Abstract
Bloodborne pathogens (BBPs) pose formidable challenges in the realm of infectious diseases, representing significant risks to both human and animal health worldwide. The review paper provides a thorough examination of bloodborne pathogens, highlighting the serious worldwide threat they pose and the effects they have on animal and human health. It addresses the potential dangers of exposure that healthcare workers confront, which have affected 3 million people annually, and investigates the many pathways by which these viruses can spread. The limitations of traditional detection techniques like PCR and ELISA have been criticized, which has led to the investigation of new detection methods driven by advances in sensor technology. The objective is to increase the amount of knowledge that is available regarding bloodborne infections as well as effective strategies for their management and detection. This review provides a thorough overview of common bloodborne infections, including their patterns of transmission, and detection techniques.
Keywords: bloodborne pathogens, biosensors, rapid detection techniques, diagnosis, transmission
Introduction
Microbes are ubiquitous and an integral part of our day-to-day lives. Some microbes are essential for our physiological functions. While most microbes are innocuous to us, some pathogens in nature can cause a spectrum of deadly diseases in humans (Balloux and van Dorp, 2017[11]). BBPs are harmful microbes that spread through blood and body fluids, posing a significant risk to healthcare professionals. HIV, HBV, and HCV are among the top BBPs reported globally (Mutangadura, 2004[110]). According to the World Health Organization (WHO), approximately 3 million healthcare workers are exposed to bloodborne viruses through percutaneous means. This exposure leads to an estimated annual occurrence of 16,000 cases of HCV, 66,000 cases of HBV, and 200 to 5,000 cases of HIV (Kermode et al., 2005[73]). To mitigate the risk of a pandemic and for safety and health concerns, precise detection of these pathogens is evident. Polymerase chain reaction (PCR) and enzyme-linked immunosorbent assay (ELISA) are the most used conventional techniques along with culture and colony counting methods to detect these pathogens (Lazcka et al., 2007[83]). While, these traditional methods, have been proven to be more time-consuming, complex, labor-intensive, and cost-ineffective. Biosensors, due to their ease of use, miniaturization, and real-time analysis capabilities, have gained attraction for accurate infectious illness diagnosis. Over the past three decades, biotechnological advancements have led to the development of biosensors (Vidic et al., 2017[152]). A biosensor is an assessment technique that has a molecular recognition molecule with biological origins into an appropriate physicochemical transducing mechanism and transforms it into an electrical signal from a biological response (Singh et al., 2014[139]). Bioreceptors can include various entities such as tissues, cells, enzymes, antibodies, nucleic acids, microorganisms, and organelles (Chen et al., 2020[23]). In the detection of BBPs, biosensors are commonly employed as transducing elements due to their exceptional sensitivity and accuracy. Various types of biosensors, including electrochemical, optical, microfluidics-based, and immunosensor-based biosensors, are utilized for this purpose. In addition to the traditional and advanced strategies, there are novel methods that have been effective at identifying BBPs like the electronic nose approach and aptamer approach. These non-conventional methods have also been proven to be equally specific, sensitive, flexible, and affordable over conventional techniques (Wilson and Baietto, 2011[154]; Li et al., 2020[88]).
Previous studies in the domain of BBPs reflect upon the nucleic acid diagnostics approaches and the general standardization to detect BBPs in humans and food animals. Moreover, studies ponder upon the overall pathogen detection and emphasize the traditional methods and advances in the field of diagnostics (Duncan et al., 2016[42]; Vidic et al., 2017[152]; Lazcka et al., 2007[83]; Li et al., 2020[89]). This study focuses on all the techniques used to detect BBPs with special emphasis on the recent advances in bloodborne diagnosis. This manuscript evaluates various methods for identifying bloodborne infections in humans and animals, categorizing them into conventional, non-conventional, and advanced approaches. It assesses the benefits of advanced detection methods and discusses prevalent BBPs and their transmission.
Bloodborne Pathogens and Their Prevalence
BBPs, including viruses and bacteria, are common in blood and bodily fluids and can cause disease in humans. They can be divided into viruses, bacteria, fungi, and parasites. Examples include Dengue, West Nile, rubella, measles, EBV, HIV, HBV, poliovirus, yellow fever, and varicella-zoster virus (VZV). HIV, HVB, and hepatitis are the top three BBPs in humans (Hunter, 2017[66]; Singhal et al., 2021[140]). Bacterial BBPs are the most common source of blood-related infections.
Some of the common bacterial BBPs are strains Staphylococcus, Bacillus, Enterococcus. Other than this Listeria monocytogenes, Escherichia coli, Klebsiella pneumoniae, Haemophilus influenzae, Yersinia pestis, Francisella tularensis, and Brucella abortus, etc. are also BBPs (Pingle et al., 2007[119]). An analysis of data from more than 200 medical facilities in 45 countries between 1997 and 2016 revealed the primary organisms that caused BSI in this survey were still E. coli and S. aureus (Diekema et al., 2019[40]). Candida spp., a fungi is the second most common source of blood-linked illnesses globally (Giri and Kindo, 2012[54]). Parasites like Cercopithifilaria, Babesia, Hepatozoon, and Theileria are some examples of parasitic BBPs that have attacked domestic animals in the countries of the Mediterranean Basin. Also, bacterial species of Anaplasma, Bartonella, Borrelia, Chlamydia/Chlamydophila, Coxiella, Ehrlichia, Francisella, Leptospira, Mycoplasma, Rickettsia, and viruses from the genus Capripoxvirus, Flavivirus, and Orthonairovirus tend to attack domestic animals in this region (Defaye et al., 2022[36]). Severe animal and human infections are caused by bloodborne parasites from the genus of Giardia, Trypanosoma, Babesia, Theileria, and Cryptosporidium. Additionally, the morbidity and mortality of animals have been linked to parasites of the genus Hepatozoon (Barbosa et al., 2017[13]). According to a study in Egypt, several pathogens affect food animals, including strains of Theileria, Borrelia, Anaplasma, Babesia, Coxiella (Abdullah et al., 2021[1]). The various BBPs prevailing in humans and food animals are enlisted in the Supplementary information (Table S1 and S2), respectively.
