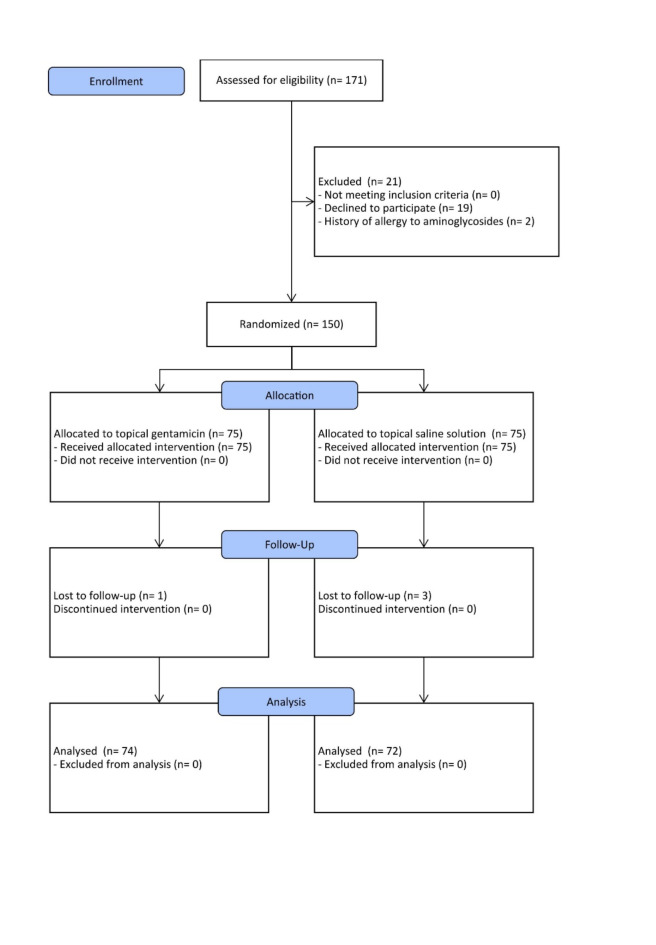
From March 2017 to March 2021, 171 patients admitted for elective incisional hernia repair met inclusion criteria. Nineteen patients were excluded from the study due to refusal to participate, and two patients were due to a history of allergy to aminoglycosides. Finally, 150 patients were randomized; 75 were assigned to the gentamicin group and 75 to the saline solution group. One patient in the gentamicin group and three in the saline solution group were lost to follow-up. Finally, data from 146 patients were included for analysis: 74 in the gentamicin group and 72 in the saline solution group. Figure 1 shows the recruitment flow chart according to CONSORT recommendations33.
Fig. 1.
CONSORT flow chart for recruitment.
Baseline characteristics of the study groups
The mean age was 67.2 ± 11.3 in the gentamicin group and 65.36 ± 14.08 in the saline solution group, with no significant differences between both groups (p = 0.384). The two groups had no significant differences regarding sex, BMI, comorbidities analyzed, anesthetic risk (ASA), and recurrent hernia (Table 1).
Table 1.
Baseline characteristics of study groups.
| Topical gentamicin (N = 74) | Topical saline solution (N = 72) | p-value | |
|---|---|---|---|
| Age, Mean ± SD | 67.2 ± 11.31 | 65.36 ± 14.08 | 0.384 |
| Sex, N (%) | 0.517 | ||
| Male | 31 (41.9) | 34 (47.2) | |
| Female | 43 (58.1) | 38 (52.8) | |
| BMI (Kg/m2), Mean ± SD | 30.29 ± 5.74 | 30.12 ± 5.04 | 0.84 |
| Comorbidities, N (%) | |||
| Diabetes mellitus | 13 (17.6) | 10 (13.9) | 0.542 |
| Heart disease | 13 (17.6) | 10 (13.9) | 0.542 |
| Chronic pulmonary disease | 9 (12.2) | 8 (11.1) | 0.843 |
| Renal disease | 0 | 3 (4.2) | 0.117 |
| Liver disease | 2 (2.7) | 0 | 0.497 |
| Obesity | 37 (50) | 38 (52.8) | 0.037 |
| Tobacco use | 9 (12.2) | 8 (11.1) | 0.843 |
| Steroid or immunosuppressant use | 2 (2.7) | 3 (4.2) | 0.679 |
| ASA, N (%) | 0.377 | ||
| ASA I | 4 (5.4) | 6 (8.3) | 0.53 |
| ASA II | 57 (77) | 48 (66.7) | 0.164 |
| ASA III | 13 (17.6) | 18 (25) | 0.272 |
| Recurrent hernia, N (%) | 16 (21.6) | 18 (25) | 0.629 |
SD: standard deviation, BMI: body mass index, ASA: American Society of Anesthesiologists classification.
Characteristics of the hernia and surgery
The mean operating time was 71.84 ± 36.19 min in the gentamicin group and 77.22 ± 41.52 min in the saline solution group, with no significant differences between both groups (p = 0.402). When analyzing the size of the hernia, location of the hernia, mesh size, plane of mesh placement, need for intestinal resection, and use of drainage between both groups, no statistically significant differences were observed (Table 2).
