Abstract
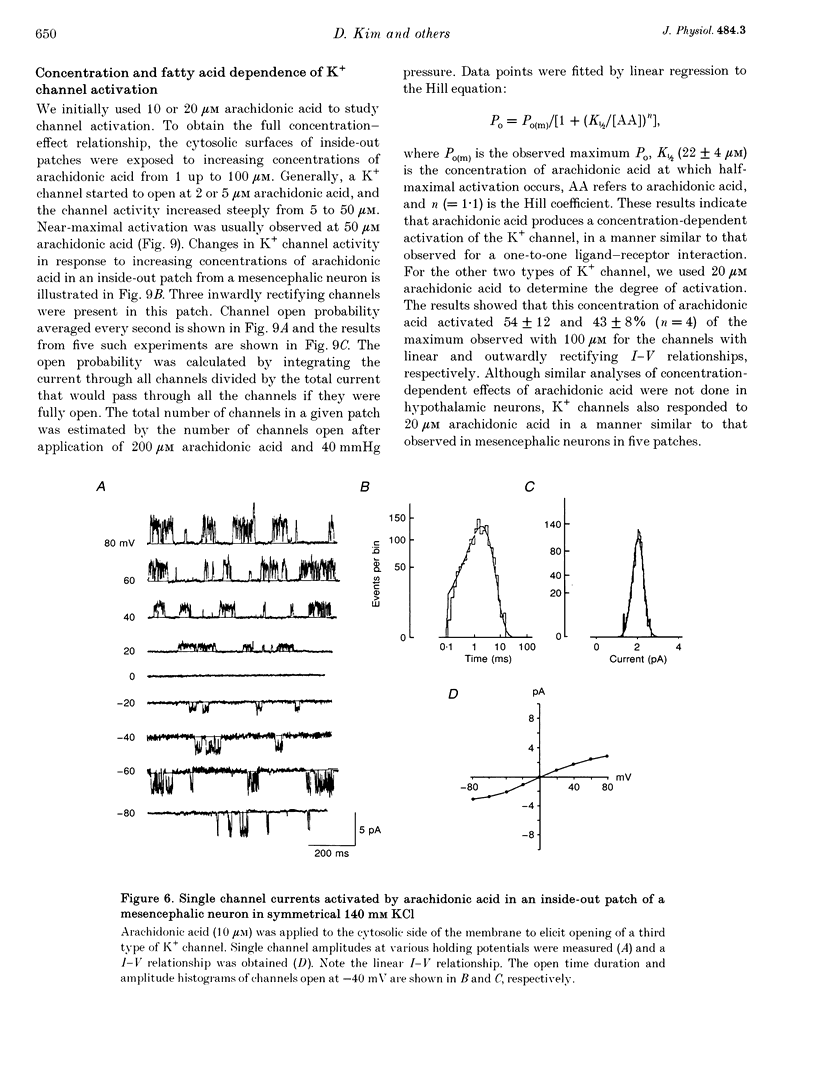
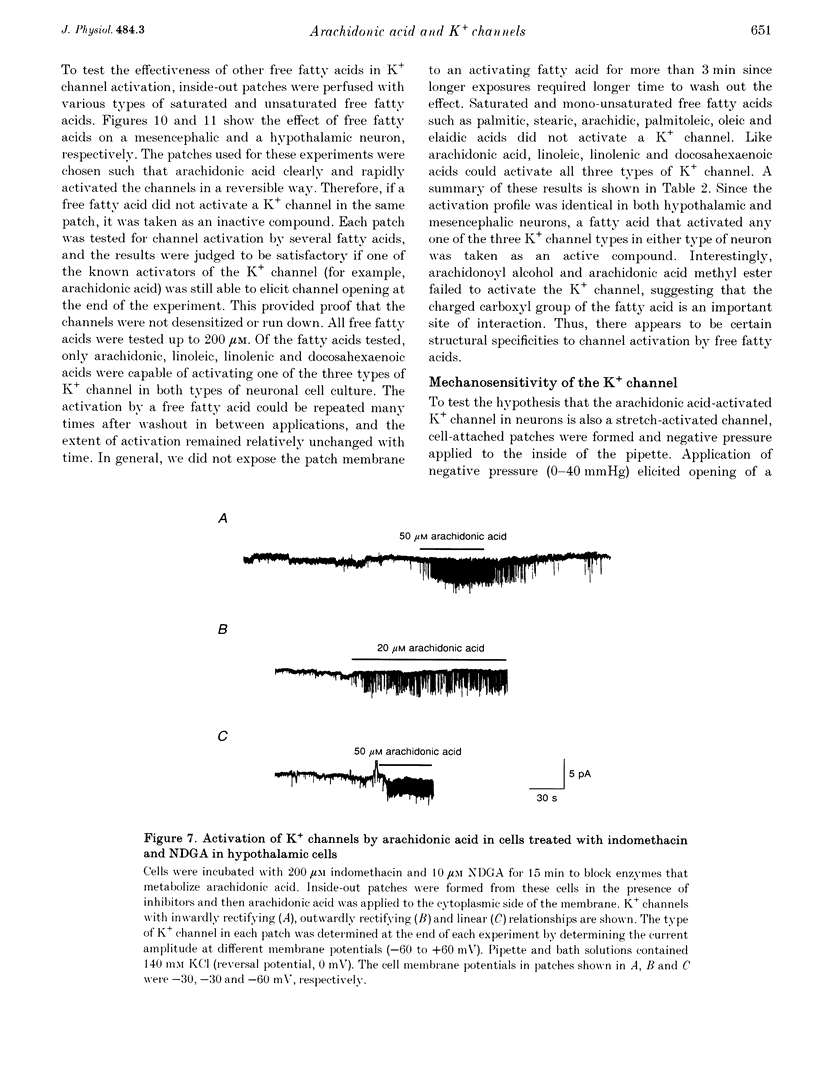
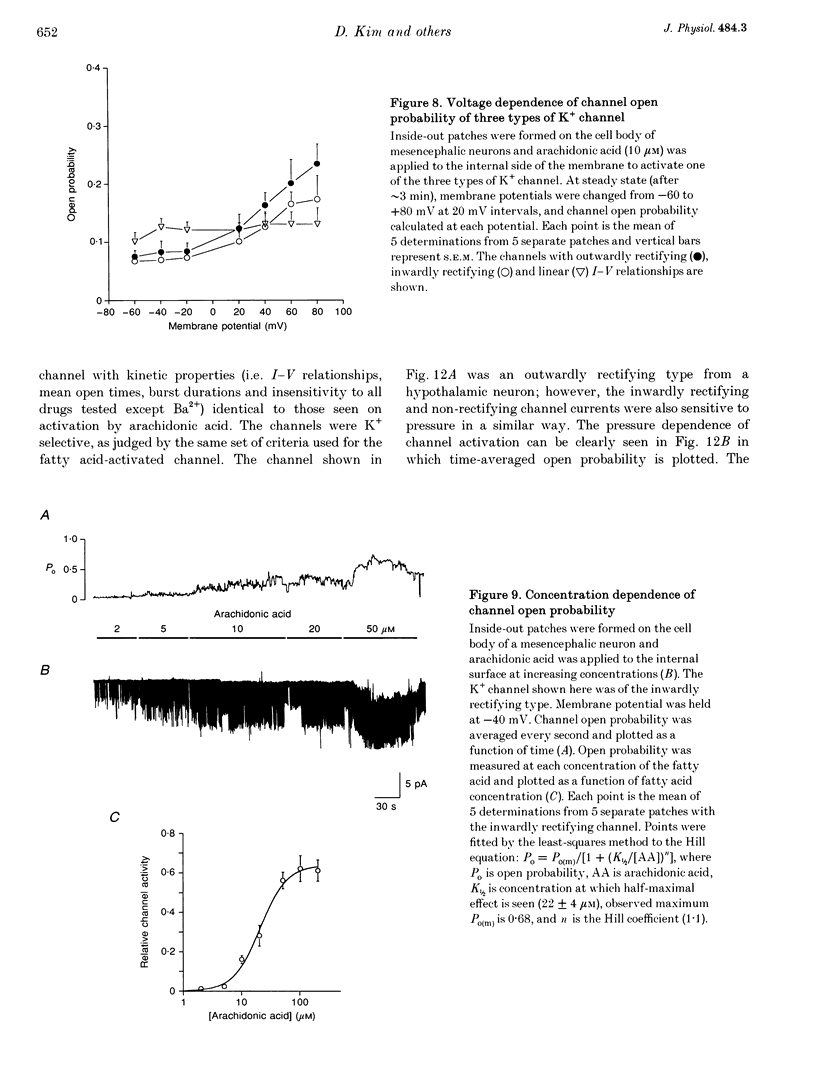
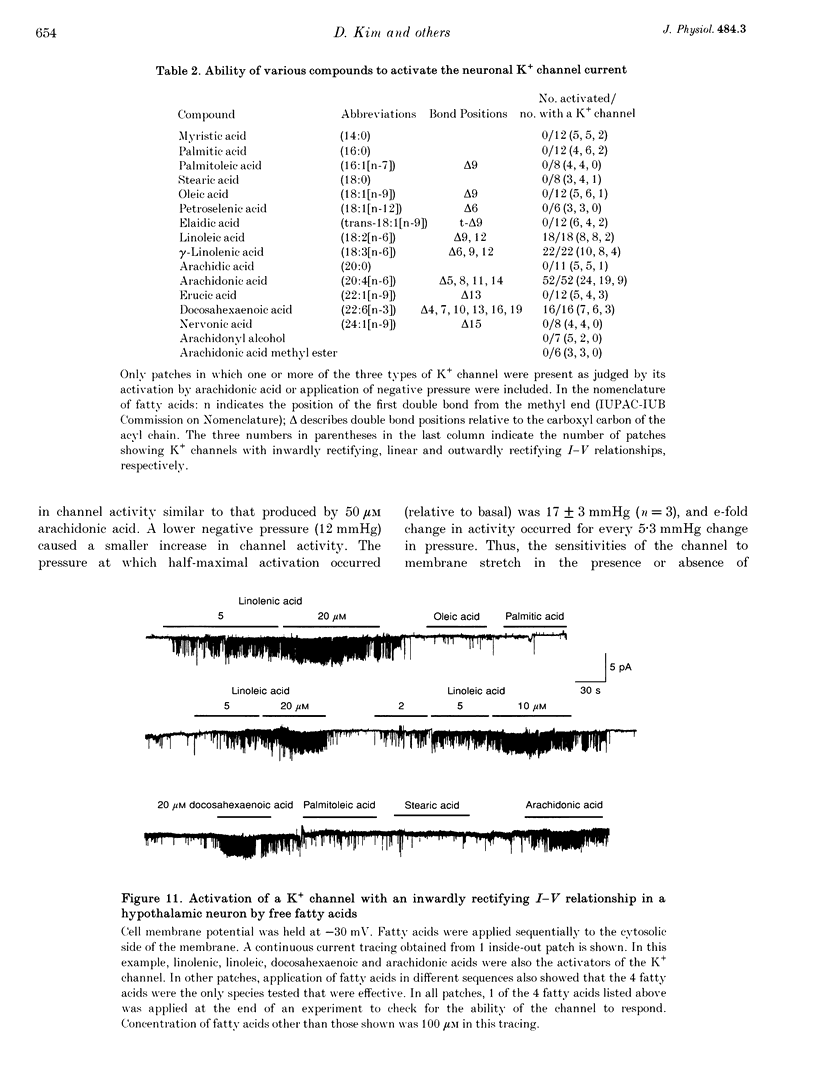
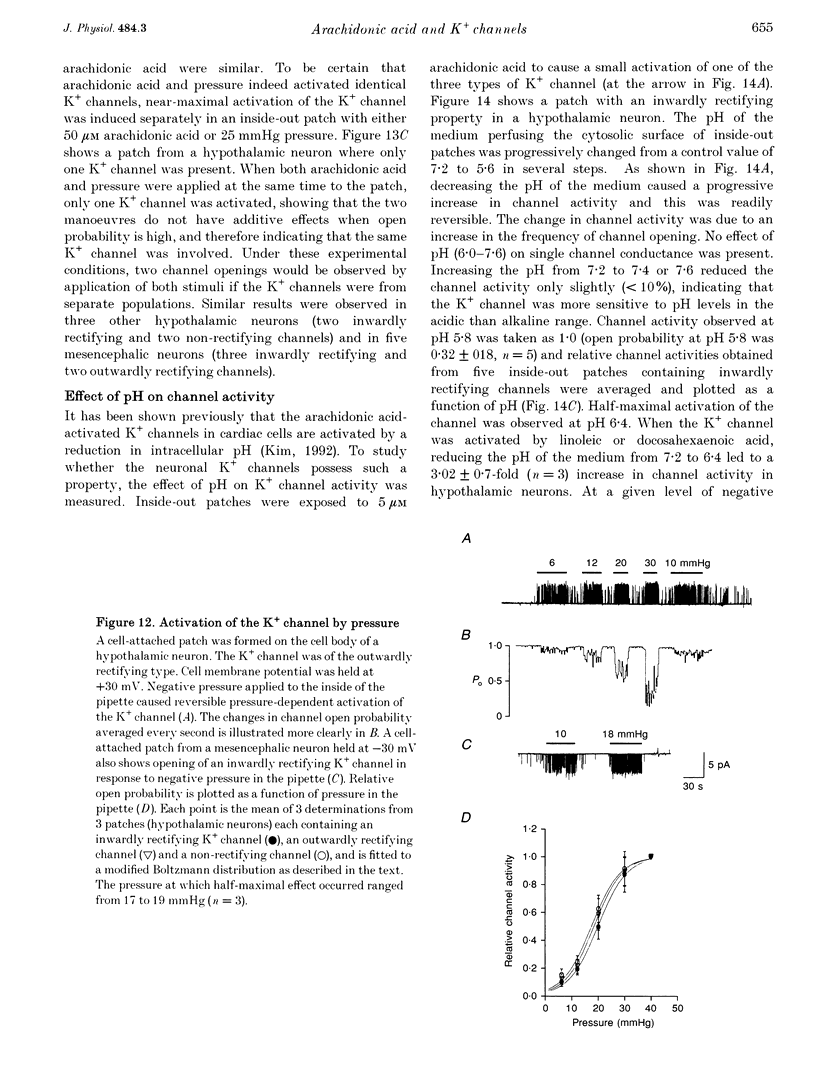
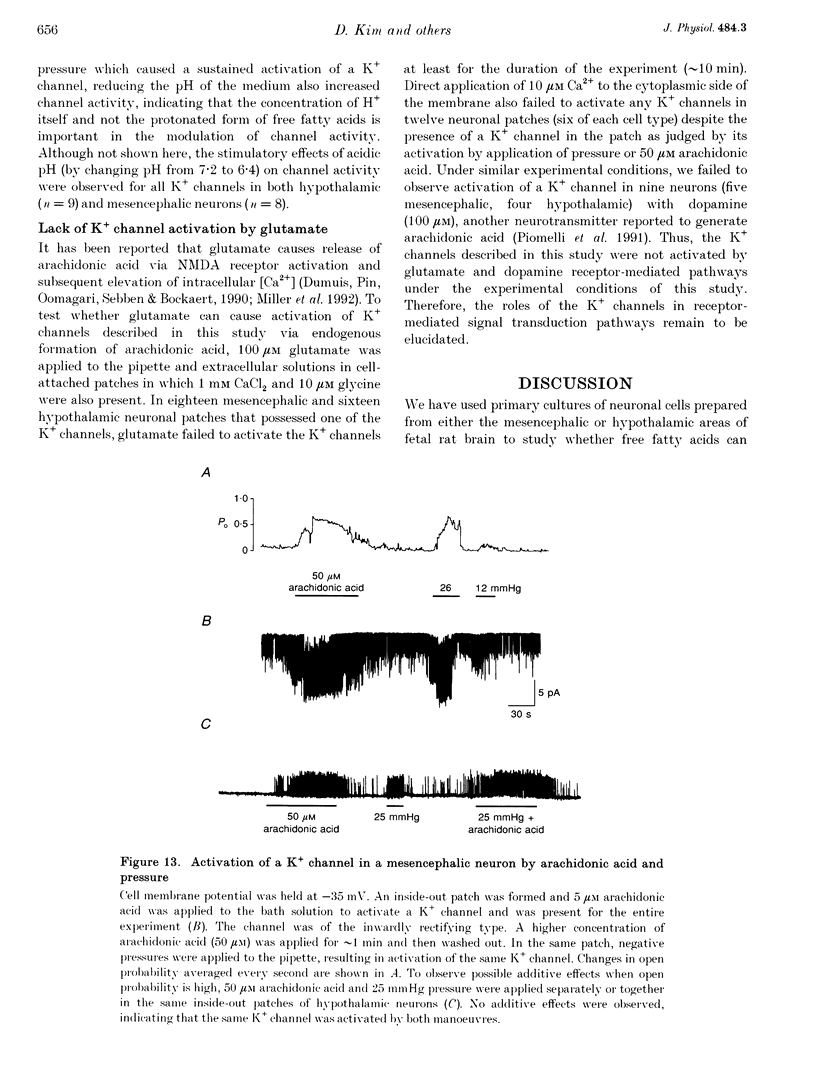
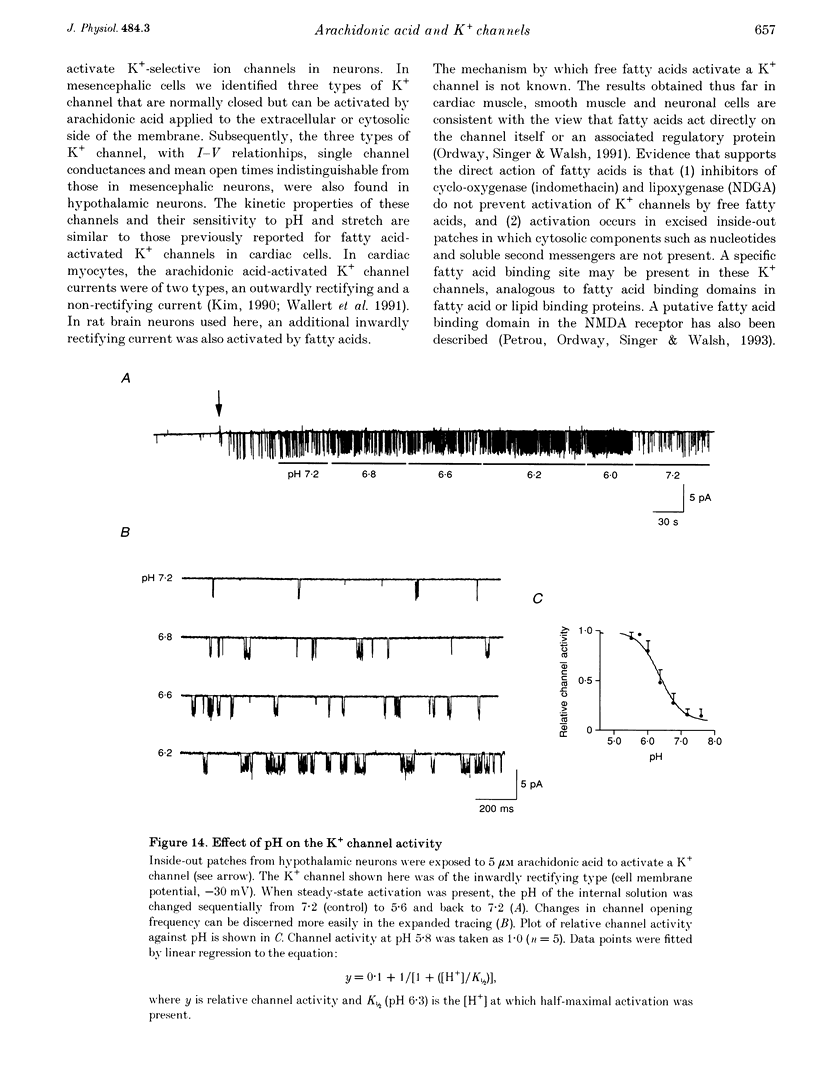
1. The presence and properties of K+ channels activated by arachidonic acid were studied in neuronal cells cultured from the mesencephalic and hypothalamic areas of rat brain. 2. Arachidonic acid produced a concentration-dependent (5-50 microM) and reversible activation of whole-cell currents. 3. In excised membrane patches, arachidonic acid applied to the cytoplasmic or extracellular side of the membrane caused opening of three types of channels whose current-voltage relationships were slightly outwardly rectifying, inwardly rectifying and linear, and whose single channel slope conductances at +60 mV were 143, 45 and 52 pS, respectively. 4. All three currents were K+ selective and blocked by 2 mM Ba2+ but not by other K+ channel blockers such as tetraethylammonium chloride, 4-aminopyridine and quinidine. The outwardly and inwardly rectifying currents were slightly voltage dependent with higher channel activity at more depolarized potentials. 