Abstract
Brain metastases (BMs) in extensive-stage small cell lung cancer (ES-SCLC) are often associated with poor survival rates and quality of life, making the timely identification of high-risk patients for BMs in ES-SCLC crucial. Patients diagnosed with ES-SCLC between 2010 and 2018 were screened from the Surveillance, Epidemiology, and End Results (SEER) database. Four different machine learning (ML) algorithms were used to create prediction models for BMs in ES-SCLC patients. The accuracy, sensitivity, specificity, AUROC, and AUPRC were compared among these models and traditional logistic regression (LR). The random forest (RF) model demonstrated the best performance and was chosen for further analysis. The AUROC and AUPRC were calculated and compared. The findings from the RF model were utilized to identify the risk factors linked to BMs in patients diagnosed with ES-SCLC. Examining 4,716 instances of ES-SCLC, the research conducted an analysis, with brain metastases arising in 1,900 cases. Through evaluation of the ROC curve and PRC concerning the RF Model, results depicted an AUROC of 0.896 (95% CI: 0.889–0.899) and AUPRC of 0.900 (95% CI: 0.895–0.904). Test accuracy measured at 0.810 (95% CI: 0.784–0.833), sensitivity at 0.797 (95% CI: 0.756–0.841), and specificity at 0.819 (95% CI: 0.754–0.879). Based on the SHAP analysis of the RF predictive model, the top 10 most relevant features were identified and ranked in order of relative importance: bone metastasis, liver metastasis, radiation, age, tumor size, primary tumor location, N-stage, race, T-stage, and chemotherapy. The research developed and validated a predictive RF model using clinical and pathological data to predict the risk of BMs in patients with ES-SCLC. This model may assist physicians in making clinical decisions that could delay the onset of BMs and improve patient survival rates.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-80425-y.
Keywords: Machine learning, Random Forest, Extensive-stage small cell lung cancer, Brain metastasis
Subject terms: Cancer, Computational biology and bioinformatics, Risk factors, Mathematics and computing
Introduction
Small-cell lung cancer (SCLC) accounts for approximately 14% of lung cancer cases, and nearly two-thirds of SCLC patients develop extensive-stage disease (ES-SCLC)1,2. More than half of ES-SCLC patients experience brain metastasis(BMs)3. Four to six cycles of chemotherapy containing cisplatin can achieve a clinical response rate of 60–70% in ES-SCLC patients, but the median survival period is only around 9 months4–6. Studies have shown that the development of BMs, especially when symptomatic, may severely impair the quality of life of ES-SCLC patients, resulting in a median survival of only 4 to 6 months7. Therefore, early assessment and identification of patients at high risk for BMs in ES-SCLC, followed by a comprehensive evaluation considering the patient’s overall condition, such as physical health and economic status, and timely implementation of appropriate interventions, can contribute to improving patient outcomes.
A clinical predictive model, which evaluates the risk of disease and the effectiveness of treatment, is an essential component of contemporary clinical practice8. Machine learning (ML) is an application of artificial intelligence that relies on data to automatically learn and improve without the need for explicit programming. Compared to traditional independent risk factor assessments for predicting cancer metastasis in patients, machine learning offers higher accuracy in predicting and diagnosing cancer metastasis. ML can autonomously identify new variables and their complex relationships within a dataset. Its application in healthcare is experiencing rapid growth and is increasingly utilized to develop new prognostic models for various diseases9. A study has indicated that the healthcare burden is skyrocketing, which is associated with the lack of early prevention and treatment for patients at risk of developing BMs in SCLC10. Machine learning is particularly well-suited for utilizing the growing big data and constantly enhancing computational capacities. This allows for the possibility of conducting large-scale analyses in a more manageable and simplified manner11.
This study aimed to comprehensively investigate the risk factors for BMs in ES-SCLC patients using population-based surveillance, epidemiology, and outcomes (SEER) databases. We have employed innovative ML techniques to build a predictive model for assessing the risk of brain metastasis in ES-SCLC patients. By predicting the probability of BMs in ES-SCLC patients, clinicians could make more informed and effective decisions, to minimize or delay the occurrence of BMs to the greatest extent possible.
Methods
Data source and study population
The SEER database, comprising 18 cancer registries in the United States, provided data on cancer incidence, therapy, and survival for around 30% of the American population. Demographic factors like income, location, age at diagnosis, ethnicity, and sex, as well as cancer-specific factors including tumor location, histological subtypes, and sites of metastasis, were included in the SEER database. For this study, we extracted information on patients histologically diagnosed with SCLC between 2010 and 2018 from the SEER database. The inclusion criteria for this study were as follows: (1) Patients who had a confirmed histological diagnosis of ES-SCLC; (2) Patients who had complete information on TNM stage and other important characteristics; (3) Patients who had complete follow-up. The exclusion criteria were as follows: (1)Patients diagnosed at autopsy or death certificate only; (2) Not the first primary malignancy; (3) TN stage was not available; (4) Patients with limited-stage small cell lung cancer; (5) Incomplete records of the patient’s other information. This study was conducted using publicly available de-identified data from the SEER database. No specific ethical approval or informed consent was required for this analysis.