Table 2.
Intraoperative data of study groups.
| Topical gentamicin (N = 74) | Topical saline solution (N = 72) | p-value | |
|---|---|---|---|
| Operative time (min), Mean ± SD | 71.84 ± 36.19 | 77.22 ± 41.52 | 0.402 |
| Hernia size (cm), Mean ± SD | |||
| Hernia length | 6.95 ± 5.28 | 7.18 ± 5.48 | 0.964 |
| Hernia width | 4.89 ± 2.92 | 5.13 ± 2.96 | 0.496 |
| Hernia size classification (EHS), N (%) | 0.839 | ||
| EHS W1 | 26 (35.1) | 22 (30.6) | |
| EHS W2 | 44 (59.5) | 46 (63.9) | |
| EHS W3 | 4 (5.6) | 4 (5.4) | |
| Hernia localization, N (%) | 0.078 | ||
| Medial | 64 (86.5) | 54 (75) | |
| Lateral | 10 (13.5) | 18 (25) | |
| Mesh size (cm2), Mean ± SD | 220.93 ± 344.51 | 254.56 ± 279.76 | 0.129 |
| Mesh placement, N (%) | 0.578 | ||
| Onlay | 27 (36.5) | 26 (36.1) | |
| Retromuscular | 39 (52.7) | 41 (56.9) | |
| Preperitoneal | 1 (1.4) | 2 (2.8) | |
| Onlay + Retromuscular | 7 (9.5) | 3 (4.2) | |
| Intestinal resection, N (%) | 1 (1.4) | 1 (1.4) | 1 |
| Surgical wound classification, N (%) | 1 | ||
| Clean (Class I) | 73 (98.6) | 72 (98.6) | |
| Clean-contaminated (Class II) | 1 (1.4) | 1 (1.4) | |
| Drain use, N (%) | 50 (67.6) | 47 (65.3) | 0.77 |
SD: standard deviation, EHS: European Hernia Society.
Outcomes of both groups
The incidence of SSI in the entire sample analyzed was 9.6%. SSI was observed in six patients (8.1%) in the gentamicin group and eight patients (11.1%) in the saline solution group, with no significant differences (p = 0.538) between both groups. Six patients in the gentamicin group had superficial infections, seven in the saline solution group had superficial infections, and one had a deep infection (Table 3). No organ/space infection was reported in any of the groups.
Table 3.
Outcomes of study groups.
| Topical gentamicin (N = 74) | Topical Saline solution (N = 72) | p-value | |
|---|---|---|---|
| Surgical site occurrence, N (%) | |||
| Any surgical site occurrence | 18 (24.3) | 21 (29.2) | 0.509 |
| Seroma | 8 (10.8) | 13 (18.1) | 0.212 |
| Hematomas | 4 (5.4) | 2 (2.8) | 0.681 |
| Wound dehiscence | 2 (2.7) | 5 (6.9) | 0.272 |
| Surgical site infection | 6 (8.1) | 8 (11.1) | 0.538 |
| Superficial surgical site infection | 6 (8.1) | 7 (9.7) | 0.732 |
| Deep surgical site infection | 0 | 1 (1.4) | 0.493 |
| Mesh infection | 0 | 1 (1.4) | 0.493 |
| Cultures performed | 3 (4.1) | 7 (9.7) | 0.206 |
| Positive culture | 2 (2.7) | 7 (9.7) | 0.095 |
| Surgical site infection treatment, N (%) | |||
| Antibiotic | 3 (4.1) | 4 (5.6) | 0.717 |
| Antibiotic and abscess drainage | 3 (4.2) | 3 (4.1) | 1 |
| Antibiotic, abscess drainage, and remove mesh | 0 | 1 (1.4) | 0.493 |
| Other complications, N (%) | 4 (5.4) | 3 (4.2) | 1 |
| Clavien Dindo, N (%) | 0.564 | ||
| I | 0 | 1 (1.4) | |
| II | 4 (5.4) | 3 (4.2) | |
| Length of stay (days), Mean ± SD | 2.32 ± 2.73 | 2.86 ± 4.15 | 0.465 |
| Mortality 30 days, N (%) | 0 | 0 | |
| Hernia recurrence, N (%) | 2 (2.7) | 6 (8.3) | 0.163 |
SD: standard deviation.
No statistically significant differences were observed in the presentation of seroma, hematoma, and surgical wound dehiscence between both groups. Some complication of the surgical wound (surgical site infection, seroma, hematoma, or wound dehiscence) was observed in 18 patients (24.3%) in the gentamicin group and 21 patients (29.2%) in the saline solution group, without significant differences (p = 0.509). Furthermore, no adverse effects or allergic reactions were reported.
