Abstract
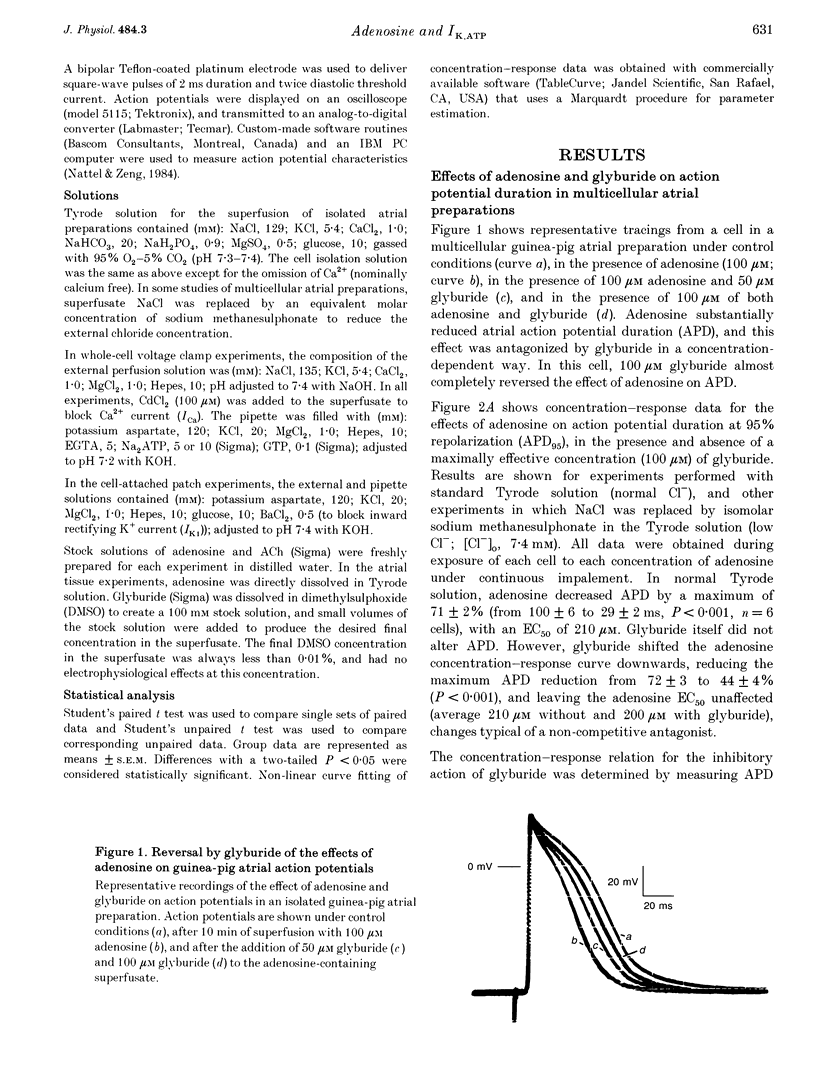
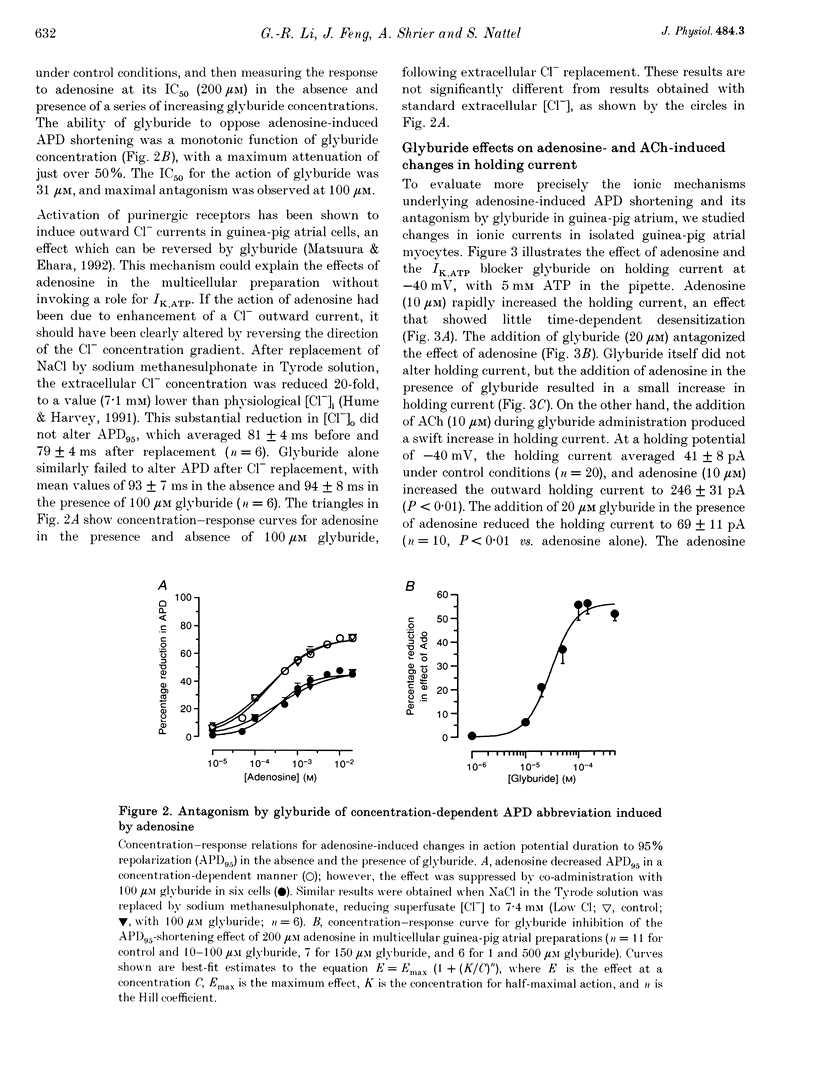
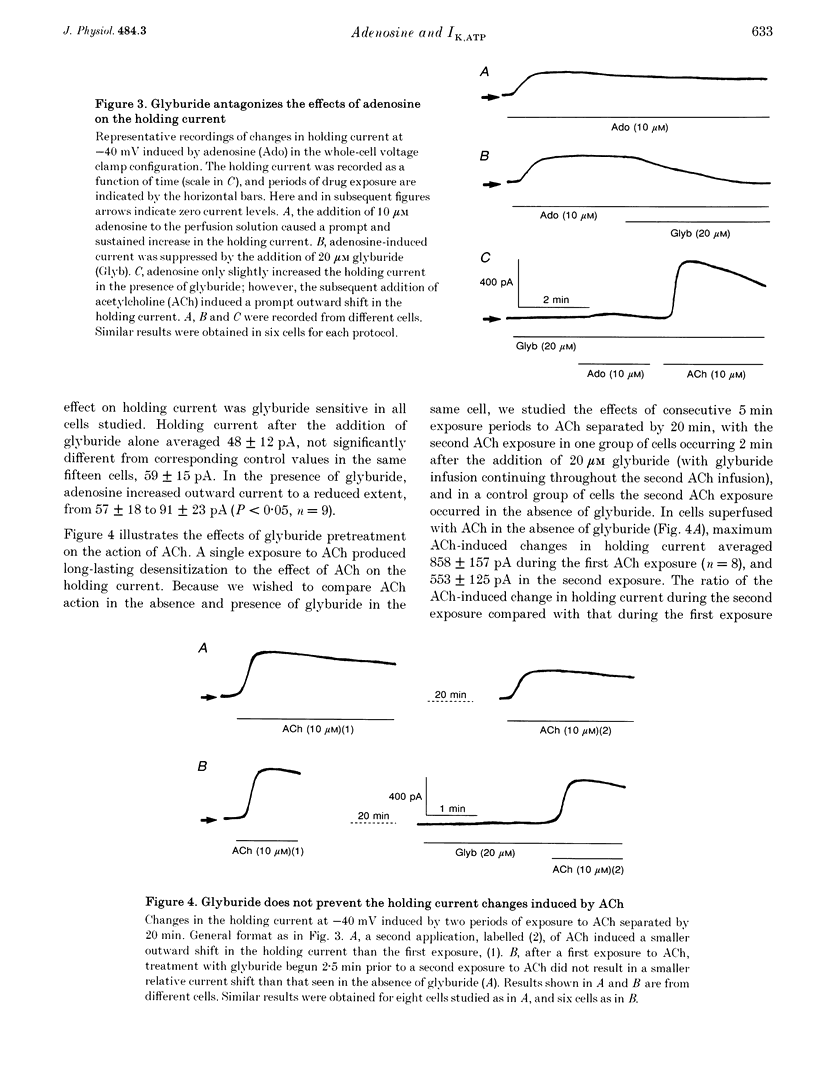
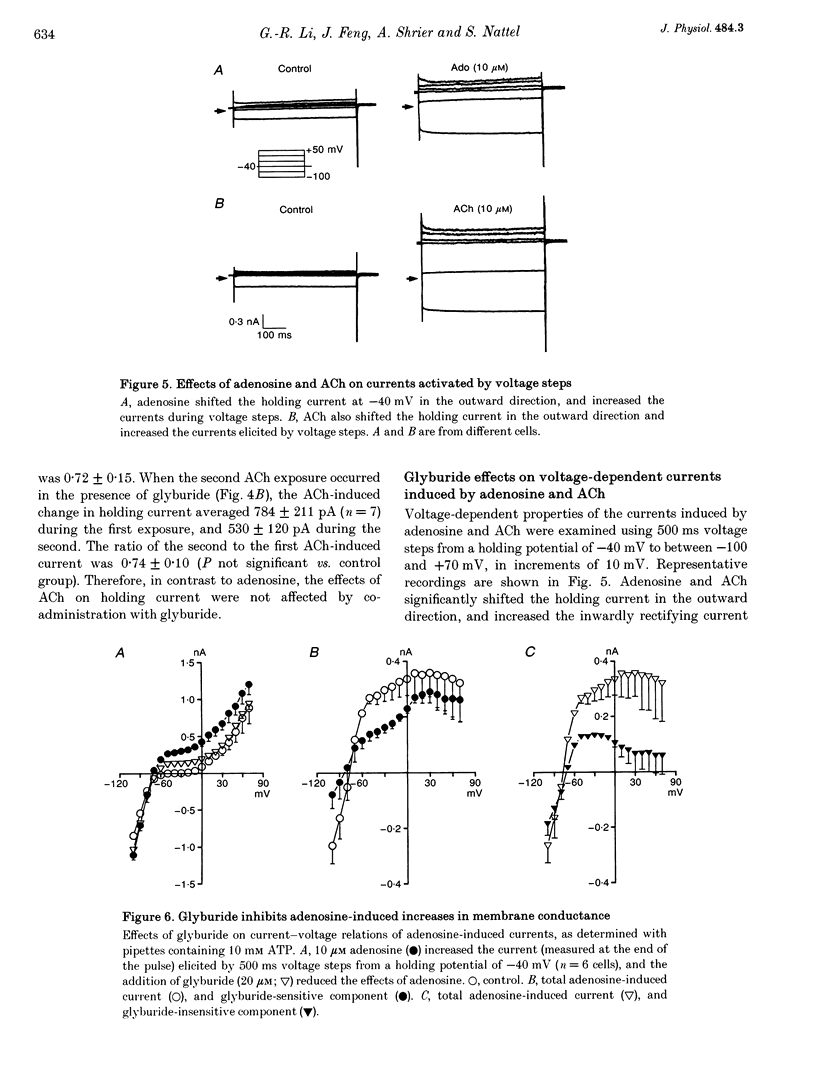
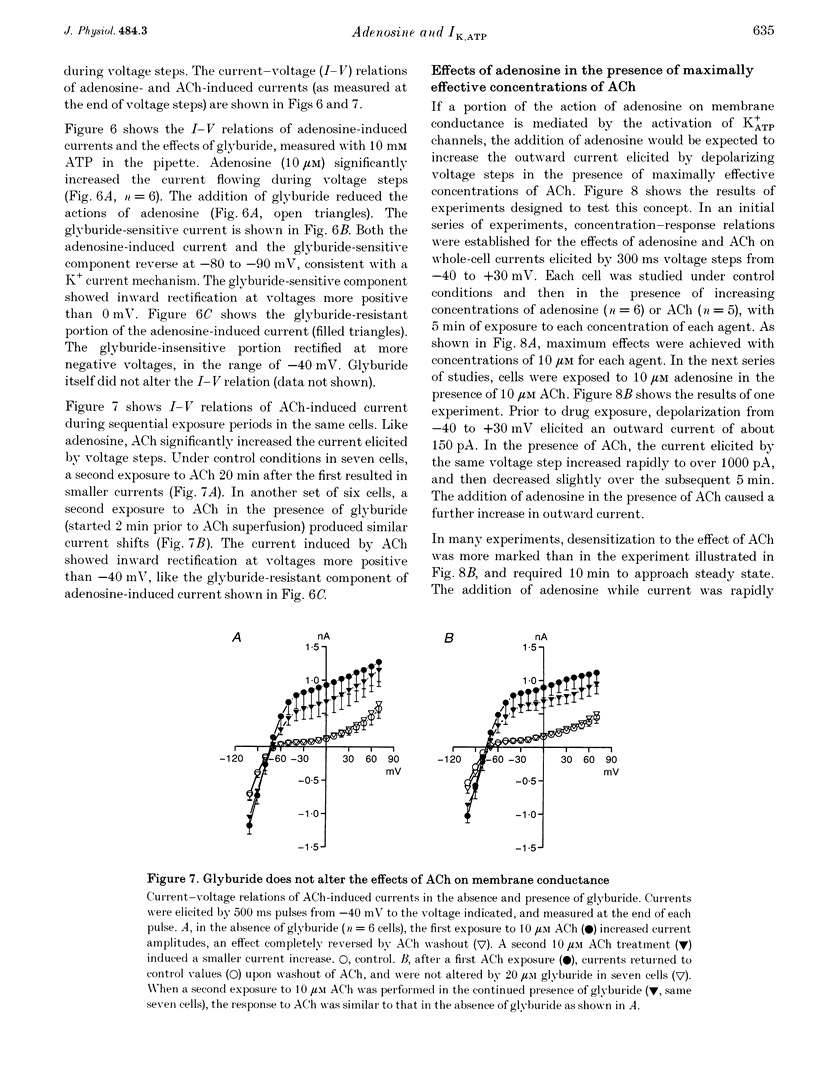
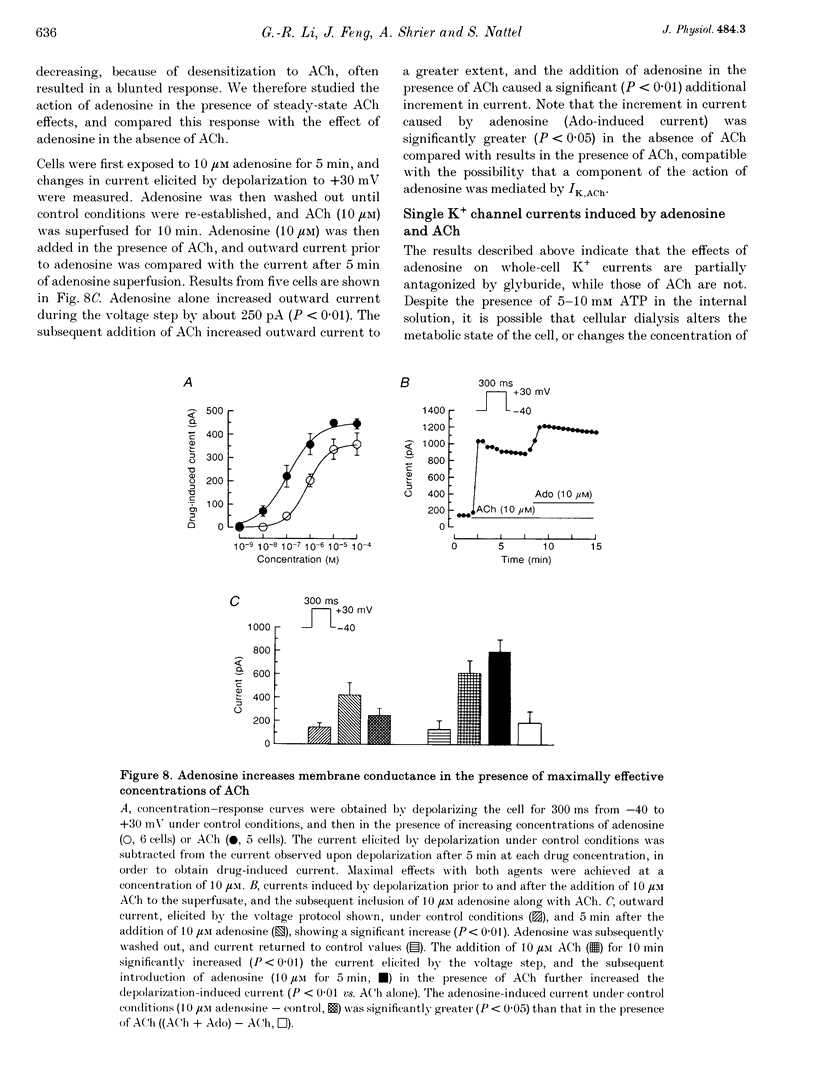
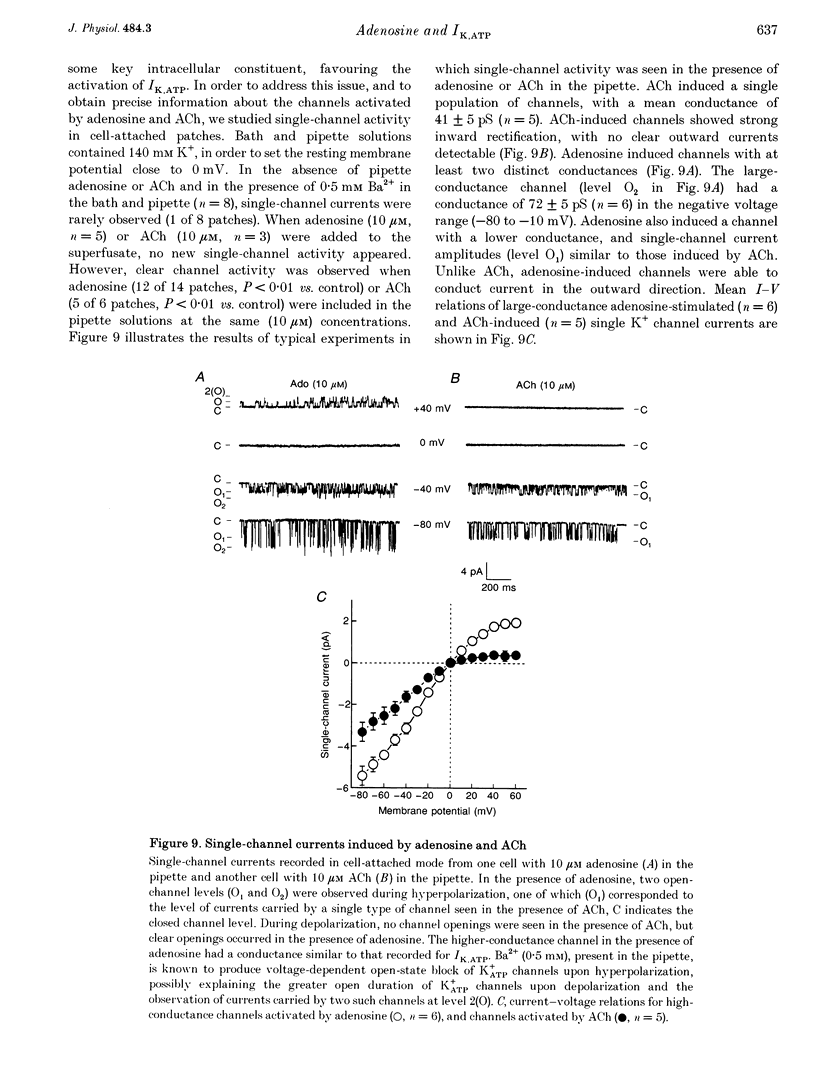
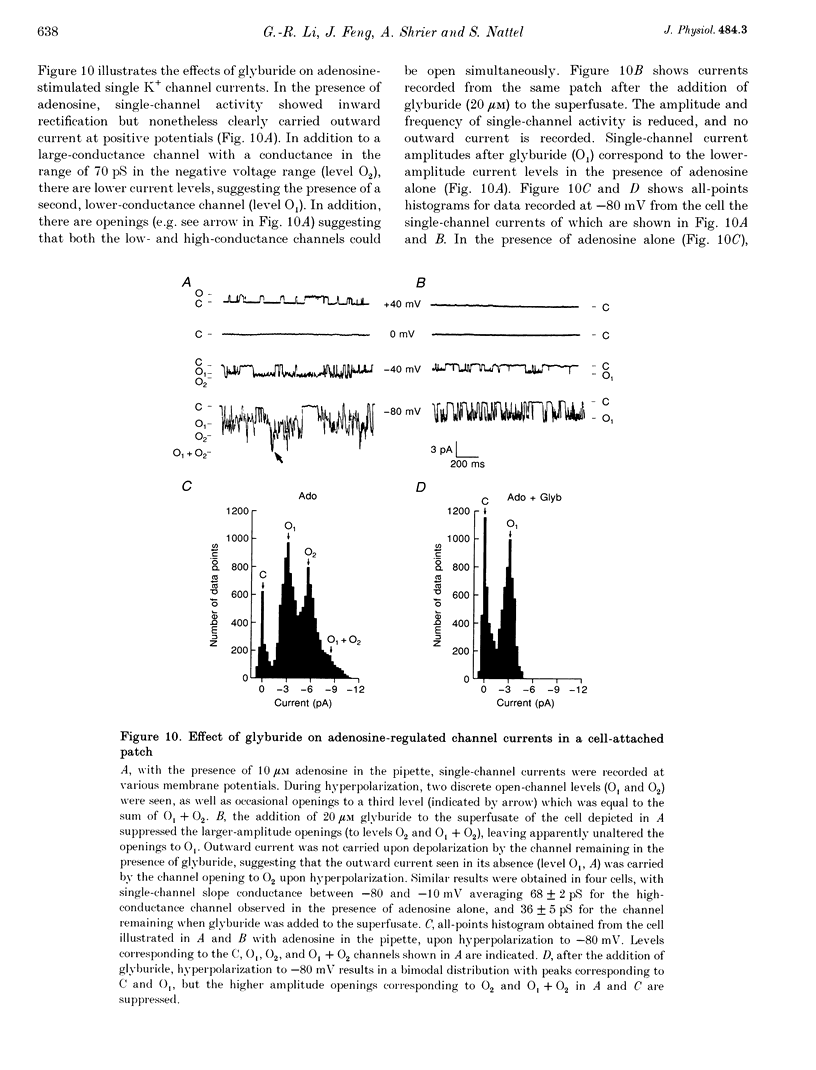
1. Adenosine caused dose-dependent action potential abbreviation in multicellular guinea-pig atrial preparations, an action antagonized by glyburide (IC50, 31 microM) in both physiological and low-chloride superfusate. 2. When 5 mM ATP was included in pipettes for whole-cell voltage clamp of isolated guinea-pig atrial myocytes, adenosine (10 microM) increased the holding current at -40 mV from 41 +/- 8 to 246 +/- 31 pA (mean +/- S.E.M., P < 0.01), and glyburide (20 microM) returned the holding current to 69 +/- 11 pA (P < 0.01 vs. adenosine alone). Acetylcholine (10 microM) also increased the holding current, but its effects were not altered by glyburide. 3. Both adenosine and acetylcholine induced an additional current component in response to 500 ms voltage steps. Glyburide partially inhibited the adenosine-induced current, but did not alter the effect of acetylcholine. In the presence of maximally effective acetylcholine concentrations, adenosine increased membrane conductance (P < 0.01), although to a lesser extent than in the absence of acetylcholine. 