5. Arachidonic acid activated the K+ channels in cells treated with cyclo-oxygenase and lipoxygenase inhibitors (indomethacin and nordihydroguaiaretic acid), indicating that arachidonic acid itself can directly activate the channels. Alcohol and methyl ester derivatives of arachidonic acid failed to activate the K+ channels, indicating that the charged carboxyl group is important for activation. 6. Certain unsaturated fatty acids (linoleic, linolenic and docosahexaenoic acids), but not saturated fatty acids (myristic, palmitic, stearic acids), also reversibly activated all three types of K+ channel. 7. All three K+ channels were activated by pressure applied to the membrane (i.e. channels were stretch sensitive) with a half-maximal pressure of approximately 18 mmHg. The K+ channels were not blocked by 100 microM GdCl3. 8. A decrease in intracellular pH (over the range 5.6-7.2) caused a reversible, pH-dependent increase in channel activity whether the channel was initially activated by arachidonic acid or stretch. 9. Glutamate, a neurotransmitter reported to generate arachidonic acid in striatal neurons, did not cause activation of the K+ channels when applied extracellularly in cell-attached patches. 10. It is suggested that the K+ channels described here belong to a distinct family of ion channels that are activated by either fatty acids or membrane stretch. Although the physiological roles of these K+ channels are not yet known, they may be involved in cellular processes such as cell volume regulation and ischaemia-induced elevation of K+ loss.