Other complications not related to the surgical wound occurred in seven patients: two patients with acute urinary retention, one patient with cardiac arrhythmias, one patient with bronchospasm, one patient with pneumonia, one patient with confusional syndrome, and one patient with paralytic ileus. No significant differences were observed between both groups.
No significant differences were also observed between both groups when comparing postoperative complications according to the Clavien-Dindo classification, hospital stay, 30-day mortality, and hernia recurrence at one year of follow-up.
No adverse events potentially attributable to the use of the topical antibiotic were reported in patients in the gentamicin group.
Details of infected patients
All surgical site infections were diagnosed after hospital discharge. In the gentamicin group, three patients were treated with oral antibiotics alone, and three patients required abscess drainage in addition to the antibiotic. In the saline solution group, four patients were treated with oral antibiotics only, three patients with oral antibiotics and abscess drainage, and one patient also required mesh removal (Table 3).
Surgical wound cultures were performed in 10 patients, and pathogenic microorganisms were isolated in 2 patients in the gentamicin group and seven in the saline solution group. Bacteria resistant to gentamicin were Enterococcus faecalis (in 1 of 2 isolates) and Pseudomonas aeruginosa (in 1 of 2 isolates). Gentamicin-resistant bacteria were isolated in two patients in the gentamicin group. The bacteria isolated in the samples and the antibiotic resistance in both groups are detailed in Table 4.
Table 4.
Type of bacteria isolated in cultures from the infected surgical site.
| Case | Allocated intervention | Bacteria isolated (R: resistant antibiotic) |
|---|---|---|
| 1 | Topical gentamicin |
Enterococcus faecalis (R: Erythromycin, Tetracycline, Cotrimoxazole, Fosfomycin, Gentamicin) Peptoniphilus harei (R: Erythromycin, Clindamycin, Tetracycline) Actinomyces turicensis (R: Metronidazole) |
| 2 | Topical gentamicin |
Pseudomonas aeruginosa (R: Gentamicin) Citrobacter freundii (R: Ampicillin, Amoxicillin/clavulanic, Cephalothin) |
| 3 | Topical gentamicin | Negative |
| 4 | Topical saline solution |
Enterococcus faecalis (R: Erythromycin, Tetracycline, Cotrimoxazole, Fosfomycin) Citrobacter koseri (R: Ampicillin) |
| 5 | Topical saline solution |
Staphylococcus aureus (R: Penicillin) Pseudomonas aeruginosa (R: Ampicillin/sulbactam, Cefotaxime, Minocycline, Cotrimoxazole) |
| 6 | Topical saline solution | Escherichia coli (R: Ampicillin, Piperacillin, Ciprofloxacin, Levofloxacin) |
| 7 | Topical saline solution |
Serratia marcescens (R: Ampicillin, Amoxicillin/clavulanic, Cefuroxime, Tobramycin) Staphylococcus aureus (R: Erythromycin, Clindamycin, Streptococcus agalactiae (R: Tetracycline) |
| 8 | Topical saline solution |
Proteus mirabilis (R: Nalidixic acid, Fosfomycin) Enterococcus faecium (R: Penicillin, Ampicillin, Cefuroxime, Imipenem, Ciprofloxacin, Levofloxacin, Erythromycin, Clindamycin, Cotrimoxazole, Streptomycin, Kanamycin) |
| 9 | Topical saline solution |
Proteus mirabilis (R: Tigecycline) Staphylococcus aureus (R: none) |
| 10 | Topical saline solution | Staphylococcus aureus (R: Penicillin) |
Discussion
In this randomized controlled trial, topical gentamicin before wound closure of incisional hernia repair with mesh did not significantly reduce the incidence of SSI. There were also no significant differences in the incidence of other local complications or adverse effects due to topical gentamicin.
SSI is a common postoperative complication associated with worse outcomes and higher costs12,15. In a recent study in the U.S., SSI was the most common infection associated with medical care, representing 42% of these infections41. The incidence of SSI varies depending on several factors, such as the type of surgical procedures, the context in which the operations are performed, patient comorbidities, surveillance criteria used, and the quality of data collection.
Overall, the incidence of SSI in clean surgical procedures is only 2%. In comparison, reported rates for wall reconstruction procedures range from 2.4 to 4.9% in inguinal hernias9,10, between 4% and 16% in ventral hernias42–45 and 23–33% in incisional hernias11,39. Although the presence of prosthetic mesh does not increase the incidence of infection46, SSI after hernia repair with mesh is a difficult complication to solve and is associated with high morbidity, occasionally requiring reintervention for removal of the infected mesh, increases the risk of hernia recurrence, and increases healthcare costs27,42,47.
Implementation of evidence-based protocols can prevent up to 50–60% of all SSIs2,4,48. The WHO and the CDC have developed guidelines for preventing SSIs2,18,49. Some practices to prevent SSI include adequate preoperative preparation, sterilization of the surgical site with antiseptics, proper surgical technique, and administration of prophylactic antibiotics4,7. The incidence of SSI in our study was 9.6% in the entire sample analyzed, lower than in previous years in our center, probably because conducting the clinical trial required stricter follow-up of these guidelines.