Transmission of bloodborne pathogens to humans and food animals
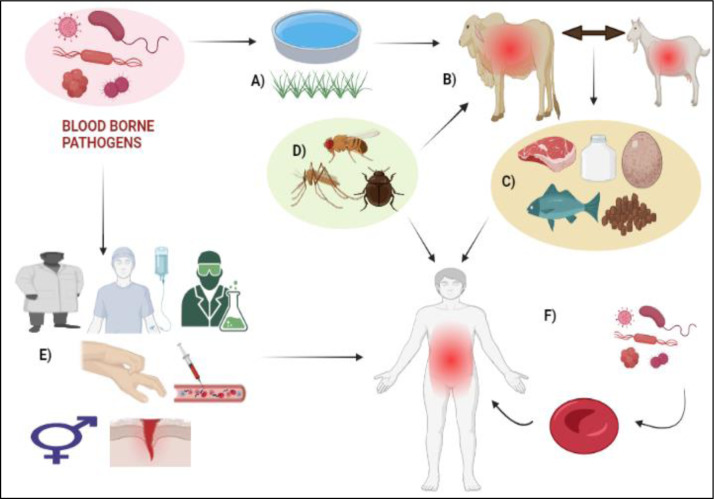
BBPs are microorganisms that can cause severe diseases when transmitted through the bloodstream. Bloodborne infections (BBIs) are the term used to describe the infection caused by the BBPs. Healthcare professionals are at a significant risk of exposure to BBPs, which can be spread through mucocutaneous contact, percutaneous damage, unintentional punctures, bites, cuts, and abrasions (Figure 1(Fig. 1)). Factors such as viral load, injury exposure, and recipient immune status influence the spread of BBPs. In Zambian healthcare, the average rate of sharp injuries per respondent is eight times higher than in the United States, indicating a higher risk of exposure to BBPs (Denault and Gardner, 2022[37]; Beltrami et al., 2000[14]; Lanphear, 1994[81]; Pirozzolo and LeMay, 2007[121]; Xeroulis et al., 2005[156]; Deuffic-Burban et al., 2011[38]; Cleveland et al., 2016[28]; Phillips et al., 2012[118]).
Figure 1. Transmission cycle of bloodborne pathogens (A) contaminated water and food B) consumption of water and food by animals, C) consuming food products like milk, meat, eggs, etc. produced from infected animals, D) insect vectors transmit bloodborne pathogens in humans as well as food animals, E) direct contact between healthcare professionals and infected patients, F) penetration of bloodborne pathogen in the bloodstream causes infectious diseases like AIDS, Hepatitis, Dengue, etc.
Food-producing animals (such as cattle, chickens, pigs, and turkeys) are the main reservoirs for several pathogens (EFSA, 2016[44]). Food-producing animals play an important role in pathogen transmission; for example, beef is claimed to have been the cause of 7 % of the 1.7 million cases of foodborne illness documented in England and Wales between 1996 and 2000 (Anderson et al., 2009[4]). Food animals, raw or undercooked meat, contaminated water, and food-processing equipment are potential sources of transmission for the Hepatitis E virus (HEV), the leading cause of enteric viral hepatitis infection globally. In Europe, domestic swine herds often exhibit high prevalence rates of HEV, making it a significant global health concern (EFSA, 2017[45]). Animals can also get ailments besides functioning as BBP transmission vectors. Transmission of BBPs in humans and animals is passed on via a varied scale of pathogens spread by arthropod routes (Figure 1(Fig. 1)) (Abebe et al., 2020[2]; Baneth, 2014[12]). Food animals such as sheep, goats, pigs, and chickens can potentially acquire Toxoplasma gondii infections. Tissue cysts are present in diseased animals, and consumption of these cysts in undercooked or raw meat can cause infection in humans. Tactyzoites found in blood products, tissue transplants, and unpasteurized milk may also be a source of transmission (Hill and Dubey, 2018[61]). Numerous species of hard ticks infest cattle, and these ticks can transmit several pathogenic diseases like bovine babesiosis, which is brought on by Babesia bigemina and Babesia bovis; bovine anaplasmosis, which is brought on by Anaplasma marginale; and heartwater, which is brought on by Ehrlichia ruminantium (Aouadi et al., 2017[6]).
The BBP located in the blood and skin of the animals that serve as its hosts are mechanically transported by the Stomoxys fly. It may spread bacteria like Trypanosoma, Besnoitia, and Rickettsia as well as viruses including African swine fever, Rift Valley, West Nile, and equine infectious anemia (Hailemariam et al., 2017[59]). Chagas disease (CD) is brought on by the protozoan parasite Trypanosoma cruzi (T. cruzi). It can be blowout by blood transfusions, organ transplantation, incidental blood exposure, eating food contaminated with infected triatomine insects, or any other situation where blood-sucking triatomine insects are present (Bern et al., 2011[15]; Jankowska et al., 2020[70]).
Detection methods of bloodborne pathogens
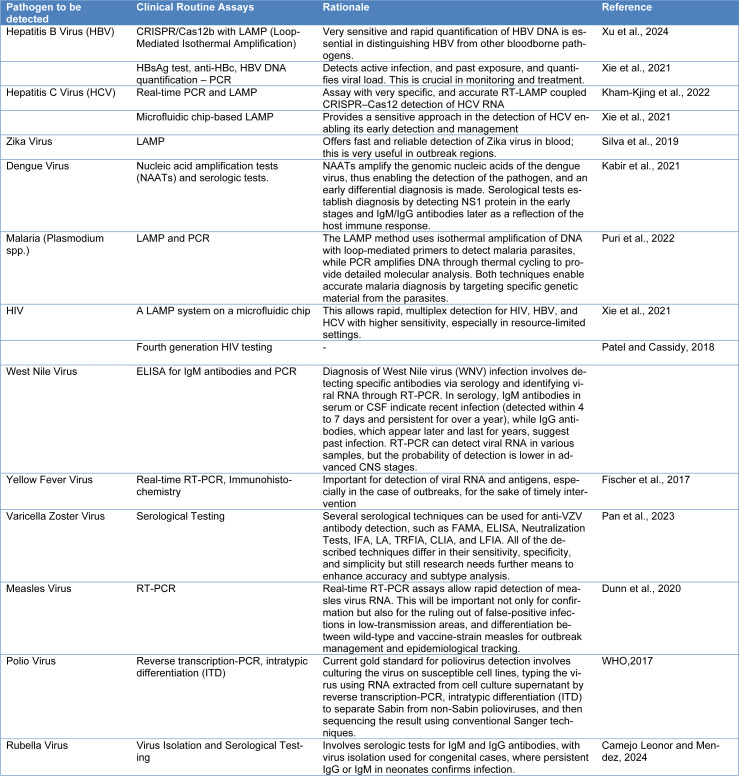
BBPs screening is crucial for preventing cross-infections and diseases transferred through blood transfusions. Biosensors are appealing analytical tools for rapid and accurate infectious disease detection because of their simplicity, ability to be miniaturized, and capacity for real-time analysis. Over the past 30 years, several biotechnological developments have created biosensors intended for the recognition and checking of pathogens (Vidic et al., 2017[152]). Advanced biosensing technologies, including aptamer-based and electronic nose techniques, have proven effective in identifying BBPs, offering quick screening for infectious illnesses and high stability (Park, 2018[115]; Turner and Magan, 2004[149]). The diagnostic methods used currently in clinical settings for detecting BBP in humans are mentioned in Table 1(Tab. 1) (References in Table 1: Camejo Leonor and Mendez, 2024[18]; Dunn et al., 2020[43]; Fischer et al., 2017[50]; Kabir et al., 2021[71]; Kham-Kjing et al., 2022[74]; Pan et al., 2023[113]; Patel and Cassidy, 2018[116]; Puri et al., 2022[123]; Silva et al., 2019[136]; WHO, 2017[153]; Xie et al., 2021[158]; Xu et al., 2024[161]).