Data preprocessing
Based on our research objectives, we extracted 36 indicators from the SEER database related to the metastasis of ES-SCLC. We filtered the necessary data, eliminating any missing or abnormal entries. Text data underwent encoding as part of the data cleaning process. Additionally, the data was standardized, normalized, and discretized to enhance analysis12. Various processing techniques were implemented on various factors, including tumor dimensions and age groups. Discriminating against non-significant variables or highly duplicative ones led to the identification of the most informative features. To equalize the imbalanced dataset, the resampling method was employed to ensure uniformity between the two categories. Ultimately, a total of 15 features were chosen for the model.
Model development
Various input variables were used to establish machine learning models in this research, such as age, gender, race, primary site of tumor, surgical procedures, radiation therapy, chemotherapy, marital status, bone metastases, liver metastases, lung metastases, laterality, tumor size, T stage, and N stage. A 7:3 split of the SEER cohort was determined using Python and the scikit-learn package13. In addition, random forests (RF)14, AdaBoosts15, extreme gradient boostings (XGB)16, logistic regression (LR)17, and support vector machine (SVM)18 were used in the prediction process. Based on a development dataset, all models are built using 10-fold cross-validation and 50 iterations, with each iteration’s samples drawn randomly from observational data19.
Python was used to export predictive models based on machine learning, resulting in the creation of five models. These models varied in discrimination and accuracy across datasets. To determine the final model, the predictive performance of each model was assessed and compared on the testing dataset. Various metrics such as accuracy, sensitivity, specificity, as well as ROC and PRC curves were utilized to evaluate model performance. The significance of input variables in predicting BMs in ES-SCLC was determined by ranking the feature importance of input variables.
Evaluation methods
In order to assess the effectiveness of ML models, the performance of five different models was analyzed in terms of accuracy, sensitivity, specificity, AUROC, and AUPRC, with a confidence interval of 95%. The RF model was chosen as the optimal model for predicting BMs in ES-SCLC research after evaluating these metrics. Each model’s AUROC was computed and compared to determine their generalization capabilities and clinical utility. In cases of imbalanced datasets, the AUPRC is a more reliable metric than the AUC for evaluating model performance. Thus, the Precision-Recall curve was plotted and the AUPRC was calculated to supplement the AUC value20. Additionally, SHAP, a cooperative game-theoretic-based technique, was utilized to provide explanations for predictions made by the best-performing ML model21. The statistical software employed for these analyses included R (v4.2.2) and Python (v3.9.13).
Result
Patient clinical characteristics
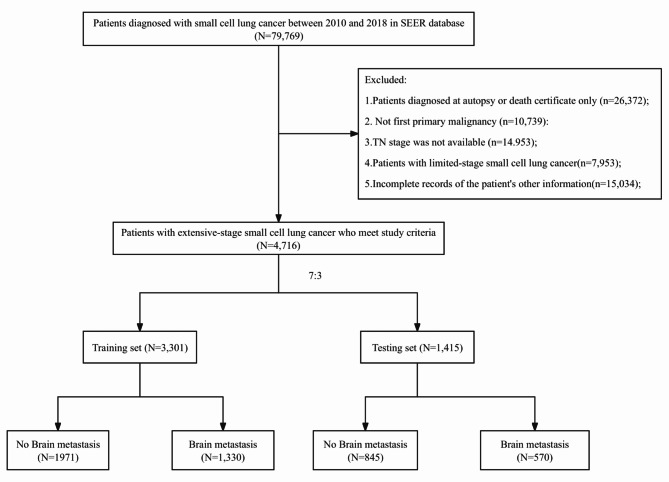
This study analyzed a total of 4,716 patients diagnosed with ES-SCLC between 2010 and 2018, using data from the SEER database. Among the 79,769 patients who did not meet the inclusion criteria, they were excluded from the study. The 4,716 ES-SCLC patients included in the analysis were divided into a training group (n = 3,301) and a testing group (n = 1,415) at a ratio of 7:3. Out of these patients, 2,816 had no brain metastases (non-BMs), while 1,900 patients had brain metastases(BMs) (Fig. 1).
Fig. 1.
The flowchart of patient selection from the SEER database. SEER Surveillance, Epidemiology, and End Results.
