Abstract
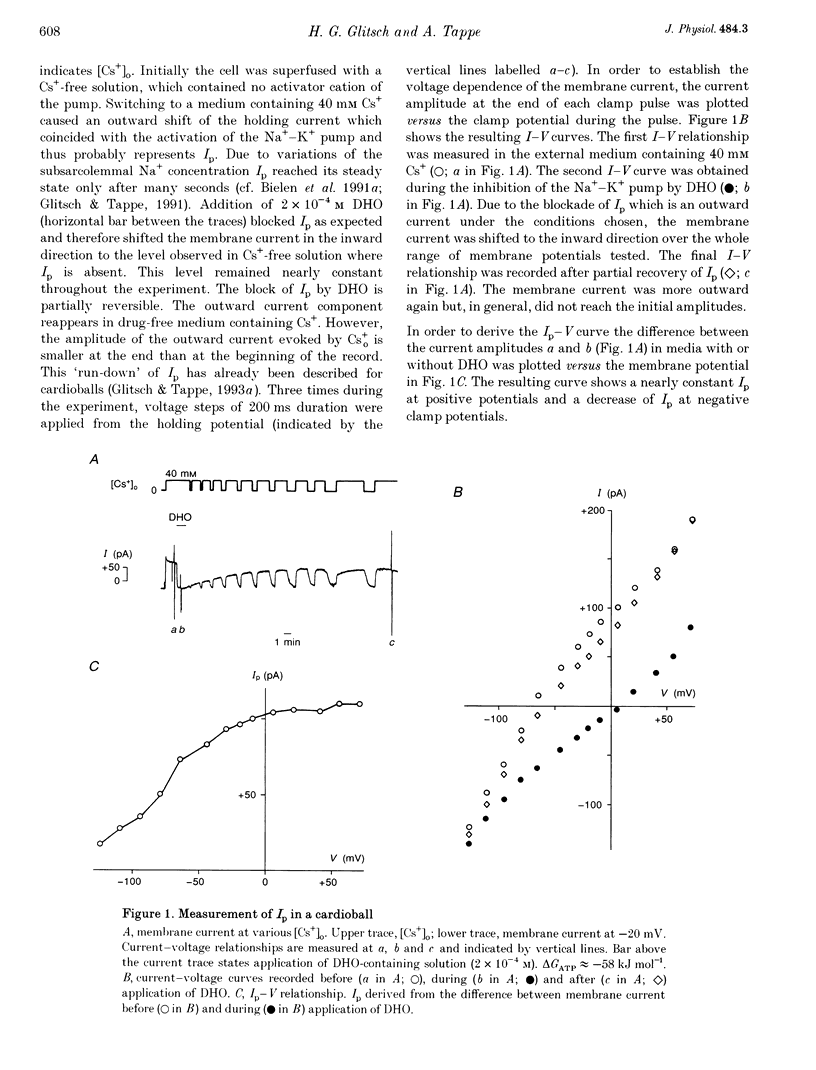
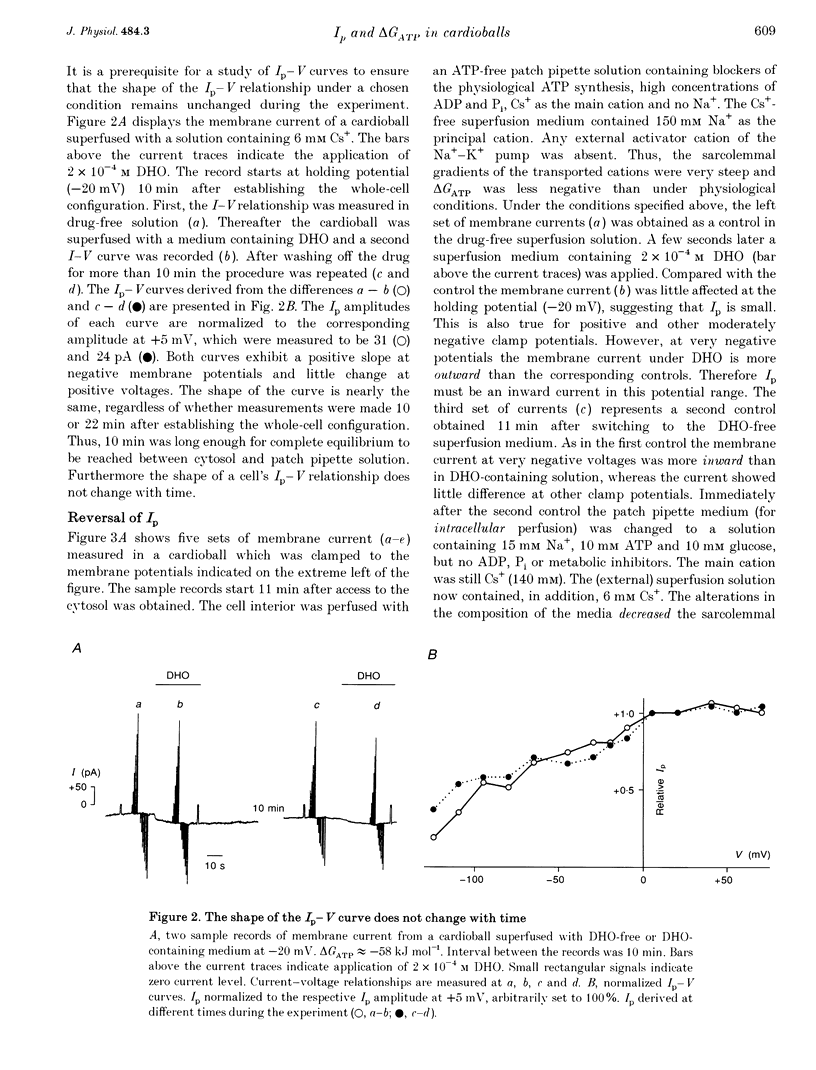
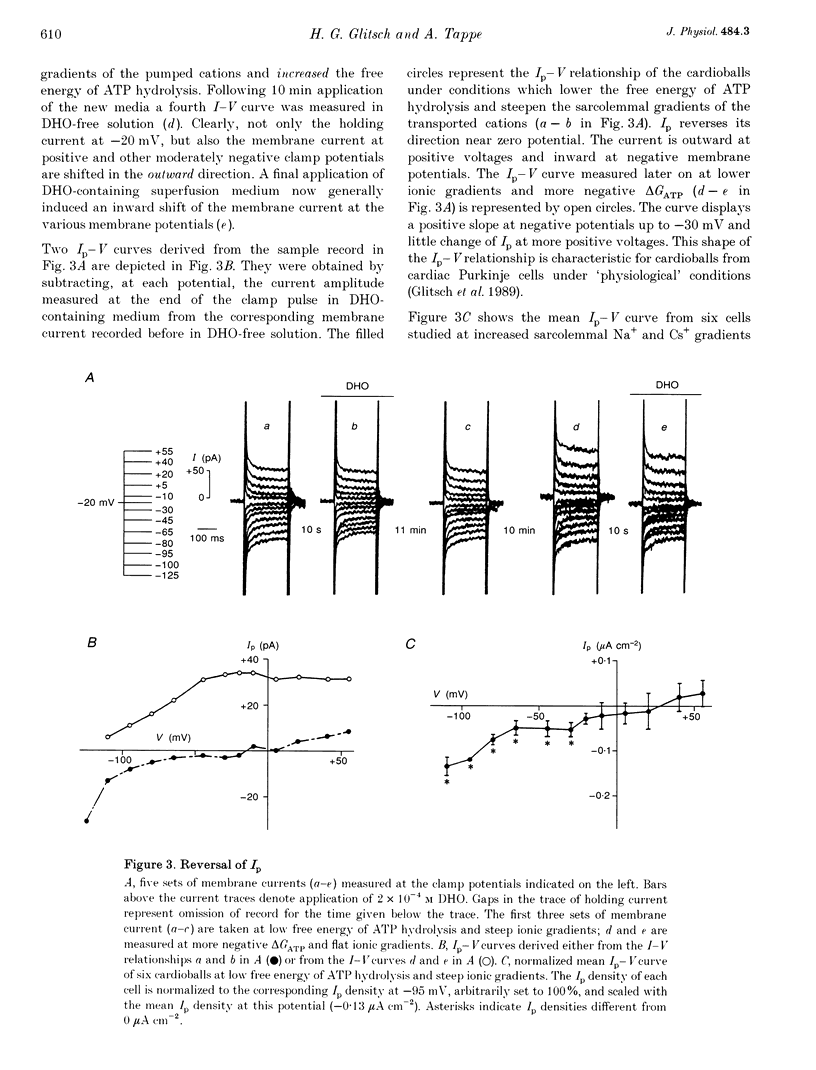
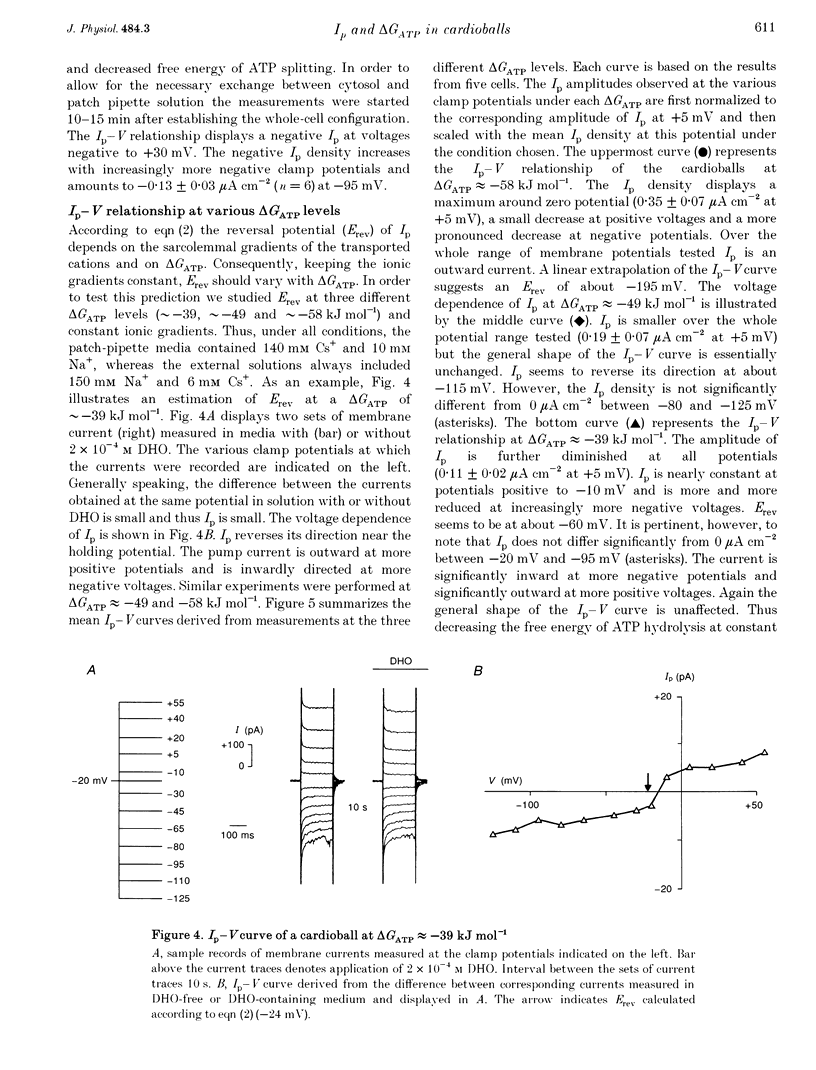
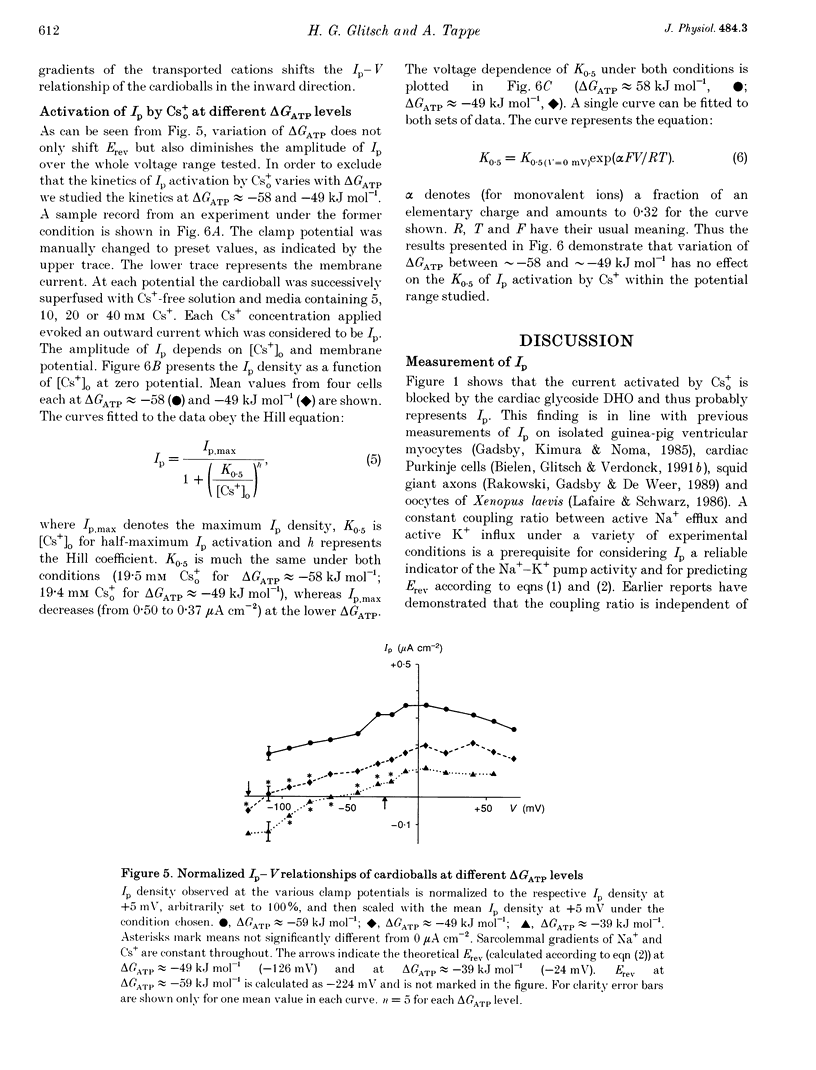
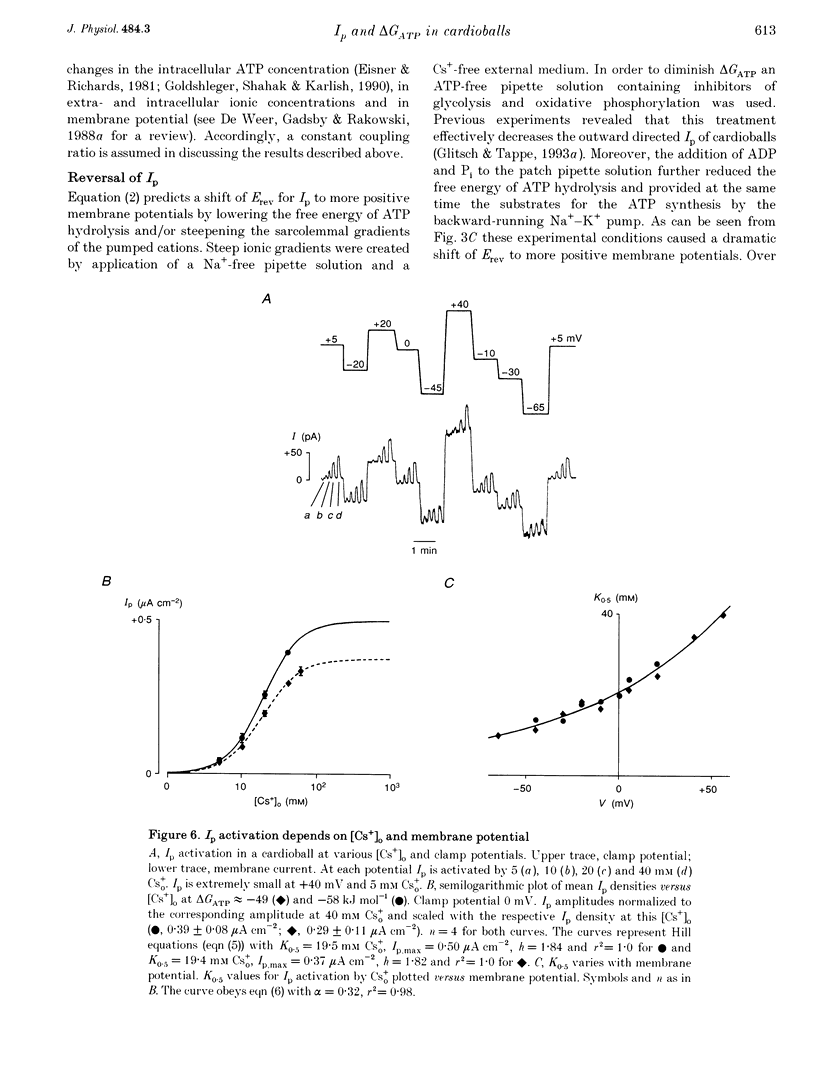
1. The Na(+)-K+ pump current, Ip, of cardioballs from isolated sheep cardiac Purkinje cells was measured at 30-34 degrees C by means of whole-cell recording. 2. Under physiological conditions Ip is an outward current. Experimental conditions which cause a less negative free energy of intracellular ATP hydrolysis (delta GATP) and steeper sarcolemmal gradients for the pumped Na+ and Cs+ ions evoked an Ip in the inward direction over a wide range of membrane potentials. The reversal of the Ip direction was reversible. 3. The inwardly directed Ip increased with increasingly negative membrane potentials and amounted to -0.13 +/- 0.03 microA cm-2 (mean +/- S.E.M.; n = 6) at -95 mV. 4. The reversal potential (Erev) of Ip was studied as a function of delta GATP at constant sarcolemmal gradients of the pumped cations. 5. In order to vary delta GATP the cell interior was dialysed with patch pipette solutions containing 10 mM ATP and different concentrations of ADP and inorganic phosphate. The media were composed to produce delta GATP levels of about -58, -49 and -39 kJ mol-1. 