Among the measures to reduce the incidence of SSI after surgery, topical antibiotics have been proposed. However, the evidence is currently low quality, so its efficacy remains uncertain20,50 and is generally reserved for at-risk patients (e.g. diabetes, immunosuppression, obesity) or high-risk procedures (e.g., clean-contaminated procedures, colorectal surgery, implantation of prosthetic materials)5,51. A recent meta-analysis concludes that the use of topical antibiotics in hernia repair does not show a significant benefit in reducing SSI52. However, the authors note that the diversity of meshes, the methods of antibiotic administration, and the variations in reporting standards do not allow for conclusions with a high level of evidence.
Commonly used antibiotics are beta-lactams, cephalosporins, aminoglycosides, glycopeptides, chloramphenicol, and bacitracin53–55 with different methods and doses of administration. To carry out this clinical trial, we chose gentamicin because it is effective against gram-negative and gram-positive bacteria such as staphylococci (S. aureus and S. epidermidis) because the local concentration achieved could be sufficient to exert a potential effect against resistant species and because it presents limited systemic toxicity56. Furthermore, favorable results have been described with topical gentamicin in experimental studies with mesh32,57,58 and clinical studies performed in inguinal hernia repair with mesh29,59,60.
Our results show a lower proportion of SSIs in the gentamicin group; however, this difference was not statistically significant. Previous clinical trials conducted in inguinal hernia surgery29, cardiothoracic surgery61 and colorectal surgery23,62 found a significant reduction in SSI. In these studies, unlike our clinical trial, gentamicin implants or sponges were used, which could favor a longer release of the antibiotic. However, other clinical trials have shown that the use of topical gentamicin, even in the form of gentamicin sponge implantation, does not produce a relevant reduction in the rate of SSI30,63–65.
Few studies analyze SSI rates, differentiating them into superficial and deep. A clinical trial carried out in patients undergoing sternotomy compared the results of using a sponge with gentamicin and a sponge with placebo, observing that the rate of superficial infection was similar in both groups; however, the rate of deep infection was reduced in the group with gentamicin61. Our study did not find significant differences in superficial or deep infection between the gentamicin and saline solution (placebo) groups. The importance of having information about deep infections in abdominal wall repair surgery is the associated risk of mesh infection. An estimated incidence of mesh infection is between 6% and 10% for incisional hernia repair24. In our study, only one patient with mesh infection was identified as belonging to the saline solution group and who required reintervention for mesh removal during study follow-up. The low incidence of mesh infection in our sample could be explained by the selection of patients for randomization, where we excluded parastomal hernias, emergency surgeries, and the presence of active infection.
The bacteria most associated with SSI are Staphylococcus aureus (30%), followed by coagulase-negative staphylococcus (13.7%), enterococcus species (11.2%), and Escherichia coli (9.6%)66. Most SSIs are caused by microorganisms at the incision site and introduced during surgery. The contaminating germ can come from the patient’s flora or a hospital-transmitted germ. The hospital microbial environment, medical conditions, and patient-specific risk factors determine the pathogenic microorganism and the risk of infection67. In our sample, the most frequently isolated bacteria were Staphylococcus aureus in four patients, Enterococcus faecalis in two patients, and Pseudomonas aeruginosa in two.
Excessive or unnecessary exposure to antibiotics is the cause of increasing resistance to these drugs68. A possible adverse effect of topical antibiotics is the risk of increasing resistance; for this reason, the WHO guidelines do not recommend their use2,18. However, the real risk of antibiotic resistance after local application has not yet been quantified, and its use must be weighed in situations where the benefit may outweigh the risk, such as in procedures with a high infection rate or devastating infectious complications. In our study, bacteria resistant to gentamicin were isolated in two patients belonging to the gentamicin group, which could be related to its local use. Furthermore, it should be considered that all patients received a prophylactic dose of intravenous cephalosporin, which may influence the microorganisms isolated in the cultures and resistance.
The results of comparing topical prophylaxis with systemic prophylaxis and whether there is a synergistic effect when using local and systemic prophylaxis are not yet known with certainty. An argument favoring topical prophylaxis is that it allows a higher antibiotic concentration at the wound level. In contrast, the blood concentration remains low, reducing complications such as ototoxicity and nephrotoxicity associated with gentamicin69. However, the greatest disadvantage of topical prophylaxis is that it occurs after making the incision, while the recommendation to reduce SSI is that there should be adequate concentrations of the antibiotic at the surgical site when starting the incision4,7.
The pharmacokinetics of locally administered antibiotics are not well known. Two previous studies on the prevention of SSI with gentamycin in hernioplasty30,59 were used as a reference to determine the dose used in this clinical trial. However, further studies are needed to analyze the sufficient dose to ensure adequate and safe drug concentrations at local and systemic levels.