Full text
PDF


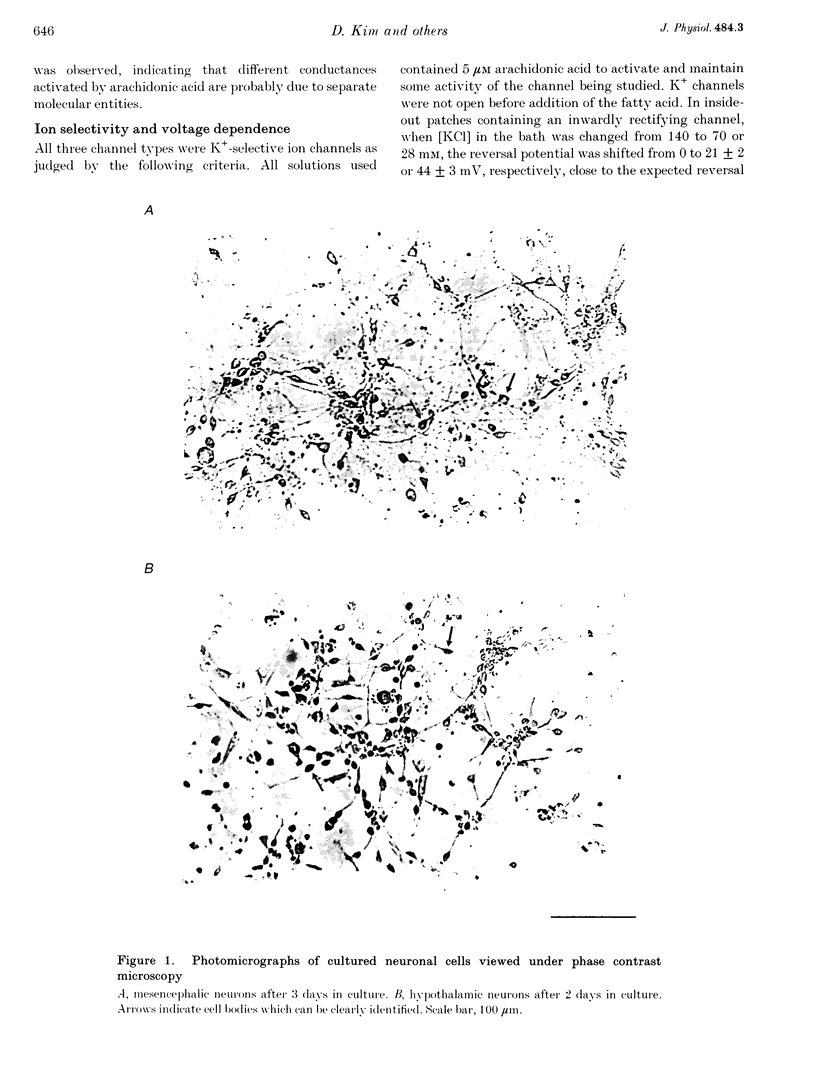

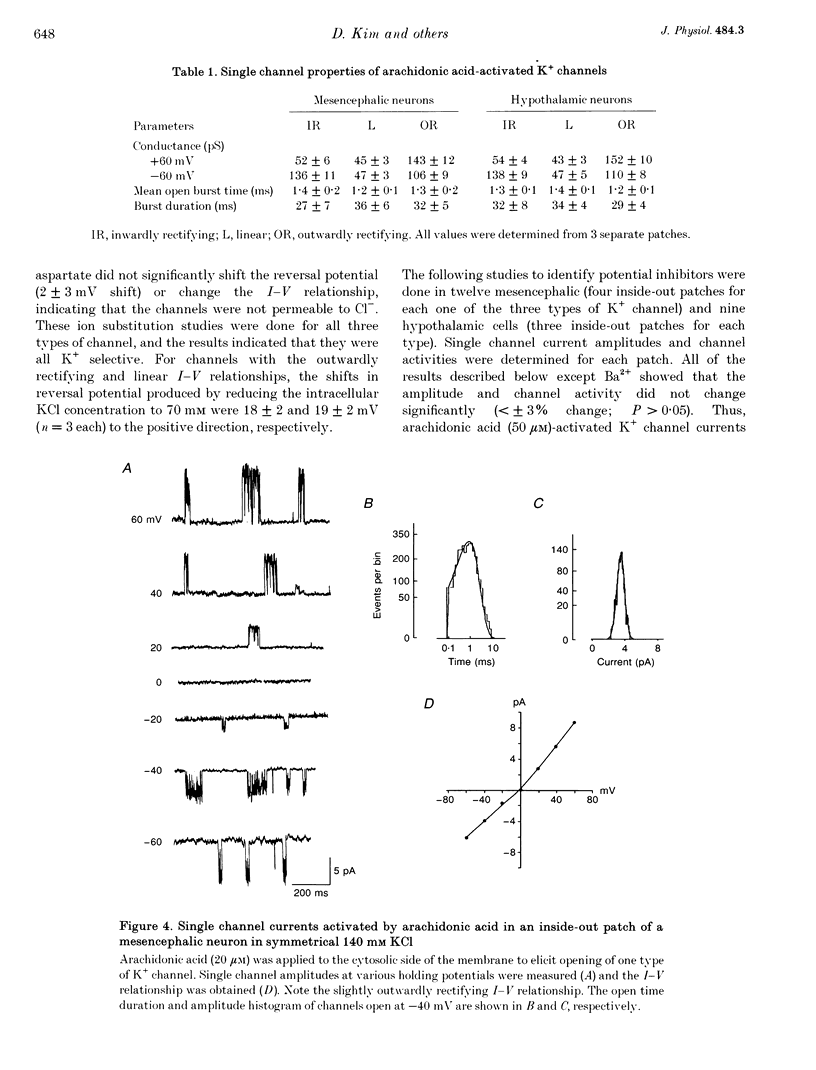
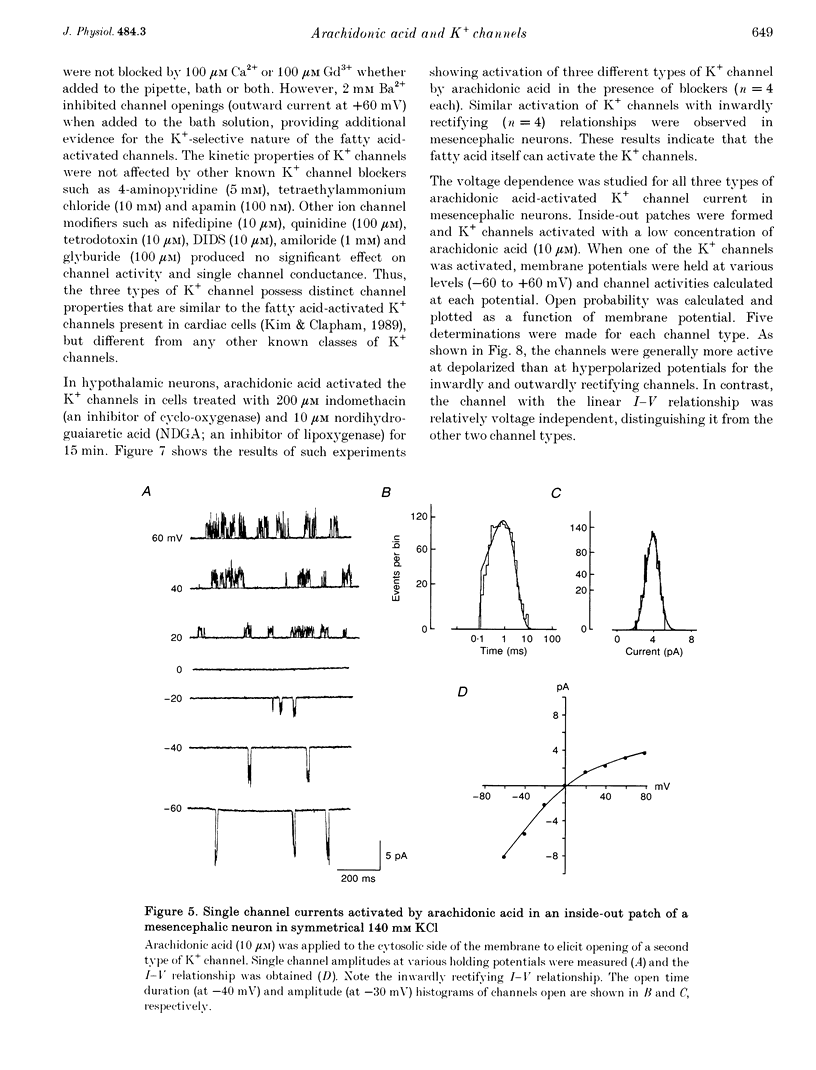




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buttner N., Siegelbaum S. A., Volterra A. Direct modulation of Aplysia S-K+ channels by a 12-lipoxygenase metabolite of arachidonic acid. Nature. 1989 Nov 30;342(6249):553–555. doi: 10.1038/342553a0. [DOI] [PubMed] [Google Scholar]
- Béhé P., Sandmeier K., Meves H. The effect of arachidonic acid on the M current of NG108-15 neuroblastoma x glioma hybrid cells. Pflugers Arch. 1992 Nov;422(2):120–128. doi: 10.1007/BF00370411. [DOI] [PubMed] [Google Scholar]
- Calabresi P., Maj R., Pisani A., Mercuri N. B., Bernardi G. Long-term synaptic depression in the striatum: physiological and pharmacological characterization. J Neurosci. 1992 Nov;12(11):4224–4233. doi: 10.1523/JNEUROSCI.12-11-04224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier T. J., Gallagher M. J., Sladek C. D. Cryopreservation and storage of embryonic rat mesencephalic dopamine neurons for one year: comparison to fresh tissue in culture and neural grafts. Brain Res. 1993 Oct 1;623(2):249–256. doi: 10.1016/0006-8993(93)91435-u. [DOI] [PubMed] [Google Scholar]