6. A less negative delta GATP shifted Erev to more positive membrane potentials. From measurements of Ip as a function of membrane potential Erev was estimated to be -195, -115 and -60 mV at delta GATP levels of approximately -58, -49 and -39 kJ mol-1, respectively. The calculated Erev amounted to -224 mV at delta GATP approximately -58 kJ mol-1, -126 mV at delta GATP approximately 49 kJ mol-1 and -24 mV at delta GATP approximately -39 kJ mol-1. 7. Possible reasons for the discrepancy between estimated and calculated Erev values are discussed. 8. Shifting delta GATP to less negative values not only altered Erev but also diminished Ip at each membrane potential tested. The maximal Ip (Ip,max), which can be activated by external Cs+ (Cs+o), decreased under these conditions, whereas [Cs+]o causing half-maximal Ip activation remained unchanged. Similarly, the voltage dependence of Ip activation by Cs+o was unaffected. 9. It is concluded that Erev of Ip varies with delta GATP at constant sarcolemmal gradients of the pumped cations. This agrees with thermodynamic considerations.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apell H. J., Nelson M. T., Marcus M. M., Läuger P. Effects of the ATP, ADP and inorganic phosphate on the transport rate of the Na+,K+-pump. Biochim Biophys Acta. 1986 May 9;857(1):105–115. doi: 10.1016/0005-2736(86)90103-3. [DOI] [PubMed] [Google Scholar]