Table 1 displays the clinical features of individuals with ES-SCLC in the BMs group and non-BMs group. The mean ages of the two groups were similar (mean (SD), 67 vs. 67, P < 0.001). Both cohorts predominantly consisted of patients aged ≥ 65 (P < 0.001). The prevalence of the White race was notably higher in both groups than other ethnicities (P < 0.001). In comparison to the non-BMs group, a greater percentage of patients in the BMs group underwent radiation therapy (75.7% vs. 35.2%, P < 0.001). Most patients were diagnosed while married (P < 0.001). The non-BMs group had a higher rate of metastases at DX-liver, DX-lung, and DX-bone compared to the BMs group. The proportion of T4 and N2 stages was relatively elevated in both groups (P < 0.001). No significant disparities were noted in sex, tumor location, surgery, or chemotherapy between the two cohorts.
Table 1.
Patient demographics and clinical characteristics.
| Characteristics, n (%) | No Brain metastasis (N = 2816) | Brain metastasis (N = 1900) | P Value |
|---|---|---|---|
| Age (years), median (range) | 67(17–85) | 67(37–85) | < 0.001 |
| Age | < 0.001 | ||
| < 65 | 993 (35.3%) | 877 (46.2%) | |
| ≥ 65 | 1823 (64.7%) | 1023 (53.8%) | |
| Sex | 0.545 | ||
| Male | 1555 (55.2%) | 1067 (56.2%) | |
| Female | 1261 (44.8%) | 833 (43.8%) | |
| Race | < 0.001 | ||
| White | 2486 (88.3%) | 1539 (81.0%) | |
| Black | 234 (8.3%) | 246 (12.9%) | |
| Asian or Pacific Islander | 87 (3.1%) | 102 (5.4%) | |
| American Indian/Alaska Native | 9 (0.3%) | 13 (0.7%) | |
| Primary.Site | 0.764 | ||
| Main bronchus | 241 (8.6%) | 149 (7.8%) | |
| Upper lobe, lung | 1432 (50.9%) | 1007 (53.0%) | |
| Middle lobe, lung | 116 (4.1%) | 79 (4.2%) | |
| Lower lobe, lung | 675 (24.0%) | 434 (22.8%) | |
| Overlapping lesion of lung | 42 (1.5%) | 30 (1.6%) | |
| Lung, NOS | 310 (11.0%) | 201 (10.6%) | |
| Surgery | 0.872 | ||
| No | 178 (6.3%) | 114 (6.0%) | |
| Yes | 2631 (93.4%) | 1782 (93.8%) | |
| Others | 7 (0.2%) | 4 (0.2%) | |
| Radiation | < 0.001 | ||
| No | 1808 (64.2%) | 447 (23.5%) | |
| Yes | 990 (35.2%) | 1439 (75.7%) | |
| Others | 18 (0.6%) | 14 (0.7%) | |
| Chemotherapy | 0.005 | ||
| No | 1051 (37.3%)) | 787 (41.4%) | |
| Yes | 1765 (62.7%) | 1113 (58.6%) | |
| Marital.status | < 0.001 | ||
| Married | 1465 (52.0%) | 988 (52.0%) | |
| Single and Unmarried | 370 (13.1%) | 326 (17.2%) | |
| Others | 981 (34.8%) | 586 (30.8%) | |
| Mets.at.DX.bone | < 0.001 | ||
| No | 951 (33.8%) | 1418 (74.6%) | |
| Yes | 1865 (66.2%) | 482 (25.4%) | |
| Mets.at.DX.liver | < 0.001 | ||
| No | 1140 (40.5%) | 1453 (76.5%) | |
| Yes | 1676 (59.5%) | 447 (23.5%) | |
| Mets.at.DX.lung | 0.123 | ||
| No | 2147 (76.2%) | 1486 (78.2%) | |
| Yes | 669 (23.8%) | 414 (21.8%) | |
| Laterality | 0.461 | ||
| Left | 1181 (41.9%) | 793 (41.7%) | |
| Right | 1576 (56.0%) | 1062 (55.9%) | |
| Paired site | 18 (0.6%) | 20 (1.1%) | |
| Others | 41 (1.5%) | 25 (1.3%) | |
| T.stage | < 0.001 | ||