Table 1. Current clinically used diagnostic techniques for the detection of bloodborne pathogens in humans.
Conventional Approaches for the Detection of Bloodborne Pathogens
PCR-based approaches to detect bloodborne pathogens
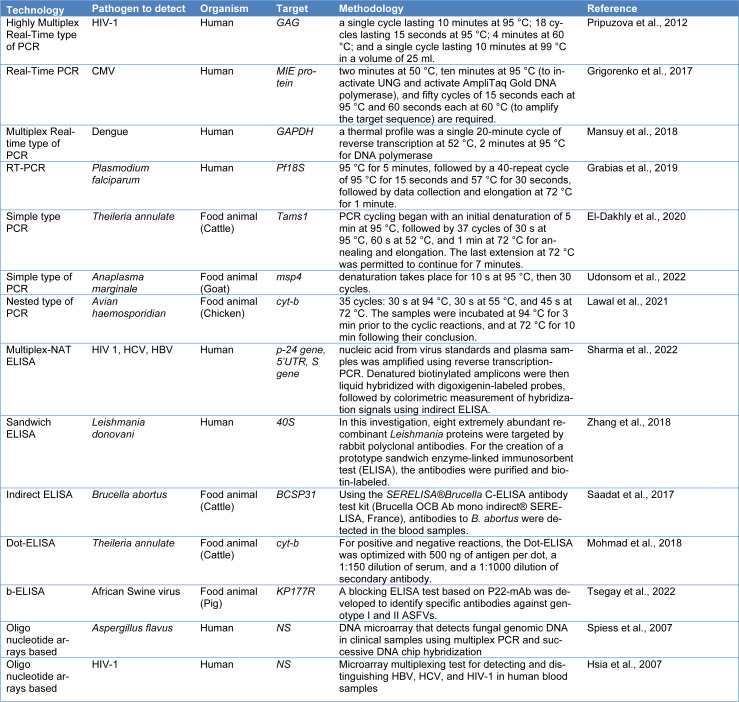
PCR is a laboratory enzymatic process that enables the amplification of short DNA segments. In PCR, target DNA is replicated using a thermostable DNA polymerase enzyme in the presence of nucleotides and primers (Yang and Rothman, 2004[163]). PCR plays a crucial role in molecular diagnostics as it allows for the sensitive and specific amplification of nucleic acids, making it a significant technique in molecular diagnostics and various fields of biological sciences (Miao et al., 2020[105]). Schijman et al. (2011[129]) conducted a groundbreaking investigation using blood samples from 32 seropositive and 10 seronegative patients to investigate blood infections brought on by the T. cruzi virus in patients from South Cone countries. They identified T. cruzi DNA at concentrations as low as 10 fg/microliter and 0.5 parasites/ml using Polymerase Chain Reaction (PCR) techniques, with sensitivity ranging from 83.3 % to 94.4 % and specificity ranging from 85 % to 95 %. With the potential to improve diagnostic capacities and disease management tactics in the fight against Chagas disease, this ground-breaking research marked a significant turning point in the global validation of PCR techniques for reliably detecting T. cruzi in human blood samples. Contini et al. (2005[29]) developed and evaluated a highly sensitive real-time PCR (Light-cycler, LC-PCR) to detect and quantify T. gondii B1 and bradyzoite-specific genes (SAG-4, MAG-1) in serum and PBMC specimens from immunocompetent subjects with or without suspected T. gondii infection. The LC-PCR targeting the B1 gene showed high sensitivity, detecting quantities as low as 102 to 10-3 parasites/ml. Cox et al. (2005[31]) collected field samples, including 245 samples of bovine blood drawn from communities in Uganda's Soroti and Tororo regions. Positive amplification was achieved even with DNA concentrations as low as 55 pg/ml, indicating the sensitivity of the PCR assay in detecting trypanosomes. However, the clinical application of PCR assays has certain limitations, including the potential for false-negative and false-positive results, background DNA contamination leading to false positives, detection sensitivity exceeding clinical significance, and limited detection capacity for simultaneous identification of multiple species, virulence factors, or drug resistance (Chen et al., 2013[21]; Schijman et al., 2011[129]; Contini et al., 2005[29]; Cox et al., 2005[31]). Table 2(Tab. 2) (References in Table 2: El-Dakhly et al., 2020[47]; Grabias et al., 2019[55]; Grigorenko et al., 2017[57]; Hsia et al., 2007[62]; Lawal et al., 2021[82]; Mansuy et al., 2018[102]; Mohmad et al., 2018[108]; Pripuzova et al., 2012[122]; Saadat et al., 2017[126]; Sharma et al., 2022[131]; Spiess et al., 2007[144]; Tsegay et al., 2022[148]; Udonsom et al., 2022[150]; Zhang et al., 2018[170]) describes the application of PCR to BBP detection.
Table 2. Conventional approaches to detect bloodborne pathogens in humans and food animals.
ELISA-based approaches to detect bloodborne pathogens
ELISA is a gold standard immunoassay used to detect and measure molecules like antibodies, antigens, proteins, glycoproteins, and hormones. It can identify biological processes quickly and cost-effectively, and detect significant infections. Antibodies are complexed with antigens, yielding quantifiable outcomes. The analyte can be any protein or a complex combination of proteins (Konstantinou, 2017[79]; de la Rica and Stevens, 2012[35]).
The ELISA test has been utilized in peptide and protein analysis a lot lately. This test is sensitive and focused, and it yields findings quickly. Due to its simplicity and quickness, it may be used in a wide range of situations. Additionally, it is more efficient because only one serum sample needs to be examined. The ELISA test does not need specialized tools or radioactive labeling, although it is virtually as sensitive as radio-immunoassays (RIA). However, compared to RIA, its dependability is poor (Aydin, 2015[8]). To investigate the biological importance of NS1 secretion in vivo and detect the presence of this protein in the sera of DENV-infected patients, Alcon et al. (2002[3]) developed a highly sensitive ELISA assay. The sensitivity of the ELISA was demonstrated to be less than 1 ng/ml when using pure dengue virus type 1 NS1 as a protein standard. Noedl et al. (2006[112]) collected a total of 700 whole blood samples from symptomatic outpatients of malaria clinics located along the Thai-Myanmar border. The HRP2 ELISA showed an overall sensitivity of 98.8 % (95 % CI, 93.6-100 %) and a specificity of 100 % (95 % CI, 99.5-100 %) for detecting P. falciparum malaria (Alcon et al., 2002[3]; Noedl et al., 2006[112]). Table 2(Tab. 2) describes the application of ELISA to BBP detection.