Surgical site occurrences are a quality measure of hospitals and surgeons70, which cause considerable morbidity, increase costs, and are associated with an increased risk of hernia recurrence71. The effect of topical gentamicin on developing other surgical site occurrences and SSI has also been described in the literature. A clinical trial in which gentamicin lavage of the axillary dissection site was performed after lymph node excision did not observe a significant reduction in the incidence of seroma72. Experimental studies have also been conducted on the effect of gentamicin on healing, with contradictory results73,74. Our results do not show statistically significant differences in the presentation of the surgical wound’s seroma, hematoma, or dehiscence.
In this clinical trial, no adverse reactions related to the use of topical gentamicin were reported. The adverse reactions described using topical antibiotics vary from contact dermatitis in dermatological surgery to intraoperative anaphylactic shock75,76. The incidence of adverse reactions related to topical antibiotics is low75,77. However, few studies have addressed this issue, so the exact risk remains unknown.
The limitations of this clinical trial include the fact that it was a study conducted in a single hospital, which reduces its external validity. One-year follow-up may not be sufficient to detect rare complications associated with surgical mesh that may occur up to five years after incisional hernia repair78. However, since the trial’s primary objective is to determine the rate of SSI, the follow-up time of this study is sufficient to meet the criteria defining SSI7. Based on data from previous clinical trials, the estimated incidence of SSI for the placebo group was higher than the real incidence detected in this study, which influenced the calculation of the sample size and, therefore, the power. These clinical trials’ inclusion and exclusion criteria could also have influenced the SSI rate reported in the open surgery groups. Among the strengths of this study is the inherent characteristic of a clinical trial that allows selection biases to be reduced. Factors associated with the development of SSI such as age, obesity, diabetes mellitus, chronic obstructive pulmonary disease, use of steroids, immunosuppressants, smoking, prolonged operative time, location of mesh placement, mesh size, and associated intestinal resection43–45, were distributed evenly among the groups analyzed. Furthermore, this study is one of the first clinical trials to analyze the use of topical gentamicin to prevent SSI in incisional hernia surgery.
Conclusions
In this clinical trial, the use of topical gentamicin in incisional hernia repair with mesh did not significantly reduce the incidence of SSI. Topical gentamicin did not significantly change the proportion of other surgical site complications. It is still necessary to carry out more studies that analyze the benefits of using topical antibiotics in preventing SSI, the risk of resistance, the pharmacokinetics of their topical application, and possible adverse effects.
Author contributions
NJ.H: project development, data collection and analysis, manuscript writing and editing. M.J: project development, data collection and analysis, manuscript editing. S.G: project development, data analysis, manuscript writing and editing. C.H: data collection, manuscript editing. O.V: data analysis, manuscript editing. M.P: data analysis, manuscript writing and editing. All authors reviewed and approved the manuscript.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Magill, S. S. et al. Multistate point-prevalence survey of health care-associated infections. N Engl. J. Med.370, 1198–1208 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berríos-Torres, S. I. et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg.152, 784–791 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Klevens, R. M. et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public. Health Rep.122, 160–166 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson, D. J. et al. Strategies to prevent surgical site infections in acute care hospitals. Infect. Control Hosp. Epidemiol.29 (Suppl 1), S51–61 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Bratzler, D. W. et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am. J. Health Syst. Pharm.70, 195–283 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Gottrup, F. Prevention of surgical-wound infections. N Engl. J. Med.342, 202–204 (2000). [DOI] [PubMed] [Google Scholar]
- 7.Mangram, A. J. et al. Guideline for prevention of surgical site infection. Am. J. Infect. Control. 27, 97–132 (1999). quiz 133-4; discussion 96 (1999). [PubMed] [Google Scholar]
- 8.Yalçin, A. N., Bakir, M., Bakici, Z., Dökmetas, I. & Sabir, N. Postoperative wound infections. J. Hosp. Infect.29, 305–309 (1995). [DOI] [PubMed] [Google Scholar]