4. Single K+ channel activity was seen in only one of eight cell-attached patches in the absence of adenosine or acetylcholine (0.5 mM Ba2+ in bath and pipette solutions). With acetylcholine (10 microM) in the pipette, inwardly rectifying channels (conductance, 41 +/- 5 pS) were seen in five of six patches. With adenosine (10 microM) in the pipette, single-channel activity was seen in twelve of fourteen patches with two populations of channels, one similar to that induced by acetylcholine and another higher-conductance channel (72 +/- 5 pS) that showed less inward rectification. Glyburide (20 microM) suppressed the high-conductance channel (68 +/- 2 pS) leaving a single channel type with a conductance of 36 +/- 5 pS and strong inward rectification. 5. We conclude that K+ATP channels contribute to the electrophysiological actions of adenosine on guinea-pig atrium in the presence of physiological intracellular ATP levels, and may therefore play a role in the cardiac electrophysiological effects of adenosine in the absence of myocardial ischaemia.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNE R. M. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am J Physiol. 1963 Feb;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- Bailey J. C., Watanabe A. M., Besch H. R., Jr, Lathrop D. A. Acetylcholine antagonism of the electrophysiological effects of isoproterenol on canine cardiac Purkinje fibers. Circ Res. 1979 Mar;44(3):378–383. doi: 10.1161/01.res.44.3.378. [DOI] [PubMed] [Google Scholar]