Array-based approaches to detectbloodborne pathogens
DNA microarrays are inverse dot blots in which a substrate is coupled to a lattice of ''probes'' with predetermined sequences. The final picture displays probes as "spots," where each spot represents a distinct probe sequence. Typically, spots are 200 to 500 micrometers (µm) apart and 100 to 200 micrometers (µm) in size. The array is loaded with targets, and any reporter molecules are employed to detect targets that combine with complementary probes. These later ones are either mechanically attached to the substrate or lithographically constructed on the spot. PCR by-products or oligonucleotides are used as probes (Call et al., 2003[17]). Microbial diagnostic microarrays (MDMs) utilize three types of probes: (I) short oligonucleotides, (II) long oligonucleotides, and (III) PCR amplicons. Short oligonucleotides often contain 1-2 mismatches, which can limit their binding ability and require the use of PCR amplification to enhance specificity. Stronger binding capacities can be used with more universal amplification techniques (like WGA-whole genome amplification), even though the discriminating ability of long oligonucleotide probes and PCR products is low (80-85 % sequence homology). Hybridization specificity is significantly influenced by the position of the mismatch in addition to probe length (Letowski et al., 2004[86]). The conventional microarray format typically consists of a flat glass slide that undergoes various surface modifications to facilitate the covalent attachment of probe molecules in research laboratories. However, this approach has drawbacks such as cost inefficiency and complex implementation across the organization. Table 2(Tab. 2) describes the application of array-based methods to BBP detection.
Advanced Approaches to Detect Bloodborne Pathogens
Biosensor-based approaches to detect bloodborne pathogens
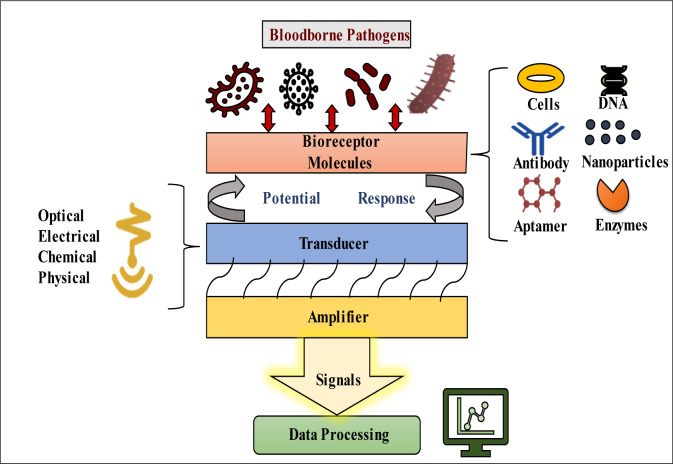
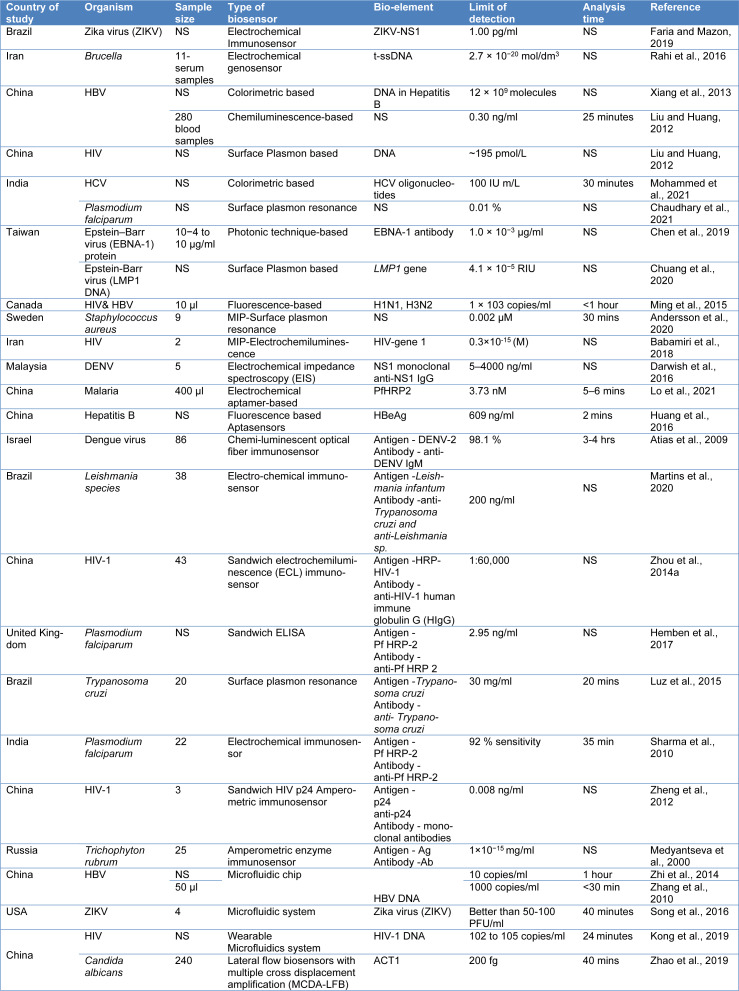
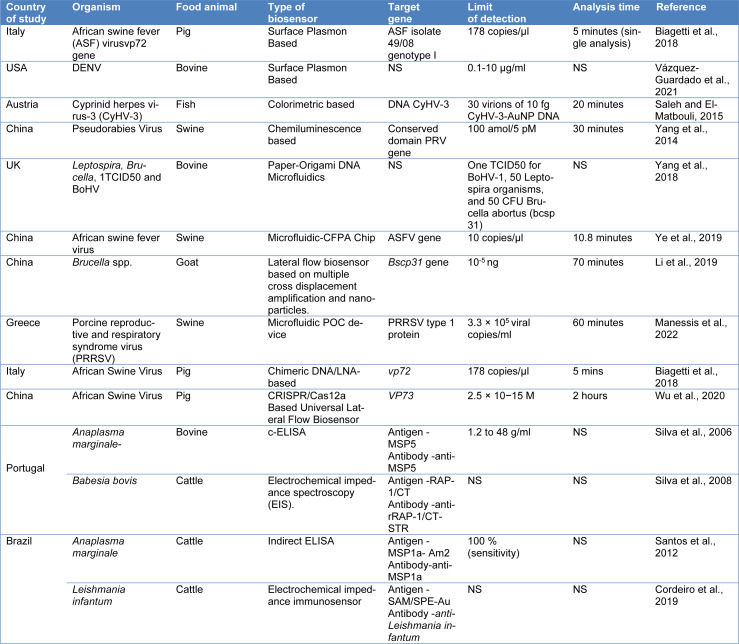
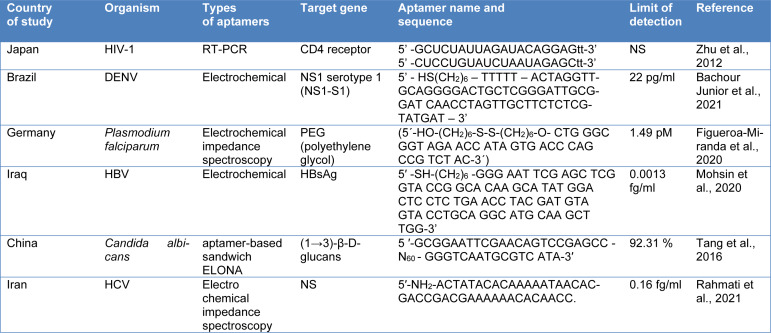
A biosensor is an analytic tool that uses a transducer to transform biological recognition of a target analyte into a quantifiable signal (Figure 2(Fig. 2)). The glucose sensor for the control of diabetes is the most well-known application of these sensors in the modern environment (Sin et al., 2014[137]). Lateral flow assays, such as home pregnancy tests, are also being extensively employed. The biosensor is not only an easy-to-use technique, but it is also an inexpensive technology with high sensitivity and specificity to rapidly identify pathogens that cause infectious diseases (Foudeh et al., 2012[51]). Biosensors are required to easily detect pathogens that cause emerging infectious diseases (EIDs). The analytical sensitivity of the device can easily detect very low levels of antigens without significant changes in selectivity. A biosensor is a cheap and robust technique that is highly desired for field applications since it provides high throughput (Pejcic et al., 2006[117]). In their study, Gray et al. (2018[56]) utilized an inexpensive component found in cell phones to identify HIV in 133 individuals. The biosensor exhibited remarkable sensitivity, specificity, low sample volumes, and rapid results. Testing different biomarkers in 102 healthy volunteers and 31 plasma samples from HIV patients, the biosensor achieved a combined sensitivity of 100 % for anti-gp41 and 64.5 % for anti-p24, with 100 % specificity, all within a 5-minute timeframe. Another study focused on a multiplex biosensor capable of identifying pathogens in physiological samples by detecting species-specific bacterial 16S ribosomal RNA sequences without the need for preamplification. The viability of this biosensor for pathogen identification and rapid diagnosis of bloodstream infections was investigated. The electrochemical biosensor demonstrated complete agreement with microbial