- 9.Sereysky, J., Parsikia, A., Stone, M. E., Castaldi, M. & McNelis, J. Predictive factors for the development of surgical site infection in adults undergoing initial open inguinal hernia repair. Hernia24, 173–178 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Dipp Ramos, R., O’Brien, W. J., Gupta, K. & Itani, K. M. F. Incidence and risk factors for long-term Mesh Explantation due to infection in more than 100,000 hernia operation patients. J. Am. Coll. Surg.232, 872–880e2 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Sauerland, S., Walgenbach, M., Habermalz, B., Seiler, C. M. & Miserez, M. Laparoscopic versus open surgical techniques for ventral or incisional hernia repair. Cochrane Database Syst. Rev.CD00778110.1002/14651858.CD007781.pub2 (2011). [DOI] [PubMed]
- 12.Badia, J. M. et al. Impact of surgical site infection on healthcare costs and patient outcomes: A systematic review in six European countries. J. Hosp. Infect.96, 1–15 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Murray, B. W., Cipher, D. J., Pham, T. & Anthony, T. The impact of surgical site infection on the development of incisional hernia and small bowel obstruction in colorectal surgery. Am. J. Surg.202, 558–560 (2011). [DOI] [PubMed] [Google Scholar]
- 14.de Lissovoy, G. et al. Surgical site infection: Incidence and impact on hospital utilization and treatment costs. Am. J. Infect. Control. 37, 387–397 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Sullivan, E., Gupta, A. & Cook, C. H. Cost and consequences of Surgical Site infections: A call to arms. Surg. Infect. (Larchmt). 18, 451–454 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Alexander, J. W., Solomkin, J. S. & Edwards, M. J. Updated recommendations for control of surgical site infections. Ann. Surg.253, 1082–1093 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Cha, H. G., Kwon, J. G., Han, H. H., Eom, J. S. & Kim, E. K. Appropriate prophylactic antibiotic use in clean wound surgery under local anesthesia. J. Korean Med. Sci.34, e135 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allegranzi, B. et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect. Dis.16, e288–e303 (2016). [DOI] [PubMed] [Google Scholar]
- 19.López-Cano, M. et al. Use of Topical antibiotics before primary incision closure to prevent surgical site infection: A meta-analysis. Surg. Infect. (Larchmt). 20, 261–270 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Chen, P. J., Hua, Y. M., Toh, H. S. & Lee, M. C. Topical antibiotic prophylaxis for surgical wound infections in clean and clean-contaminated surgery: A systematic review and meta-analysis. BJS Open.5, (2021). [DOI] [PMC free article] [PubMed]
- 21.Zhang, M., Feng, H., Gao, Y., Gao, X. & Ji, Z. Effect of topical antibiotics on the prevention and management of wound infections: A meta-analysis. Int. Wound J.20, 4015–4022 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Heal, C. F., Banks, J. L., Lepper, P., Kontopantelis, E. & van Driel, M. L. Meta-analysis of randomized and quasi-randomized clinical trials of topical antibiotics after primary closure for the prevention of surgical-site infection. Br. J. Surg.104, 1123–1130 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Rutten, H. J. & Nijhuis, P. H. Prevention of wound infection in elective colorectal surgery by local application of a gentamicin-containing collagen sponge. Eur. J. Surg. Suppl. 31–35 (1997). [PubMed]
- 24.Cobb, W. S., Carbonell, A. M., Kalbaugh, C. L., Jones, Y. & Lokey, J. S. Infection risk of open placement of intraperitoneal composite mesh. Am. Surg.75, 762–767 (2009). discussion 767-8. [PubMed] [Google Scholar]
- 25.Plymale, M. A., Ragulojan, R., Davenport, D. L. & Roth, J. S. Ventral and incisional hernia: The cost of comorbidities and complications. Surg. Endosc. 31, 341–351 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Ventral Hernia Working Group. Incisional ventral hernias: Review of the literature and recommendations regarding the grading and technique of repair. Surgery148, 544–558 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Cobb, W. S. et al. Open retromuscular mesh repair of complex incisional hernia: Predictors of wound events and recurrence. J. Am. Coll. Surg.220, 606–613 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Nissen, A. T. et al. Health-Related Quality of Life after ventral hernia repair with biologic and synthetic mesh. Ann. Plast. Surg.82, S332–S338 (2019). [DOI] [PubMed] [Google Scholar]
- 29.Musella, M., Guido, A. & Musella, S. Collagen tampons as aminoglycoside carriers to reduce postoperative infection rate in prosthetic repair of groin hernias. Eur. J. Surg.167, 130–132 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Praveen, S. & Rohaizak, M. Local antibiotics are equivalent to intravenous antibiotics in the prevention of superficial wound infection in inguinal hernioplasty. Asian J. Surg.32, 59–63 (2009). [DOI] [PubMed] [Google Scholar]