- Collier T. J., Sladek C. D., Gallagher M. J., Gereau R. W., 4th, Springer J. E. Diffusible factor(s) from adult rat sciatic nerve increases cell number and neurite outgrowth of cultured embryonic ventral mesencephalic tyrosine hydroxylase-positive neurons. J Neurosci Res. 1990 Nov;27(3):394–399. doi: 10.1002/jnr.490270318. [DOI] [PubMed] [Google Scholar]
- Dumuis A., Pin J. P., Oomagari K., Sebben M., Bockaert J. Arachidonic acid released from striatal neurons by joint stimulation of ionotropic and metabotropic quisqualate receptors. Nature. 1990 Sep 13;347(6289):182–184. doi: 10.1038/347182a0. [DOI] [PubMed] [Google Scholar]
- Fraser D. D., Hoehn K., Weiss S., MacVicar B. A. Arachidonic acid inhibits sodium currents and synaptic transmission in cultured striatal neurons. Neuron. 1993 Oct;11(4):633–644. doi: 10.1016/0896-6273(93)90075-3. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kandel E. R., O'Dell T. J. Are adult learning mechanisms also used for development? Science. 1992 Oct 9;258(5080):243–245. doi: 10.1126/science.1411522. [DOI] [PubMed] [Google Scholar]
- Kato K., Clark G. D., Bazan N. G., Zorumski C. F. Platelet-activating factor as a potential retrograde messenger in CA1 hippocampal long-term potentiation. Nature. 1994 Jan 13;367(6459):175–179. doi: 10.1038/367175a0. [DOI] [PubMed] [Google Scholar]