| T1 | 220 (7.8%) | 165 (8.7%) | |
| T2 | 811 (28.8%) | 666 (35.1%) | |
| T3 | 150 (5.3%) | 107 (5.6%) | |
| T4 | 1635 (58.1%) | 962 (50.6%) | |
| N.stage | < 0.001 | ||
| N0 | 405 (14.4%) | 370 (19.5%) | |
| N1 | 265 (9.4%) | 212 (11.2%) | |
| N2 | 1531 (54.4%) | 951 (50.1%) | |
| N3 | 615 (21.8%) | 367 (19.3%) | |
| Tumor.Size | < 0.001 | ||
| Median(IQR) | 50(33–71) | 58(35–73) | |
Feature analysis of common clinical variables
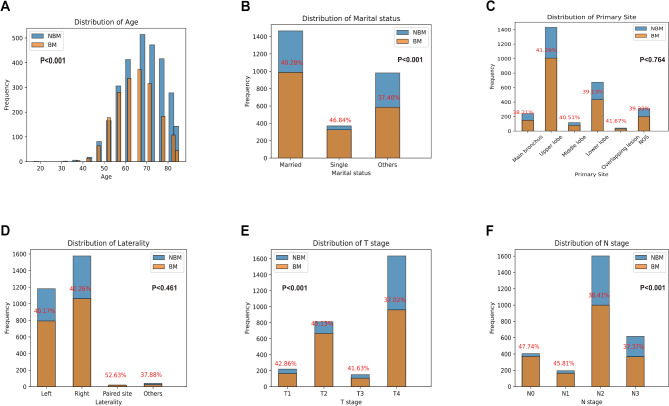
Based on the SEER database, a statistical analysis was conducted on six common clinical variables of ES-SCLC patients included in the study (Fig. 2). The research findings indicate that BMs in ES-SCLC patients predominantly occurred between the ages of 50 and 80 (P < 0.001). Among patients within this age range, those around 65 and 70 years old were more prone to developing BMs, with a relatively higher risk (Fig. 2A). In the diagnosis of ES-SCLC, we have observed that when patients have marital statuses such as divorce or widowhood, the likelihood of BMs occurrence is higher (P < 0.001) (Fig. 2B). The risk of BMs in ES-SCLC patients was not statistically significantly correlated with primary tumor site (P = 0.764) or laterality (P = 0.461) (Fig. 2C–D). The risk of BMs was found to be similar between stage T3 and T4 stage (P < 0.001) ES-SCLC patients (Fig. 2E). Additionally, when considering the stage of lymph node metastasis, it was observed that the risk of BMs was slightly higher in stage N2 and N3 stage ES-SCLC patients compared to those in stage N2 (P < 0.001) (Fig. 2F).
Fig. 2.
Common clinical variable characteristics analysis of ES-SCLC is based on the SEER database.
Model performance
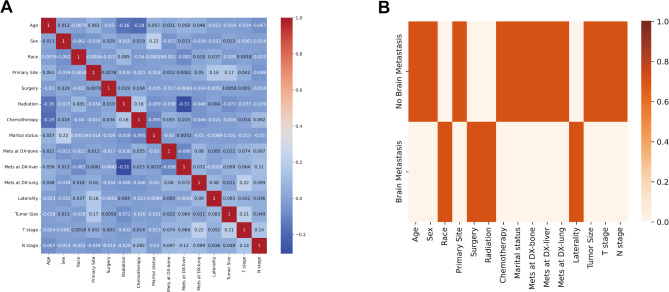
Based on the statistical analysis results, a total of 15 clinical variables were identified. Figure 3 illustrates the correlation analysis conducted among these clinical variables. The results indicated that there appears to be a correlation between BMs in ES-SCLC and factors such as race, surgery, radiation, and laterality. Clinical variables associated with non-BMs cases might include age, sex, primary tumor site, chemotherapy, marital status, metastasis at DX-liver, metastasis at DX-lung, metastasis at DX-bone, tumor size, T stage, and N stage.
Fig. 3.
Correlation analysis of clinical variables. (A) The correlation heatmap between clinical variables. (B) The correlation heatmap between ES-SCLC BMs or non-BMs and 15 clinical variables.
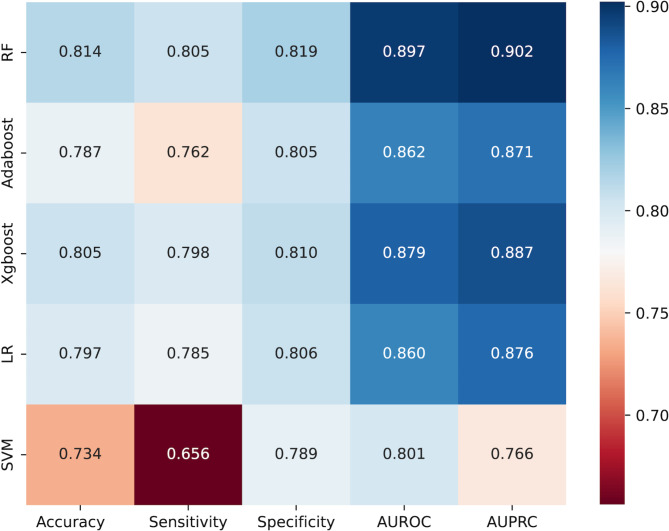
In order to evaluate the predictive abilities of the five models, a ten-fold cross-validation was conducted using the training dataset. Figure 4 illustrates the comparison of model performances, revealing that the RF model demonstrates superior results compared to other models in AUROC, AUPRC, sensitivity, accuracy, and specificity. Consequently, we suggest employing the RF model as the optimal classifier for predicting ES-SCLC brain metastasis.