- 31.Junge, K. et al. Improved collagen type I/III ratio at the interface of gentamicin-supplemented polyvinylidenfluoride mesh materials. Langenbecks Arch. Surg.392, 465–471 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Klink, C. D. et al. In vitro and in vivo characteristics of gentamicin-supplemented polyvinylidenfluoride mesh materials. J. Biomed. Mater. Res. A. 100, 1195–1202 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Schulz, K. F., Altman, D. G., Moher, D. & CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ340, c332 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American Society of Anesthesiologists. ASA Physical Status Classification System. (2021). https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system
- 35.Muysoms, F. E. et al. Classification of primary and incisional abdominal wall hernias. Hernia13, 407–414 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guillaumes, S. & O’Callaghan, C. A. [Spanish adaptation of the free OxMaR software for minimization and randomization of clinical studies]. Gac Sanit.33, 395–397 (2019). [DOI] [PubMed] [Google Scholar]
- 37.CatSalut. Programa de prevenció de les infeccions quirúrgiques a Catalunya PREVINQ-CAT. https://catsalut.gencat.cat/ca/proveidors-professionals/vincat/prevencio-infeccio/metodologia-resultats/objectiu-3/previnq-cat/
- 38.Dindo, D., Demartines, N. & Clavien, P. A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg.240, 205–213 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Itani, K. M. F. et al. Comparison of laparoscopic and open repair with mesh for the treatment of ventral incisional hernia: A randomized trial. Arch. Surg.145, 322–328 (2010). discussion 328. [DOI] [PubMed] [Google Scholar]
- 40.Rogmark, P. et al. Short-term outcomes for open and laparoscopic midline incisional hernia repair: A randomized multicenter controlled trial: The ProLOVE (prospective randomized trial on open versus laparoscopic operation of ventral eventrations) trial. Ann. Surg.258, 37–45 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Weiner-Lastinger, L. M. et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect. Control Hosp. Epidemiol.41, 1–18 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luijendijk, R. W. et al. A comparison of suture repair with mesh repair for incisional hernia. N Engl. J. Med.343, 392–398 (2000). [DOI] [PubMed] [Google Scholar]
- 43.Dunne, J. R., Malone, D. L., Tracy, J. K. & Napolitano, L. M. Abdominal wall hernias: Risk factors for infection and resource utilization. J. Surg. Res.111, 78–84 (2003). [DOI] [PubMed] [Google Scholar]
- 44.Finan, K. R., Vick, C. C., Kiefe, C. I., Neumayer, L. & Hawn, M. T. Predictors of wound infection in ventral hernia repair. Am. J. Surg.190, 676–681 (2005). [DOI] [PubMed] [Google Scholar]
- 45.White, T. J., Santos, M. C. & Thompson, J. S. Factors affecting wound complications in repair of ventral hernias. Am. Surg.64, 276–280 (1998). [PubMed] [Google Scholar]
- 46.Grant, A. M. & EU Hernia Trialists Collaboration. Open mesh versus non-mesh repair of groin hernia: Meta-analysis of randomised trials based on individual patient data [corrected]. Hernia6, 130–136 (2002). [DOI] [PubMed] [Google Scholar]
- 47.Iqbal, C. W. et al. Long-term outcome of 254 complex incisional hernia repairs using the modified Rives-Stoppa technique. World J. Surg.31, 2398–2404 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Cherla, D. V. et al. Decreasing surgical site infections after ventral hernia repair: A quality-improvement initiative. Surg. Infect. (Larchmt). 18, 780–786 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allegranzi, B. et al. New WHO recommendations on preoperative measures for surgical site infection prevention: An evidence-based global perspective. Lancet Infect. Dis.16, e276–e287 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Tong, Q. J. et al. A systematic review and meta-analysis on the use of prophylactic topical antibiotics for the prevention of uncomplicated wound infections. Infect. Drug Resist.11, 417–425 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huiras, P., Logan, J. K., Papadopoulos, S. & Whitney, D. Local antimicrobial administration for prophylaxis of surgical site infections. Pharmacotherapy32, 1006–1019 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Bontekoning, N. et al. Topical antimicrobial treatment of mesh for the reduction of surgical site infections after hernia repair: A systematic review and meta-analysis. Hernia28, 691–700 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McHugh, S. M., Collins, C. J., Corrigan, M. A., Hill, A. D. K. & Humphreys, H. The role of topical antibiotics used as prophylaxis in surgical site infection prevention. J. Antimicrob. Chemother.66, 693–701 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Sadava, E. E., Krpata, D. M., Gao, Y., Novitsky, Y. W. & Rosen, M. J. Does presoaking synthetic mesh in antibiotic solution reduce mesh infections? An experimental study. J. Gastrointest. Surg.17, 562–568 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Al-Shehri, M. Y. et al. Topical ampicillin for prophylaxis against wound infection in acute appendicitis. Ann. Saudi Med.14, 233–236 (1994). [DOI] [PubMed] [Google Scholar]
- 56.Friberg, O. et al. Antibiotic concentrations in serum and wound fluid after local gentamicin or intravenous dicloxacillin prophylaxis in cardiac surgery. Scand. J. Infect. Dis.35, 251–254 (2003). [DOI] [PubMed] [Google Scholar]