- Keyser D. O., Alger B. E. Arachidonic acid modulates hippocampal calcium current via protein kinase C and oxygen radicals. Neuron. 1990 Oct;5(4):545–553. doi: 10.1016/0896-6273(90)90092-t. [DOI] [PubMed] [Google Scholar]
- Kim D. A mechanosensitive K+ channel in heart cells. Activation by arachidonic acid. J Gen Physiol. 1992 Dec;100(6):1021–1040. doi: 10.1085/jgp.100.6.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Clapham D. E. Potassium channels in cardiac cells activated by arachidonic acid and phospholipids. Science. 1989 Jun 9;244(4909):1174–1176. doi: 10.1126/science.2727703. [DOI] [PubMed] [Google Scholar]
- Linden D. J., Routtenberg A. cis-Fatty acids, which activate protein kinase C, attenuate Na+ and Ca2+ currents in mouse neuroblastoma cells. J Physiol. 1989 Dec;419:95–119. doi: 10.1113/jphysiol.1989.sp017863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuko S., Nakajima S., Nakajima Y. Dissociated high-purity dopaminergic neuron cultures from the substantia nigra and the ventral tegmental area of the postnatal rat. Neuroscience. 1992 Jul;49(2):347–364. doi: 10.1016/0306-4522(92)90101-7. [DOI] [PubMed] [Google Scholar]
- Meves H. Modulation of ion channels by arachidonic acid. Prog Neurobiol. 1994 Jun;43(2):175–186. doi: 10.1016/0301-0082(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Miller B., Sarantis M., Traynelis S. F., Attwell D. Potentiation of NMDA receptor currents by arachidonic acid. Nature. 1992 Feb 20;355(6362):722–725. doi: 10.1038/355722a0. [DOI] [PubMed] [Google Scholar]