Fig. 4.
Performance comparison of different models.
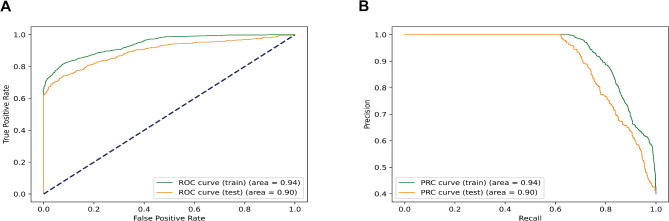
The ROC curve and precision-recall curve for the RF model are illustrated in Fig. 5, displaying an AUROC of 0.896 (95% CI: 0.889–0.899) and an AUPRC of 0.900 (95% CI: 0.895–0.904). The RF model achieved a test accuracy score of 0.810 (95% CI: 0.784–0.833) along with a sensitivity of 0.797 (95% CI: 0.756–0.841) and a specificity of 0.819 (95% CI: 0.754–0.879). Moreover, the feasibility of the AdaBoost model in predicting BMs in ES-SCLC was also demonstrated through confusion matrix analysis (Supplemental Fig. 1).
Fig. 5.
The receiver operating characteristic curve and the precision-recall curve on the training and test dataset. (A) AUROC. (B) AUPRC.
The significance of factors in ML algorithms
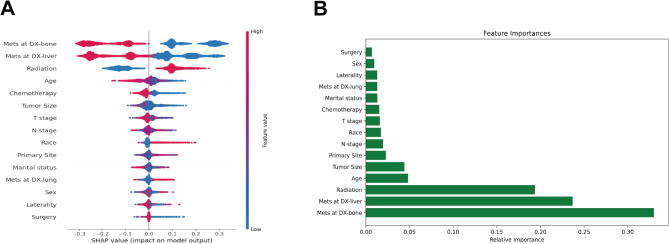
SHAP was utilized in the analysis of the RF model in this study. Generally, higher SHAP values associated with features suggest a higher likelihood of the target event taking place. During the SHAP analysis, red is used to signify feature values that have a positive effect on the model, while blue is reserved for feature values that have a negative impact22. The results of the study revealed that out of the top ten feature variables, bone metastasis held the most significance, followed closely by liver metastasis, radiation, patient age, tumor size, primary tumor site, N-stage, ethnicity, T-stage, and the use of chemotherapy (Fig. 6).
Fig. 6.
Relative importance of variables based on SHAP for RF prediction model. SHAP, Shapley’s Additive explanations.
Discussion
This study utilized data from the SEER database to gather information on extensive-stage small cell lung cancer(ES-SCLC) patients with or without brain metastasis (BMs). The 15 feature variables were carefully selected and determined, and four simple and practical machine learning (ML) models and traditional logistic regression (LR) were developed and validated. The performance of these models was compared in terms of accuracy, sensitivity, specificity, AUROC, and AUPRC. These research findings indicated that the Random forest (RF) model exhibited the highest predictive ability for BMs in ES-SCLC patients, as observed in both the training and testing models. The application of this model assisted clinical practitioners in accurately assessing the risk of BMs in ES-SCLC patients, enabling timely prophylactic cranial irradiation (PCI) treatment, thereby enhancing survival rates and delaying the reduction of BMs risk. Based on current understanding, this research was groundbreaking in utilizing machine learning algorithms to forecast the likelihood of BMs in ES-SCLC.
The RF is an ensemble learning method that employs bagging technology to combine multiple decision trees for tasks such as classification, regression, and others. During its training phase, it constructs and aggregates the outputs of numerous decision trees—producing a majority vote of classes for classification problems and an average of predicted values for regression problems23. This technique’s primary benefit lies in its exceptional stability, effectively preventing overfitting in training24, particularly beneficial for smaller datasets. Consequently, it enhances accuracy not only during testing but also when implementing the model on samples. While the prevailing notion suggested that random forest classifiers could yield satisfactory outcomes without systematic hyperparameter adjustments, we conducted hyperparameter optimization to both mitigate overfitting and enhance positive prediction accuracy.
Utilizing SHAP values, this research evaluated the influence of individual factors. In the visualization of SHAP for variable importance, it was observed that each of the 15 variables analyzed made unique contributions to the model. Feature importance analysis and ranking of the variables identified the top ten features associated with BMs in ES-SCLC: bone metastasis, liver metastasis, radiation, age, tumor size, primary tumor location, N staging, race, T staging, and chemotherapy.