- 57.Binnebösel, M. et al. Impact of gentamicin-supplemented polyvinylidenfluoride mesh materials on MMP-2 expression and tissue integration in a transgenic mice model. Langenbecks Arch. Surg.395, 413–420 (2010). [DOI] [PubMed] [Google Scholar]
- 58.Suárez-Grau, J. M. et al. Antibiotic embedded absorbable prosthesis for prevention of surgical mesh infection: Experimental study in rats. Hernia19, 187–194 (2015). [DOI] [PubMed] [Google Scholar]
- 59.Deysine, M. Infection control in a hernia clinic: 24 year results of aseptic and antiseptic measure implementation in 4620 ‘clean cases’. Hernia10, 25–29 (2006). [DOI] [PubMed] [Google Scholar]
- 60.Daood, M. et al. Comparison of topical antibiotics plus intravenous antibiotics Versus intravenous antibiotics alone in prevention of surgical site infection in inguinal hernia repair: Topical vs. intravenous antibiotics in preventing SSIs in Hernia Repair. J. Health Rehabilitation Res.4, 1–6 (2024). [Google Scholar]
- 61.Schimmer, C. et al. Gentamicin-collagen sponge reduces sternal wound complications after heart surgery: A controlled, prospectively randomized, double-blind study. J. Thorac. Cardiovasc. Surg.143, 194–200 (2012). [DOI] [PubMed] [Google Scholar]
- 62.Guzmán, V., Gomez, G., Guerrero, T. S., Lluck, M. C. & Delgado, F. J. Effectiveness of collagen-gentamicin implant for treatment of ‘dirty’ abdominal wounds. World J. Surg.23, 123–126 (1999). discussion 126-7. [DOI] [PubMed] [Google Scholar]
- 63.Bennett-Guerrero, E. et al. Effect of an implantable gentamicin-collagen sponge on sternal wound infections following cardiac surgery: A randomized trial. JAMA304, 755–762 (2010). [DOI] [PubMed] [Google Scholar]
- 64.Emile, S. H., Elfallal, A. H., Abdel-Razik, M. A., El-Said, M. & Elshobaky, A. A randomized controlled trial on irrigation of open appendectomy wound with gentamicin- saline solution versus saline solution for prevention of surgical site infection. Int. J. Surg.81, 140–146 (2020). [DOI] [PubMed] [Google Scholar]
- 65.Bennett-Guerrero, E. et al. Gentamicin-collagen sponge for infection prophylaxis in colorectal surgery. N Engl. J. Med.363, 1038–1049 (2010). [DOI] [PubMed] [Google Scholar]
- 66.Tubre, D. J., Schroeder, A. D., Estes, J., Eisenga, J. & Fitzgibbons, R. J. Surgical site infection: The ‘Achilles Heel’ of all types of abdominal wall hernia reconstruction. Hernia22, 1003–1013 (2018). [DOI] [PubMed] [Google Scholar]
- 67.Page, C. P. et al. Antimicrobial prophylaxis for surgical wounds. Guidelines for clinical care. Arch. Surg.128, 79–88 (1993). [DOI] [PubMed] [Google Scholar]
- 68.Shlaes, D. M. et al. Society for healthcare epidemiology of america and infectious diseases society of America joint committee on the prevention of antimicrobial resistance: Guidelines for the prevention of antimicrobial resistance in hospitals. Clin. Infect. Dis.25, 584–599 (1997). [DOI] [PubMed] [Google Scholar]
- 69.Moore, R. D., Lietman, P. S. & Smith, C. R. Clinical response to aminoglycoside therapy: Importance of the ratio of peak concentration to minimal inhibitory concentration. J. Infect. Dis.155, 93–99 (1987). [DOI] [PubMed] [Google Scholar]
- 70.Bruce, J., Russell, E. M., Mollison, J. & Krukowski, Z. H. The quality of measurement of surgical wound infection as the basis for monitoring: A systematic review. J. Hosp. Infect.49, 99–108 (2001). [DOI] [PubMed] [Google Scholar]
- 71.Gignoux, B. et al. Incidence and risk factors for incisional hernia and recurrence: Retrospective analysis of the French national database. Colorectal Dis.23, 1515–1523 (2021). [DOI] [PubMed] [Google Scholar]
- 72.Ruiz-Tovar, J. et al. Effect of gentamicin lavage of the axillary surgical bed after lymph node dissection on drainage discharge volume. Breast22, 874–878 (2013). [DOI] [PubMed] [Google Scholar]
- 73.Binnebösel, M. et al. Intraperitoneally applied gentamicin increases collagen content and mechanical stability of colon anastomosis in rats. Int. J. Colorectal Dis.24, 433–440 (2009). [DOI] [PubMed] [Google Scholar]
- 74.Vaneerdeweg, W., Hendriks, J. M., Lauwers, P. R., Ieven, M. & Eyskens, E. J. Effect of gentamicin-containing sponges on the healing of colonic anastomoses in a rat model of peritonitis. Eur. J. Surg.166, 959–962 (2000). [DOI] [PubMed] [Google Scholar]
- 75.Gette, M. T., Marks, J. G. & Maloney, M. E. Frequency of postoperative allergic contact dermatitis to topical antibiotics. Arch. Dermatol.128, 365–367 (1992). [PubMed] [Google Scholar]
- 76.Damm, S. Intraoperative anaphylaxis associated with bacitracin irrigation. Am. J. Health Syst. Pharm.68, 323–327 (2011). [DOI] [PubMed] [Google Scholar]
- 77.Gehrig, K. A. & Warshaw, E. M. Allergic contact dermatitis to topical antibiotics: Epidemiology, responsible allergens, and management. J. Am. Acad. Dermatol.58, 1–21 (2008). [DOI] [PubMed] [Google Scholar]
- 78.Kokotovic, D., Bisgaard, T. & Helgstrand, F. Long-term recurrence and complications associated with elective incisional hernia repair. JAMA316, 1575–1582 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.