- Belardinelli L., Fenton R. A., West A., Linden J., Althaus J. S., Berne R. M. Extracellular action of adenosine and the antagonism by aminophylline on the atrioventricular conduction of isolated perfused guinea pig and rat hearts. Circ Res. 1982 Nov;51(5):569–579. doi: 10.1161/01.res.51.5.569. [DOI] [PubMed] [Google Scholar]
- Belardinelli L., Giles W. R., West A. Ionic mechanisms of adenosine actions in pacemaker cells from rabbit heart. J Physiol. 1988 Nov;405:615–633. doi: 10.1113/jphysiol.1988.sp017352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belardinelli L., Isenberg G. Actions of adenosine and isoproterenol on isolated mammalian ventricular myocytes. Circ Res. 1983 Sep;53(3):287–297. doi: 10.1161/01.res.53.3.287. [DOI] [PubMed] [Google Scholar]
- Belardinelli L., Isenberg G. Isolated atrial myocytes: adenosine and acetylcholine increase potassium conductance. Am J Physiol. 1983 May;244(5):H734–H737. doi: 10.1152/ajpheart.1983.244.5.H734. [DOI] [PubMed] [Google Scholar]
- Belardinelli L., Linden J., Berne R. M. The cardiac effects of adenosine. Prog Cardiovasc Dis. 1989 Jul-Aug;32(1):73–97. doi: 10.1016/0033-0620(89)90015-7. [DOI] [PubMed] [Google Scholar]
- Dart C., Standen N. B. Adenosine-activated potassium current in smooth muscle cells isolated from the pig coronary artery. J Physiol. 1993 Nov;471:767–786. doi: 10.1113/jphysiol.1993.sp019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J., Maier-Rudolph W., von Beckerath N., Mehrke G., Günther K., Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990 Mar 16;247(4948):1341–1344. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- Drury A. N., Szent-Györgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929 Nov 25;68(3):213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely S. W., Mentzer R. M., Jr, Lasley R. D., Lee B. K., Berne R. M. Functional and metabolic evidence of enhanced myocardial tolerance to ischemia and reperfusion with adenosine. J Thorac Cardiovasc Surg. 1985 Oct;90(4):549–556. [PubMed] [Google Scholar]
- Fan Z., Makielski J. C. Intracellular H+ and Ca2+ modulation of trypsin-modified ATP-sensitive K+ channels in rabbit ventricular myocytes. Circ Res. 1993 Mar;72(3):715–722. doi: 10.1161/01.res.72.3.715. [DOI] [PubMed] [Google Scholar]
- Froldi G., Belardinelli L. Species-dependent effects of adenosine on heart rate and atrioventricular nodal conduction. Mechanism and physiological implications. Circ Res. 1990 Oct;67(4):960–978. doi: 10.1161/01.res.67.4.960. [DOI] [PubMed] [Google Scholar]
- Gross G. J., Auchampach J. A. Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ Res. 1992 Feb;70(2):223–233. doi: 10.1161/01.res.70.2.223. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Adenosine receptors in frog sinus venosus: slow inhibitory potentials produced by adenine compounds and acetylcholine. J Physiol. 