- Bahinski A., Nakao M., Gadsby D. C. Potassium translocation by the Na+/K+ pump is voltage insensitive. Proc Natl Acad Sci U S A. 1988 May;85(10):3412–3416. doi: 10.1073/pnas.85.10.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechem M., Pott L., Rennebaum H. Atrial muscle cells from hearts of adult guinea-pigs in culture: a new preparation for cardiac cellular electrophysiology. Eur J Cell Biol. 1983 Sep;31(2):366–369. [PubMed] [Google Scholar]
- Bielen F. V., Glitsch H. G., Verdonck F. Changes of the subsarcolemmal Na+ concentration in internally perfused cardiac cells. Biochim Biophys Acta. 1991 Jun 18;1065(2):269–271. doi: 10.1016/0005-2736(91)90239-5. [DOI] [PubMed] [Google Scholar]
- Bielen F. V., Glitsch H. G., Verdonck F. Dependence of Na+ pump current on external monovalent cations and membrane potential in rabbit cardiac Purkinje cells. J Physiol. 1991 Oct;442:169–189. doi: 10.1113/jphysiol.1991.sp018788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielen F. V., Glitsch H. G., Verdonck F. Na+ pump current-voltage relationships of rabbit cardiac Purkinje cells in Na(+)-free solution. J Physiol. 