- Nichols C. G., Lederer W. J. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991 Dec;261(6 Pt 2):H1675–H1686. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- O'Dell T. J., Hawkins R. D., Kandel E. R., Arancio O. Tests of the roles of two diffusible substances in long-term potentiation: evidence for nitric oxide as a possible early retrograde messenger. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11285–11289. doi: 10.1073/pnas.88.24.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordway R. W., Singer J. J., Walsh J. V., Jr Direct regulation of ion channels by fatty acids. Trends Neurosci. 1991 Mar;14(3):96–100. doi: 10.1016/0166-2236(91)90069-7. [DOI] [PubMed] [Google Scholar]
- Ordway R. W., Walsh J. V., Jr, Singer J. J. Arachidonic acid and other fatty acids directly activate potassium channels in smooth muscle cells. Science. 1989 Jun 9;244(4909):1176–1179. doi: 10.1126/science.2471269. [DOI] [PubMed] [Google Scholar]
- Petrou S., Ordway R. W., Singer J. J., Walsh J. V., Jr A putative fatty acid-binding domain of the NMDA receptor. Trends Biochem Sci. 1993 Feb;18(2):41–42. doi: 10.1016/0968-0004(93)90050-w. [DOI] [PubMed] [Google Scholar]
- Piomelli D., Pilon C., Giros B., Sokoloff P., Martres M. P., Schwartz J. C. Dopamine activation of the arachidonic acid cascade as a basis for D1/D2 receptor synergism. Nature. 1991 Sep 12;353(6340):164–167. doi: 10.1038/353164a0. [DOI] [PubMed] [Google Scholar]