1979 Aug;293:23–49. doi: 10.1113/jphysiol.1979.sp012877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homeister J. W., Hoff P. T., Fletcher D. D., Lucchesi B. R. Combined adenosine and lidocaine administration limits myocardial reperfusion injury. Circulation. 1990 Aug;82(2):595–608. doi: 10.1161/01.cir.82.2.595. [DOI] [PubMed] [Google Scholar]
- Hume J. R., Harvey R. D. Chloride conductance pathways in heart. Am J Physiol. 1991 Sep;261(3 Pt 1):C399–C412. doi: 10.1152/ajpcell.1991.261.3.C399. [DOI] [PubMed] [Google Scholar]
- Iijima T., Irisawa H., Kameyama M. Membrane currents and their modification by acetylcholine in isolated single atrial cells of the guinea-pig. J Physiol. 1985 Feb;359:485–501. doi: 10.1113/jphysiol.1985.sp015598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Tung R. T., Sugimoto T., Kobayashi I., Takahashi K., Katada T., Ui M., Kurachi Y. On the mechanism of G protein beta gamma subunit activation of the muscarinic K+ channel in guinea pig atrial cell membrane. Comparison with the ATP-sensitive K+ channel. J Gen Physiol. 1992 Jun;99(6):961–983. doi: 10.1085/jgp.99.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch G. E., Codina J., Birnbaumer L., Brown A. M. Coupling of ATP-sensitive K+ channels to A1 receptors by G proteins in rat ventricular myocytes. Am J Physiol. 1990 Sep;259(3 Pt 2):H820–H826. doi: 10.1152/ajpheart.1990.259.3.H820. [DOI] [PubMed] [Google Scholar]