Relevant studies indicated that bone metastasis and liver metastasis are significant risk factors for SCLC brain metastasis25–27. This finding further corroborates our research results. In our study, bone metastasis and liver metastasis emerged as the two most notable variables for BMs in ES-SCLC. It was noteworthy that past research did not conduct distinctive analyses between limited-stage and extensive-stage patients, and we were the first to systematically explore the role of bone metastasis and liver metastasis as risk factors for BMs in ES-SCLC.
There is currently controversy over whether age was a risk factor for ES-SCLC-induced BMs. Multiple studies indicated that age is not a key risk factor for BMs in ES-SCLC patients28–30. However, in the systematic review and meta-analysis conducted by Zeng Haiyan et al.31 regarding the risk factors for BMs in patients with SCLC (induced ES-SCLC), a comprehensive analysis of the conclusions from 14 studies revealed that age (< 65 years) is a risk factor for BMs. The explanation for this finding may be that younger SCLC patients typically have a longer lifespan32,33, allowing for more time to experience BMs. A research led by et al.34 delved into the predictive value of clinical features in relation to the occurrence and consequences of simultaneous brain metastases (BMs) in patients with small cell lung cancer (SCLC), revealing that older age was established as a notable risk factor for synchronous BMs in individuals with SCLC. This is consistent with our results. Age was the fourth factor associated with BMs in our study of ES-SCLC.
The size of the tumor was identified as another significant risk factor in the development of distant metastasis from malignant tumors. An analysis conducted by Zheng et al.35 revealed that the size of the primary tumor can predict the occurrence of SCLC-BM. Research conducted by Chen et al.36 regarding the factors associated with brain metastasis following PCI in limited-stage SCLC indicated that individuals with tumors ≥ 5 cm are at a higher risk of developing brain metastasis (Hazard Ratio: 1.781, 95% Confidence Interval: 1.044–3.039, P = 0.034). Similarly, a retrospective study by Farooqi AS et al.37 showed that tumors ≥ 5 cm increase the risk of brain metastasis (HR 1.77, 95% CI 1.22–2.55, P = 0.002). Our research results are essentially consistent with theirs.
It was noteworthy that in our study, radiotherapy ranks third among factors associated with BMs, which appeared contradictory to general clinical knowledge. Considering that the SEER database did not comprehensively record patients’ radiotherapy details, survival curve analysis of patients who received or did not receive radiotherapy reveals a significantly higher survival period for those who underwent radiotherapy (P < 0.001)( Supplemental Fig. 2). Radiotherapy has also been shown to provide benefits to patients with ES-SCLC in recent clinical studies. Several literature reports suggested that thoracic radiotherapy is an independent prognostic factor for improving the survival of ES-SCLC patients38,39, indicating that thoracic radiotherapy may enhance overall survival. This aligns with our analyzed survival curve results, possibly because radiotherapy extended the survival time of ES-SCLC patients, allowing for more time to experience BMs.
Although there were previous reports on predictive models of BMs in SCLC based on the SEER database, these articles limited scope and only focused on the occurrence of BMs in SCLC. Therefore, we were the first to develop and validate a prediction model for BMs in ES-SCLC using the SEER database. Our study included up to 15 clinically available variables, which were used in both the development and validation of the model. Additionally, all predictors related to BMs in ES-SCLC were obtained through sequencing.
While our study had its strengths, there were also some limitations. Firstly, it was a retrospective study, which came with the inherent data biases typical of retrospective research. Secondly, due to a significant amount of missing data for some variables in multiple imputations within the SEER dataset, we choose to exclude these missing data in the manuscript, potentially introducing bias to the results. Lastly, the absence of key variables in the SEER database, such as KPS, ECOG scores, blood biochemical indicators, and detailed survival times, limits further optimization of our model. In future research, we plan to address these limitations by incorporating additional clinical factors to enhance the predictive power of the model, thereby providing more comprehensive support to healthcare professionals in their decision-making.
In summary, five ML models were created to forecast BMs in ES-SCLC. Among these models, it was observed that the RF model showcased the most reliable predictive capacity, showcasing outstanding discriminative performance not solely in the evaluation and validation sets, but also attaining the maximum levels of AUROC, AUPRC, sensitivity, specificity, and accuracy. We hope the RF model-based web calculator can help clinicians identify patients at high risk of BMs from ES-SCLC, enabling early and effective interventions to prevent and delay the onset of BMs and improve patient survival. We hope that the RF model-based online calculator will help clinicians identify high-risk ES-SCLC patients for BMs, facilitating the selection of early and appropriate interventions, such as Prophylactic Cranial Irradiation or Brain MRI surveillance. These early interventions aim to prevent or delay BMs and improve patient survival.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Yongzhong Wu and Yunyun Zhang designed the study.Siwei Zeng, Ze Yuan, Dingyi Yang, Yong Jiang, and Qiang Wang conducted data analysis.Erha Munai conceived the project and wrote the manuscript.Dan Tao revised and approved the paper.All authors contributed to the article and approved the submitted version.