- Piomelli D., Volterra A., Dale N., Siegelbaum S. A., Kandel E. R., Schwartz J. H., Belardetti F. Lipoxygenase metabolites of arachidonic acid as second messengers for presynaptic inhibition of Aplysia sensory cells. Nature. 1987 Jul 2;328(6125):38–43. doi: 10.1038/328038a0. [DOI] [PubMed] [Google Scholar]
- Premkumar L. S., Gage P. W., Chung S. H. Coupled potassium channels induced by arachidonic acid in cultured neurons. Proc Biol Sci. 1990 Oct 22;242(1303):17–22. doi: 10.1098/rspb.1990.0097. [DOI] [PubMed] [Google Scholar]
- Rouzaire-Dubois B., Gérard V., Dubois J. M. Modification of K+ channel properties induced by fatty acids in neuroblastoma cells. Pflugers Arch. 1991 Nov;419(5):467–471. doi: 10.1007/BF00370790. [DOI] [PubMed] [Google Scholar]
- Schweitzer P., Madamba S., Siggins G. R. Arachidonic acid metabolites as mediators of somatostatin-induced increase of neuronal M-current. Nature. 1990 Aug 2;346(6283):464–467. doi: 10.1038/346464a0. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek C. D., Gallagher M. J. The stimulation of vasopressin gene expression in cultured hypothalamic neurons by cyclic adenosine 3',5'-monophosphate is reversible. Endocrinology. 1993 Sep;133(3):1320–1330. doi: 10.1210/endo.133.3.7689952. [DOI] [PubMed] [Google Scholar]