- Kurachi Y., Nakajima T., Sugimoto T. On the mechanism of activation of muscarinic K+ channels by adenosine in isolated atrial cells: involvement of GTP-binding proteins. Pflugers Arch. 1986 Sep;407(3):264–274. doi: 10.1007/BF00585301. [DOI] [PubMed] [Google Scholar]
- Leipert B., Becker B. F., Gerlach E. Different endothelial mechanisms involved in coronary responses to known vasodilators. Am J Physiol. 1992 Jun;262(6 Pt 2):H1676–H1683. doi: 10.1152/ajpheart.1992.262.6.H1676. [DOI] [PubMed] [Google Scholar]
- Lerman B. B., Belardinelli L. Cardiac electrophysiology of adenosine. Basic and clinical concepts. Circulation. 1991 May;83(5):1499–1509. doi: 10.1161/01.cir.83.5.1499. [DOI] [PubMed] [Google Scholar]
- Liu G. S., Thornton J., Van Winkle D. M., Stanley A. W., Olsson R. A., Downey J. M. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991 Jul;84(1):350–356. doi: 10.1161/01.cir.84.1.350. [DOI] [PubMed] [Google Scholar]
- Matsuura H., Ehara T. Activation of chloride current by purinergic stimulation in guinea pig heart cells. Circ Res. 1992 Apr;70(4):851–855. doi: 10.1161/01.res.70.4.851. [DOI] [PubMed] [Google Scholar]
- Mauser M., Hoffmeister H. M., Nienaber C., Schaper W. Influence of ribose, adenosine, and "AICAR" on the rate of myocardial adenosine triphosphate synthesis during reperfusion after coronary artery occlusion in the dog. Circ Res. 1985 Feb;56(2):220–230. doi: 10.1161/01.res.56.2.220. [DOI] [PubMed] [Google Scholar]