Funding
The current study was supported by grants from the Chongqing Science and Health Joint Medical Research Project (No. 2023GGXM002 to YZ Wu), National Natural Science Foundation Project (No. 82073347 to YZ Wu) and Chongqing Talent Plan (No. cstc2022ycjh-bgzxm0208 to YZ Wu).
Data availability
The dataset analysed during the current study are available in the Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/). All data analysed during this study are included in the supplementary file.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Erha Munai and Siwei Zeng contributed equally as the first authors.
Contributor Information
Yongzhong Wu, Email: cqmdwyz@163.com.
Yunyun Zhang, Email: 1120303669@qq.com.
Dan Tao, Email: taodan@cqu.edu.cn.
References
- 1.Chen, W. et al. Cancer statistics in China, 2015. CA Cancer J. Clin.66(2), 115–132. 10.3322/caac.21338 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Jett, J. R. et al. Treatment of small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest, 143(5 Suppl): p. e400S-e419S. DOI: 10.1378/chest.12-2363 (2013). [DOI] [PubMed]
- 3.Seute, T. et al. Neurologic disorders in 432 consecutive patients with small cell lung carcinoma. Cancer100(4), 801–806. 10.1002/cncr.20043 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Chen, Y., Chen, L. & Zhong, D. Comparing the adverse effects of platinum in combination with etoposide or irinotecan in previously untreated small-cell lung cancer patients with extensive disease: a network meta-analyses. Thorac. Cancer8(3), 170–180. 10.1111/1759-7714.12420 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aupérin, A. et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic cranial irradiation overview Collaborative Group. N Engl. J. Med.341(7), 476–484. 10.1056/nejm199908123410703 (1999). [DOI] [PubMed] [Google Scholar]
- 6.Guo, S., Liang, Y. & Zhou, Q. Complement and correction for meta-analysis of patients with extensive-stage small cell lung cancer managed with irinotecan/cisplatin versus etoposide/cisplatin as first-line chemotherapy. J. Thorac. Oncol.6(2), 406–408. 10.1097/JTO.0b013e3182061d8c (2011). Author reply 408. [DOI] [PubMed] [Google Scholar]
- 7.Greenspoon, J. N. et al. Selecting patients with extensive-stage small cell lung cancer for prophylactic cranial irradiation by predicting brain metastases. J. Thorac. Oncol.6(4), 808–812. 10.1097/JTO.0b013e31820d782d (2011). [DOI] [PubMed] [Google Scholar]
- 8.Moons, K. G. et al. Prognosis and prognostic research: what, why, and how? Bmj, 338: p. b375. DOI: 10.1136/bmj.b375 (2009). [DOI] [PubMed]
- 9.Alaa, A. M. et al. Cardiovascular disease risk prediction using automated machine learning: a prospective study of 423,604 UK Biobank participants. PLoS One14(5), e0213653. 10.1371/journal.pone.0213653 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shan, Q. et al. A new nomogram and risk classification system for predicting survival in small cell lung cancer patients diagnosed with brain metastasis: a large population-based study. BMC Cancer21(1), 640. 10.1186/s12885-021-08384-5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shek, A. et al. Machine learning-enabled multitrust audit of stroke comorbidities using natural language processing. Eur. J. Neurol.28(12), 4090–4097. 10.1111/ene.15071 (2021). [DOI] [PubMed] [Google Scholar]
- 12.Hulsen, T. et al. From Big Data to Precision Medicine. Front. Med. (Lausanne)6, 34. 10.3389/fmed.2019.00034 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang, D. & Oh, S. Balanced training/test set sampling for proper evaluation of classification models. Intell. Data Anal.24, 5–18. 10.3233/IDA-194477 (2020). [Google Scholar]
- 14.Breiman, L. Random forests. Mach. Learn.45, 5–32. 10.1023/A:1010933404324 (2001). [Google Scholar]
- 15.Xiong, G. et al. Myocardial perfusion analysis in cardiac computed tomography angiographic images at rest. Med. Image Anal.24(1), 77–89. 10.1016/j.media.2015.05.010 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen, T. & Guestrin, C. XGBoost, in Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. pp. 785–794. (2016).