analysis, successfully detecting various types of microbes present in the tested samples (Gray et al., 2018[56]). Different types of biosensors are typically briefed in Table 3(Tab. 3) (References in Table 3: Andersson et al., 2020[5]; Atias et al., 2009[7]; Babamiri et al., 2018[9]; Chaudhary et al., 2021[20]; Chen et al., 2019[24]; Chuang et al., 2020[27]; Darwish et al., 2016[34]; Faria and Mazon, 2019[48]; Hemben et al., 2017[60]; Huang et al., 2016[65]; Kong et al., 2019[78]; Liu and Huang, 2012[94]; Lo et al., 2021[95]; Luz et al., 2015[98]; Martins et al., 2020[103]; Medyantseva et al., 2000[104]; Ming et al., 2015[106]; Mohammed et al., 2021[107]; Rahi et al., 2016[124]; Sharma et al., 2010[130]; Song et al., 2016[142]; Xiang et al., 2013[157]; Zhang et al., 2010[169]; Zhao et al., 2019[171]; Zheng et al., 2012[172]; Zhi et al., 2014[173]; Zhou et al., 2014[174]) and Table 4(Tab. 4) (References in Table 4: Biagetti et al., 2018[16]; Cordeiro et al., 2019[30]; Li et al., 2019[90]; Manessis et al., 2022[101]; Saleh and El-Matbouli, 2015[127]; Santos et al., 2012[128]; Silva et al., 2006[134], 2008[135]; Vázquez-Guardado et al., 2021[151]; Wu et al., 2020[155]; Yang et al., 2014[162], 2018[164]; Ye et al., 2019[165]) with the technology specification.
Figure 2. A schematic diagram illustrating the working principle of a biosensor.
Table 3. Currently used biosensors to detect bloodborne pathogens in humans.
Table 4. Currently used biosensor to detect bloodborne pathogens in food animals.
Electrochemical biosensor to detect bloodborne pathogens
The fundamental principle of an electrochemical biosensor is to convert biological events, such as enzyme-substrate interactions and antigen-antibody interactions, into electrical signals, such as current, voltage, or impedance (Cho et al., 2020[26]). To obtain valuable information regarding flaviviral infections (such as DENV, ZIKV, and JEV), various electrochemical detection methods have been developed and utilized. These methods include potentiometry (measuring the potential of an indicator electrode), conductometry (measuring conductivity or resistance) and amperometry/voltammetry (measuring current as a function of imposed potential) (Khristunova et al., 2020[76]). The combination of amino-reduced graphene oxide (NH2-rGO) and β-cyclodextrin (-CD) was utilized to modify the surface of a glassy carbon electrode (GCE) to detect the HIV gene using differential pulse voltammetry (DPV). The electrochemical biosensor exhibited excellent sensitivity and selectivity, achieving a limit of detection (LOD) of 8.7 fM. The electrochemical biosensor was developed by Li (2020[89]) for detecting the HIV segment in human serum samples, demonstrating successful detection of the target using electrochemical methods (Li, 2020[89]). To detect the Listeria hlyA gene, an impedimetric biosensor was created. It worked by immobilizing a DNA-detecting probe on a poly-5-carboxy indole (5C Pin) polymer. When it came to target DNA concentration, this label-free biosensor showed a detectable linear range of 1 × 10-4 to 1 × 10-12 M (moles per liter). After being covalently immobilized on the 5C Pin polymer, the target DNA sequence underwent further hybridization with the probe. Charge transfer resistance, expressed in ohms (Ω), was used to assess the biosensor's performance and provide information on changes in electrical impedance at the electrode interface. With potential uses in clinical diagnostics and food safety, this novel method shows promise for the extremely sensitive and specific identification of Listeria hlyA gene sequences (Kashish et al., 2015[72]). Electrochemical biosensors provide quick, precise, and sensitive results at an affordable cost. Nanomaterials and nanocomposites enhance sensitivity. Microfluidic systems integrate with biosensors for miniaturized platforms. Challenges include stability, repeatability, low LOD values, and sample sensitivity (Singh et al., 2021[138]).