1993 Jun;465:699–714. doi: 10.1113/jphysiol.1993.sp019701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. B., Johnson E. A., Kootsey J. M. Electrical and biochemical properties of an enzyme model of the sodium pump. J Membr Biol. 1983;74(2):139–153. doi: 10.1007/BF01870503. [DOI] [PubMed] [Google Scholar]
- Chmouliovsky M., Straub R. W. Increase in ATP by reversal of the Na-K-pump in mammalian non-myelinated nerve fibres. Pflugers Arch. 1974;350(4):309–320. doi: 10.1007/BF00592639. [DOI] [PubMed] [Google Scholar]
- De Weer P., Rakowski R. F. Current generated by backward-running electrogenic Na pump in squid giant axons. 1984 May 31-Jun 6Nature. 309(5967):450–452. doi: 10.1038/309450a0. [DOI] [PubMed] [Google Scholar]
- Efthymiadis A., Schwarz W. Conditions for a backward-running Na+/K+ pump in Xenopus oocytes. Biochim Biophys Acta. 1991 Sep 10;1068(1):73–76. doi: 10.1016/0005-2736(91)90062-d. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Richards D. E. The interaction of potassium ions and ATP on the sodium pump of resealed red cell ghosts. J Physiol. 1981;319:403–418. doi: 10.1113/jphysiol.1981.sp013917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Gadsby D. C., Kimura J., Noma A. Voltage dependence of Na/K pump current in isolated heart cells. Nature. 1985 May 2;315(6014):63–65. doi: 10.1038/315063a0. [DOI] [PubMed] [Google Scholar]