- Misler S., Gillis K., Tabcharani J. Modulation of gating of a metabolically regulated, ATP-dependent K+ channel by intracellular pH in B cells of the pancreatic islet. J Membr Biol. 1989 Jul;109(2):135–143. doi: 10.1007/BF01870852. [DOI] [PubMed] [Google Scholar]
- Nattel S., Zeng F. D. Frequency-dependent effects of antiarrhythmic drugs on action potential duration and refractoriness of canine cardiac Purkinje fibers. J Pharmacol Exp Ther. 1984 Apr;229(1):283–291. [PubMed] [Google Scholar]
- Nichols C. G., Lederer W. J. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991 Dec;261(6 Pt 2):H1675–H1686. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Olafsson B., Forman M. B., Puett D. W., Pou A., Cates C. U., Friesinger G. C., Virmani R. Reduction of reperfusion injury in the canine preparation by intracoronary adenosine: importance of the endothelium and the no-reflow phenomenon. Circulation. 1987 Nov;76(5):1135–1145. doi: 10.1161/01.cir.76.5.1135. [DOI] [PubMed] [Google Scholar]
- Pelzer D., Trautwein W. Currents through ionic channels in multicellular cardiac tissue and single heart cells. Experientia. 1987 Dec 1;43(11-12):1153–1162. doi: 10.1007/BF01945515. [DOI] [PubMed] [Google Scholar]
- Quayle J. M., Standen N. B., Stanfield P. R. The voltage-dependent block of ATP-sensitive potassium channels of frog skeletal muscle by caesium and barium ions. J Physiol. 1988 Nov;405:677–697. doi: 10.1113/jphysiol.1988.sp017355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J., Baumann G., Gerlach E. Adenosine as inhibitor of myocardial effects of catecholamines. Pflugers Arch. 1977 Nov 25;372(1):29–35. doi: 10.1007/BF00582203. [DOI] [PubMed] [Google Scholar]