Optical biosensor to detect bloodborne pathogens
Optical biosensors, including colorimetric, fluorescence, SPR, optic fibers, and biological luminescence, are compact diagnostic devices used for pathogen detection, generating electrical signals (Damborský et al., 2016[32]; Eksin and Erdem, 2023[46]). These provide an alternative technique for viral detection, offering reliability, user-friendliness, and cost-effectiveness. They also reduce the reliance on nucleic acid amplification. These biosensors have been used to identify pathogens such as HIV, Ebola, norovirus, and influenza virus, utilizing techniques like fluorescence, surface plasmons, and colorimetry. Nano-biosensors enable targeted and single-virus scanning for infection detection (Maddali et al., 2020[99]). Colorimetric biosensors utilize ligand-target interactions that result in a visible color change, enabling simple and portable optical quantification (Piriya VS et al., 2017[120]). To quantify fluorescence, a fluorophore substance must be activated at one wavelength to produce light emission at another wavelength. Fluorescent dye-labeled reporter molecules provide for sensitive analyte detection (Li et al., 2013[87]). A fluorescent biosensor for HBV DNA sequence recognition based on gold nanorods (AuNRs) was created by Lu et al. (2013[96]). By mixing fluorescein-tagged single-stranded DNA (FAM-ssDNA) with the AuNRs solution, they produced a ternary complex known as FAM-ssDNA-CTABAuNRs. According to Lu et al. (2013[96]), this led to fluorescence resonance energy transfer (FRET) from FAM to AuNRs, which decreased the fluorescence intensity of FAM. One effective method for identifying molecular species at the single-molecule level is surface-enhanced Raman scattering (SERS). The sensitivity and chemical specificity of current optical detection techniques can be substantially exceeded by attaching molecules to nanostructured substrates, hence increasing the Raman signal (Lin et al., 2023[91]). An essential aspect of surface-enhanced Raman scattering (SERS) is surface plasmon resonance (SPR), which takes place at the interface between a metal and a dielectric. SPR occurs when longitudinal electron waves, induced by light that is polarized parallel to the surface, cause a concentration of oscillating electrons (Lazcka et al., 2007[83]). Liu and Huang (2012[94]) used DNA-silver nanoparticle (AgNP) conjugates in a range of 0.30 to 2.0 nmol/L to quantitatively detect HIV DNA using a sandwich approach based on SPR (Liu and Huang, 2012[94]). To detect changes in mass on the probe/transducer surface, piezoelectric sensors that employ quartz crystal microbalances (QCM) measure resonance frequency fluctuations (Lazcka et al., 2007[83]).
Aptamer-based biosensor to detect bloodborne pathogens
Aptamer-based biosensors, also known as aptasensors, are biosensors that use aptamers for identification. They are versatile, can be easily changed, and have predictable secondary structures. They are effective in detecting blood infections and are known for their rapid detection sensitivity due to their integration with signal amplification techniques (Liu et al., 2021[93]). However, the absence of excellent aptamers for therapeutically significant targets is one of the field's current constraints. To enhance its accuracy and dependability, the aptamer technology must also undergo comprehensive testing in a clinical sample matrix (Zhou et al., 2014[175]). A simple method was devised to construct aptamer-based fluorescence biosensors for the quantitative measurement of Hepatitis B antigen (HBeAg). The molecular detection element in this technique is an HBeAg aptamer that has been fluorescence-labeled, and a tiny DNA molecule that is similar to the aptamer is used as a competitor. It is reported that this approach yields a limit of detection (LOD) for HBeAg of 609 nanograms per milliliter (ng/ml). Fluorescence tests were performed on blood serums that were positive and negative for HBeAg after this biosensor was established, and statistical significance was noted (p < 0.05). Notably, it just takes two minutes to finish the detection test in its whole. These recently identified aptamers have the potential to enhance the chronic hepatitis B diagnosis process (Huang et al., 2016[65]). Furthermore, a different aptamer-based biosensor was created with good specificity for the diagnosis of the Hepatitis C virus (HCV) and the identification of the virus core antigen in infected individuals. A random library of 60 RNAs, consisting of roughly 1015 sequences, was used to generate high-affinity aptamers using the systematic evolution of ligands by the exponential enrichment (SELEX) approach. Subsequent protein chip tests revealed that the chosen aptamers bound to the HCV core antigen selectively, but not to the other HCV antigen, NS5. The concentrations and the limit of detection are accurately represented in nanograms per milliliter (ng/ml), giving a clear picture of the sensitivity of the detection. These biosensors based on aptamers have a great deal of promise to improve HCV infection detection and treatment (Lee et al., 2007[85]).
Immunosensor to detect bloodborne pathogens
Affinity solid-state-based biosensors, known as immunosensors, are utilized to monitor specific analytes, such as antigens (Ag), by establishing a stable interaction between the Ag and an antibody (Ab) that acts as the binding agent. This immune reaction generates a measurable signal through a transducer, as described by Gil Rosa et al. (2022[53]). Using highly specific antibody-antigen bond, a variety of biomolecules, including viruses, bacteria, protein markers, nucleic acids, and other tiny particles, are identified (Mahato et al., 2018[100]). According to one of the investigations, a gold film electrode from a recordable compact disc (CD-trade) was used to create an electrochemical immunosensor for the NS1 protein (Cavalcanti et al., 2012[19]). The immunosensor responded linearly to NS1 concentrations between 1 and 100 ng/ml, with a 0.33 ng/ml detection limit. With a detection limit of 200 fg/ml, a highly sensitive label-free immunosensor was created for the detection of HIV-1. It is a potential strategy for viral identification and evaluation of biological and medicinal samples (Lee et al., 2013[84]). Additionally, a completely novel amperometric immunosensor for the HIV-1 p24 antigen (p24Ag) detection was found. With a relatively low detection limit of 0.0064 ng/ml (S/N = 3), the predicted immunosensor demonstrated good electrochemical sensitivity to the presence of p24 in a concentration range of 0.01 to 60.00 ng/ml (Kheiri et al., 2011[75]). An ultrasensitive immunosensor for measuring hepatitis B surface antigen at a detection limit of 0.36 pg/ml exists, according to one of the assessments. Hepatitis B surface antigen was detectable by the immunosensor in the linear concentration range of 1.7 to 1920 pg/ml (Shourian et al., 2015[133]). Immunosensors have a significant deal of potential to develop into effective measuring devices because of their real-time estimation, constrained sample intake, relatively inexpensive price, and easy apparatus operation (Janik-Karpinska et al., 2022[69]).