- Gadsby D. C., Rakowski R. F., De Weer P. Extracellular access to the Na,K pump: pathway similar to ion channel. Science. 1993 Apr 2;260(5104):100–103. doi: 10.1126/science.7682009. [DOI] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. Driving the sodium pump backwards to form adenosine triphosphate. Nature. 1966 Sep 24;211(5056):1414–1415. doi: 10.1038/2111414a0. [DOI] [PubMed] [Google Scholar]
- Garrahan P. J., Glynn I. M. The incorporation of inorganic phosphate into adenosine triphosphate by reversal of the sodium pump. J Physiol. 1967 Sep;192(1):237–256. doi: 10.1113/jphysiol.1967.sp008298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glitsch H. G., Krahn T., Pusch H. The dependence of sodium pump current on internal Na concentration and membrane potential in cardioballs from sheep Purkinje fibres. Pflugers Arch. 1989 May;414(1):52–58. doi: 10.1007/BF00585626. [DOI] [PubMed] [Google Scholar]
- Glitsch H. G., Tappe A. The Na+/K+ pump of cardiac Purkinje cells is preferentially fuelled by glycolytic ATP production. Pflugers Arch. 1993 Jan;422(4):380–385. doi: 10.1007/BF00374294. [DOI] [PubMed] [Google Scholar]
- Goldshleger R., Shahak Y., Karlish S. J. Electrogenic and electroneutral transport modes of renal Na/K ATPase reconstituted into proteoliposomes. J Membr Biol. 1990 Feb;113(2):139–154. doi: 10.1007/BF01872888. [DOI] [PubMed] [Google Scholar]