- 17.Stoltzfus, J. C. Logistic regression: a brief primer. Acad. Emerg. Med.18(10), 1099–1104. 10.1111/j.1553-2712.2011.01185.x (2011). [DOI] [PubMed] [Google Scholar]
- 18.Lapin, M., Hein, M. & Schiele, B. Learning using privileged information: SVM + and weighted SVM. Neural Netw.53, 95–108. 10.1016/j.neunet.2014.02.002 (2014). [DOI] [PubMed] [Google Scholar]
- 19.Yadaw, A. S. et al. Clinical features of COVID-19 mortality: development and validation of a clinical prediction model. Lancet Digit. Health2(10), e516–e. 10.1016/s2589-7500(20)30217-x (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muschelli, J. ROC and AUC with a binary predictor: a potentially misleading Metric. J. Classif.37(3), 696–708. 10.1007/s00357-019-09345-1 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gramegna, A. & Giudici, P. SHAP and LIME: an evaluation of discriminative power in Credit Risk. Front. Artif. Intell.4, 752558. 10.3389/frai.2021.752558 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lundberg, S. M. et al. Explainable machine-learning predictions for the prevention of hypoxaemia during surgery. Nat. Biomed. Eng.2(10), 749–760. 10.1038/s41551-018-0304-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pellegrino, E. et al. Machine learning random forest for predicting oncosomatic variant NGS analysis. Sci. Rep.11 (1), 21820. 10.1038/s41598-021-01253-y (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, J. et al. ForestQC: quality control on genetic variants from next-generation sequencing data using random forest. PLoS Comput. Biol.15(12), e1007556. 10.1371/journal.pcbi.1007556 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hao, Y. & Li, G. Risk and prognostic factors of brain metastasis in lung cancer patients: a Surveillance, Epidemiology, and end results population–based cohort study. Eur. J. Cancer Prev.32(5), 498–511. 10.1097/cej.0000000000000790 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rong, Y. T., Zhu, Y. C. & Wu, Y. A novel nomogram predicting cancer-specific survival in small cell lung cancer patients with brain metastasis. Transl Cancer Res.11(12), 4289–4302. 10.21037/tcr-22-1561 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang, G. H., Liu, Y. J. & De Ji, M. Risk factors, prognosis, and a New Nomogram for Predicting Cancer-Specific Survival among Lung Cancer patients with Brain Metastasis: a retrospective study based on SEER. Lung200 (1), 83–93. 10.1007/s00408-021-00503-0 (2022). [DOI] [PubMed] [Google Scholar]
- 28.Crockett, C. et al. Prophylactic cranial irradiation (PCI), hippocampal avoidance (HA) whole brain radiotherapy (WBRT) and stereotactic radiosurgery (SRS) in small cell lung cancer (SCLC): where do we stand? Lung Cancer162, 96–105. 10.1016/j.lungcan.2021.10.016 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Takahashi, T. et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol.18(5), 663–671. 10.1016/s1470-2045(17)30230-9 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Moher, D. et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med., 6(7): p. e1000097. DOI: 10.1371/journal.pmed.1000097 (2009). [DOI] [PMC free article] [PubMed]
- 31.Zeng, H. et al. Risk factors for brain metastases in patients with small cell Lung Cancer: a systematic review and Meta-analysis. Front. Oncol.12, 889161. 10.3389/fonc.2022.889161 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu, H. et al. Risk factors for brain metastases in completely resected small cell lung cancer: a retrospective study to identify patients most likely to benefit from prophylactic cranial irradiation. Radiat. Oncol.9, 216. 10.1186/1748-717x-9-216 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sahmoun, A. E. et al. Anatomical distribution of small cell lung cancer: effects of lobe and gender on brain metastasis and survival. Anticancer Res.25(2a), 1101–8DOI (2005). [PubMed] [Google Scholar]
- 34.Zhou, G. et al. Predictive value of clinical characteristics on risk and prognosis of synchronous brain metastases in small-cell lung cancer patients: a population-based study. Cancer Med.12(2), 1195–1203. 10.1002/cam4.4978 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng, Y. et al. Risk factors for brain metastasis in patients with small cell lung cancer without prophylactic cranial irradiation. Strahlenther Onkol194(12), 1152–1162. 10.1007/s00066-018-1362-7 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Chen, M. Y. et al. Factors affecting the risk of Brain Metastasis in Limited-Stage Small Cell Lung Cancer after prophylactic cranial irradiation. Cancer Manag Res.14, 1807–1814. 10.2147/cmar.S347449 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farooqi, A. S. et al. Prophylactic cranial irradiation after definitive chemoradiotherapy for limited-stage small cell lung cancer: do all patients benefit? Radiother Oncol.122(2), 307–312. 10.1016/j.radonc.2016.11.012 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qi, J. et al. Thoracic Radiotherapy benefits Elderly extensive-stage small cell Lung Cancer patients with distant metastasis. Cancer Manag Res.11, 10767–10775. 10.2147/cmar.S221225 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puglisi, M. et al. Treatment options for small cell lung cancer - do we have more choice? Br. J. Cancer102(4), 629–638. 10.1038/sj.bjc.6605527 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset analysed during the current study are available in the Surveillance, Epidemiology, and End Results (SEER) database (https://seer.cancer.gov/). All data analysed during this study are included in the supplementary file.