Microfluidics-based biosensor to detect bloodborne pathogens
Microfluidic biosensors are integrated chip devices that combine various activities, offering reduced reagent and specimen consumption, flexible liquid manipulation, and reduced detection times due to their integration with characterization techniques (Xing et al., 2022[160]). Many microfluidic LOC devices have been used in a variety of assays and biosensors, such as paper microfluidic assays, magnetic bead cell sorting assays, particle immunoassays, and microfluidic ELISAs for pathogen detection. These advancements have raised the levels of specificity, sensitivity, usability, and mobility (Fronczek, 2013[52]). There are many instances where microfluidic approaches have proven to detect pathogens well. According to Huang and Huang (2019[64]), SERS may be utilized to quickly identify E. coli strains that cause sepsis when combined with microfluidic microwell technology. Conventional detection methods don't require long culture durations since the bacteria in the microwell may be contained there, increasing the effective bacterium concentration by up to 107 folds (Huang and Huang, 2019[64]). Micro- and nanotechnologies have permitted clear advances in HIV diagnosis, with the commercial availability of microfluidics-based CD4+ T cell counts utilizing a drop of blood from a finger prick (Damhorst et al., 2015[33]). In contrast to conventional methods, the employment of fluorescent microsphere-based lateral flow immunoassay strips (FM-LFIAs) for standard swine fever (CSFV) detection yielded a sensitivity of 5.28 ng/ml, covering a linear range of values from 9.77 to 625 ng/ml (Xie et al., 2020[159]). Ikeda et al. (2009[67]) showed how to use an on-chip flow cytometer and a disposable microfluidic device to detect Listeria monocytogenes (pathogenic bacteria) in milk (Ikeda et al., 2009[67]). Hsieh et al. devised a nanofluidic pre-concentrator and a nanoslit Fano resonance biosensor to identify latent membrane protein 1 (LMP1) for diagnosing Epstein-Barr virus (EBV). The cost-effective nano-slit plasmonic sensor chip can be produced on a large scale using nanoimprinting and aluminium deposition. The nonporous membrane was designed as an ion-selective channel integrated into the sensor chip to enhance the concentration of LMP1 proteins. Subsequently, a sensor chip employing anti-LMP1 IgG was developed to detect LMP1, achieving a limit of detection (LOD) of 100 pg/ml, while the sensing range spanned from 100 pg/ml to 10 g/ml. (Hsieh et al., 2022[63]). Studies show microfluidics offer accurate, convenient, and quick live pathogen detection techniques. However, they lack sensitivity and have limited capacity for high sample quantities. Obstacles like usability, cold storage, and electricity-free operations need to be addressed for low-resource applications (Spatola Rossi et al., 2023[143]).
Non-biosensing technology to detect bloodborne pathogens
Aptamer-based approaches to detect bloodborne pathogens
Aptamers are molecules having properties in a fashion like antibodies with complex 3-D structures. These single-strand nucleic acids (DNA, RNA) work by forming an affinity bond with their target molecules. Due to their increased stability, aptamers, which are typically 22-100 nucleobases in length, are capable of denaturation and renaturation. The variable region edged by constant regions in the aptamers allows the amplification and identification of sequences (Devi et al., 2021[39]; Sharma and Shukla, 2014[132]). Aptamers have been employed for pathogen detection and concentration applications because of their excellent specificity and affinity in binding to their target, which is dependent on the main sequence which is described in Table 5(Tab. 5) (References in Table 5: Bachour Junior et al., 2021[10]; Figueroa-Miranda et al., 2020[49]; Mohsin et al., 2020[109]; Rahmati et al., 2021[125]; Tang et al., 2016[146]; Zhu et al., 2012[176]) (Nagarkatti et al., 2014[111]). The detection and recognition of targets rely on factors such as the length of nucleic acid molecules within aptamers, the primary sequence, and various environmental variables. In addition, the hydrogen bonds, the forces of van der Waals, hydrophobic contacts, and the combination of complementarity in form all contribute to the intermolecular interaction that occurs between the target and aptamers. As a result, the aptamers gain distinctive qualities such as in vitro creation, stable temperature, pH intervals, non-toxicity, and better immune response (Yue et al., 2021[167]).
Table 5. Aptamer-based techniques to detect bloodborne pathogens.
Electronic nose-based approaches to detect bloodborne pathogens
An electronic nose (E-nose) is a device with chemical sensors that mimic human olfactory perceptions. It identifies volatile organic molecules with varying specificity and uses a signal-pre-processing unit and pattern recognition system. The gas sensor array converts molecular signals into electrical signals, generating unique odor patterns. E-nose offers rapid sensing and less bias, providing more consistent measurements between devices (Chen et al., 2013[22]; Ye et al., 2021[166]; Kumar et al., 2020[80]; Tan and Xu, 2020[145]). An illustration of the concept of utilizing an electronic nose to identify multiple categories of volatile organic compounds (VOCs) is present in the gas phase of blood samples. In this investigation, sera from 24 control cattle from para-TB non-suspect herds and sera from 43 dairy cattle with spontaneously occurring para-TB infections were obtained. Additionally, 24 sera from control cattle that were not infected and 26 sera from naturally brucellosis-infected animals were collected. At the population level, the e-nose could only discriminate sera from brucellosis and paratuberculosis-infected animals from healthy animals. The study's findings therefore demonstrate the potential of VOC analysis for the distinction of viral illnesses in animals, despite the limitations of the technology. Also, disease-specific volatiles must be found utilizing techniques like gas-chromatography. It is necessary to develop an e-nose approach so that VOC analysis can eventually be used as a cutting-edge diagnostic test (Pardo and Sberveglieri, 2010[114]).
Detection Challenges of Bloodborne Pathogens in Humans and Food Animals
Due to the characteristics of these bloodborne pathogens (BBPs) and the difficulty of detection techniques, detecting infections caused by them in humans and animals involves several difficulties (Dong et al., 2008[41]). Acquiring suitable samples for testing can be difficult, particularly when it comes to BBI and it may be necessary to perform intrusive operations that put the patient in danger or discomfort, such as blood draws or tissue biopsies (Smalls and Fischbach, 1982[141]). During the window period, the viral load is low, and antibodies or antigens may not be detected, resulting in false-negative findings (Taylor et al., 2014[147]). The accuracy of diagnostic testing is impacted by viral mutations in conditions like human immunodeficiency virus (HIV) infections (Guatelli et al., 1989[58]). Cross-reactivity occurs when antibodies or antigens that are identical to the target pathogen are present in diagnostic testing, leading to false-positive findings (Klarkowski et al., 2013[77]). Several BBPs, like HIV, HCV, and HBV, can develop chronic infections without exhibiting symptoms for a protracted length of time. As a result, afflicted people can go untreated and unwittingly spread the infections to others (Ludlam et al., 2006[97]). Food animals can contract bloodborne pathogens like Salmonella or the bovine viral diarrhea virus, causing persistent infections without visible symptoms. Multiple diseases can co-infect food animals, making diagnosis more challenging. Sensitivity and specificity are crucial for accurate detection of BBPs in humans and animals. However, accessibility and availability of diagnostic tests can be constrained in resource-constrained areas or during public health emergencies (Jain et al., 2021[68]; Chen et al., 2014[25]; Zadran et al., 2022[168]).