- Guynn R. W., Veech R. L. The equilibrium constants of the adenosine triphosphate hydrolysis and the adenosine triphosphate-citrate lyase reactions. J Biol Chem. 1973 Oct 25;248(20):6966–6972. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kammermeier H., Schmidt P., Jüngling E. Free energy change of ATP-hydrolysis: a causal factor of early hypoxic failure of the myocardium? J Mol Cell Cardiol. 1982 May;14(5):267–277. doi: 10.1016/0022-2828(82)90205-x. [DOI] [PubMed] [Google Scholar]
- Kennedy B. G., Lunn G., Hoffman J. F. Effects of altering the ATP/ADP ratio on pump-mediated Na/K and Na/Na exchanges in resealed human red blood cell ghosts. J Gen Physiol. 1986 Jan;87(1):47–72. doi: 10.1085/jgp.87.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafaire A. V., Schwarz W. Voltage dependence of the rheogenic Na+/K+ ATPase in the membrane of oocytes of Xenopus laevis. J Membr Biol. 1986;91(1):43–51. doi: 10.1007/BF01870213. [DOI] [PubMed] [Google Scholar]
- Lant A. F., Whittam R. The influence of ions on the labelling of adenosine triphosphate in red cell ghosts. J Physiol. 1968 Dec;199(2):457–484. doi: 10.1113/jphysiol.1968.sp008663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew V. L., Glynn I. M., Ellory J. C. Net synthesis of ATP by reversal of the sodium pump. Nature. 1970 Feb 28;225(5235):865–866. doi: 10.1038/225865a0. [DOI] [PubMed] [Google Scholar]
- Omay H. S., Schwarz W. Voltage-dependent stimulation of Na+/K(+)-pump current by external cations: selectivity of different K+ congeners. Biochim Biophys Acta. 1992 Feb 17;1104(1):167–173. doi: 10.1016/0005-2736(92)90146-d. [DOI] [PubMed] [Google Scholar]
- Rakowski R. F., Gadsby D. C., De Weer P. Stoichiometry and voltage dependence of the sodium pump in voltage-clamped, internally dialyzed squid giant axon. J Gen Physiol. 1989 May;93(5):903–941. doi: 10.1085/jgp.93.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowski R. F., Vasilets L. A., LaTona J., Schwarz W. A negative slope in the current-voltage relationship of the Na+/K+ pump in Xenopus oocytes produced by reduction of external [K+]. J Membr Biol. 1991 Apr;121(2):177–187. doi: 10.1007/BF01870531. [DOI] [PubMed] [Google Scholar]
- SCHATZMANN H. J. Herzglykoside als Hemmstoffe für den aktiven Kalium- und Natriumtransport durch die Erythrocytenmembran. Helv Physiol Pharmacol Acta. 1953;11(4):346–354. [PubMed] [Google Scholar]
- Soejima M., Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984 Apr;400(4):424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]