- Sukharev S. I., Martinac B., Arshavsky V. Y., Kung C. Two types of mechanosensitive channels in the Escherichia coli cell envelope: solubilization and functional reconstitution. Biophys J. 1993 Jul;65(1):177–183. doi: 10.1016/S0006-3495(93)81044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandorpe D. H., Small D. L., Dabrowski A. R., Morris C. E. FMRFamide and membrane stretch as activators of the Aplysia S-channel. Biophys J. 1994 Jan;66(1):46–58. doi: 10.1016/S0006-3495(94)80749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroel A. Suppression of neuronal potassium A-current by arachidonic acid. FEBS Lett. 1993 Dec 6;335(2):184–188. doi: 10.1016/0014-5793(93)80726-b. [DOI] [PubMed] [Google Scholar]
- Wallert M. A., Ackerman M. J., Kim D., Clapham D. E. Two novel cardiac atrial K+ channels, IK.AA and IK.PC. J Gen Physiol. 1991 Nov;98(5):921–939. doi: 10.1085/jgp.98.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. H., Errington M. L., Lynch M. A., Bliss T. V. Arachidonic acid induces a long-term activity-dependent enhancement of synaptic transmission in the hippocampus. Nature. 1989 Oct 26;341(6244):739–742. doi: 10.1038/341739a0. [DOI] [PubMed] [Google Scholar]
- Yang X. C., Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989 Feb 24;243(4894 Pt 1):1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]