- Song Y., Thedford S., Lerman B. B., Belardinelli L. Adenosine-sensitive afterdepolarizations and triggered activity in guinea pig ventricular myocytes. Circ Res. 1992 Apr;70(4):743–753. doi: 10.1161/01.res.70.4.743. [DOI] [PubMed] [Google Scholar]
- Thornton J. D., Liu G. S., Olsson R. A., Downey J. M. Intravenous pretreatment with A1-selective adenosine analogues protects the heart against infarction. Circulation. 1992 Feb;85(2):659–665. doi: 10.1161/01.cir.85.2.659. [DOI] [PubMed] [Google Scholar]
- Trube G., Hescheler J. Inward-rectifying channels in isolated patches of the heart cell membrane: ATP-dependence and comparison with cell-attached patches. Pflugers Arch. 1984 Jun;401(2):178–184. doi: 10.1007/BF00583879. [DOI] [PubMed] [Google Scholar]
- Visentin S., Wu S. N., Belardinelli L. Adenosine-induced changes in atrial action potential: contribution of Ca and K currents. Am J Physiol. 1990 Apr;258(4 Pt 2):H1070–H1078. doi: 10.1152/ajpheart.1990.258.4.H1070. [DOI] [PubMed] [Google Scholar]
- Zünkler B. J., Lenzen S., Männer K., Panten U., Trube G. Concentration-dependent effects of tolbutamide, meglitinide, glipizide, glibenclamide and diazoxide on ATP-regulated K+ currents in pancreatic B-cells. Naunyn Schmiedebergs Arch Pharmacol. 1988 Feb;337(2):225–230. doi: 10.1007/BF00169252. [DOI] [PubMed] [Google Scholar]