Conclusion and Recommendations
BBPs pose serious threats to the welfare of food animals as well as to human health due to their adverse effects. Through a variety of mechanisms of transmission, these viruses can enter circulation and cause various life-threatening infectious illnesses, known as BBIs. To maintain safety and stop the spread of illness, early detection of BBIs is essential. For the detection of these BBPs, techniques like PCR, ELISA, and array-based analysis have been used historically; nevertheless, they come with complexity and time-consuming requirements. However, the non-conventional methods have become very specific and sensitive substitutes for the identification of BBPs. Among these, biosensor technology is a particularly innovative means of detecting BBP. Biosensors based on optical, microfluidic, and immunosensor technologies have proven to be more sensitive and precise than others, and they also take less time and expenditure to operate. Future developments in biosensing technologies could potentially lead to improved BBP detection performance. The development of biosensors that are optimized for incredibly accurate BBP detection needs to be the top priority for future research projects. To successfully advise researchers, this entails addressing major knowledge gaps in the creation of biosensors and taking important references in BBP detection into consideration. Furthermore, the development of the next generation of biosensing technology, which may result from the integration of diverse biosensing approaches would allow for immediate and thorough monitoring of BBPs. The necessity of transferring lab-scale research into large-scale industrial output must be made widely known. To achieve the highest standards of safety for consumers and healthcare professionals, as well as to ensure the safety of food animals, this transformation is essential. Overall, the continued advancement and adoption of biosensor technology offer promising prospects for early and efficient detection of BBPs. By harnessing the capabilities of biosensors and addressing key challenges, we can significantly enhance our ability to monitor and manage infectious diseases, ultimately safeguarding public health worldwide.
Notes
Rachna Verma and Asaad Khalid (Health Research Center, Jazan University, P. O. Box 114, Jazan, 82511, Saudi Arabia; E-mail: drasaad@gmail.com) contributed equally as corresponding author.
Declaration
Data availability
All data related to this study has been included in the manuscript.
Declaration of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
The authors gratefully acknowledge the support of the Deanship of Graduate Studies and Scientific Research, Jazan University, Saudi Arabia, for this research through the Research Funding Program (Project Number: RG24-L05).
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Research involving human participants and/or animals
Not applicable.
Supplementary Material
References
- 1.Abdullah HH, Amanzougaghene N, Dahmana H, Louni M, Raoult D, Mediannikov O. Multiple vector-borne pathogens of domestic animals in Egypt. PLoS Negl Trop Dis. 2021;15(9):e0009767. doi: 10.1371/journal.pntd.0009767. doi: 10.1371/journal.pntd.0009767. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abebe E, Gugsa G, Ahmed M. Review on major food-borne zoonotic bacterial pathogens. J Trop Med. 2020;2020:4674235. doi: 10.1155/2020/4674235. doi: 10.1155/2020/4674235. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alcon S, Talarmin A, Debruyne M, Falconar A, Deubel V, Flamand M, et al. Enzyme-linked immunosorbent assay specific to dengue virus type 1 Nonstructural Protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J Clin Microbiol. 2002;40:376–381. doi: 10.1128/JCM.40.02.376-381.2002. doi: 10.1128/JCM.40.02.376-381.2002. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson RC, Ricke SC, Lungu B, Johnson MG, Oliver C, Horrocks SM, et al. Food safety issues and the microbiology of Beef. In: Ricke SC, editor. Microbiologically safe foods. Chichester: Wiley-Blackwell; 2009. pp. 113–145. Available from: [DOI] [Google Scholar]
- 5.Andersson T, Bläckberg A, Lood R, Ertürk Bergdahl G. Development of a molecular imprinting-based surface plasmon resonance biosensor for rapid and sensitive detection of Staphylococcus aureus alpha hemolysin from human serum. Front Cell Infect Microbiol. 2020;10:571578. doi: 10.3389/fcimb.2020.571578. doi: 10.3389/fcimb.2020.571578. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aouadi A, Leulmi H, Boucheikhchoukh M, Benakhla A, Raoult D, Parola P. Molecular evidence of tick-borne hemoprotozoan-parasites (theileria ovis and Babesia ovis) and bacteria in ticks and blood from small ruminants in northern Algeria. Comp Immunol Microbiol Infect Dis. 2017;50:34–9. doi: 10.1016/j.cimid.2016.11.008. doi: 10.1016/j.cimid.2016.11.008. Available from: [DOI] [PubMed] [Google Scholar]
- 7.Atias D, Liebes Y, Chalifa-Caspi V, Bremand L, Lobel L, Marks RS, et al. Chemiluminescent optical fiber immunosensor for the detection of IGM antibody to dengue virus in humans. Sens Actuators B Chem. 2009;140:206–15. doi: 10.1016/j.snb.2009.03.044. doi: 10.1016/j.snb.2009.03.044. Available from: [DOI] [Google Scholar]
- 8.Aydin S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides. 2015;72:4–15. doi: 10.1016/j.peptides.2015.04.012. doi: 10.1016/j.peptides.2015.04.012. Available from: [DOI] [PubMed] [Google Scholar]
- 9.Babamiri B, Salimi A, Hallaj R. A molecularly imprinted electrochemiluminescence sensor for ultrasensitive HIV-1 gene detection using EuS nanocrystals as luminophore. Biosens Bioelectron. 2018;117:332–9. doi: 10.1016/j.bios.2018.06.003. doi: 10.1016/j.bios.2018.06.003. Available from: [DOI] [PubMed] [Google Scholar]
- 10.Bachour Junior B, Batistuti MR, Pereira AS, de Sousa Russo EM, Mulato M. Electrochemical aptasensor for NS1 detection: Towards a fast dengue biosensor. Talanta. 2021;233:122527. doi: 10.1016/j.talanta.2021.122527. doi: 10.1016/j.talanta.2021.122527. Available from: [DOI] [PubMed] [Google Scholar]
- 11.Balloux F, van Dorp L. Q&A: What are pathogens, and what have they done to and for us? BMC Biol. 2017;15(1):91. doi: 10.1186/s12915-017-0433-z. doi: 10.1186/s12915-017-0433-z. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baneth G. Tick-borne infections of animals and humans: A common ground. Int J Parasitol. 2014;44:591–6. doi: 10.1016/j.ijpara.2014.03.011. doi: 10.1016/j.ijpara.2014.03.011. Available from: [DOI] [PubMed] [Google Scholar]
- 13.Barbosa A, Reiss A, Jackson B, Warren K, Paparini A, Gillespie G, et al. Prevalence, genetic diversity and potential clinical impact of blood-borne and enteric protozoan parasites in native mammals from northern Australia. Vet Parasitol. 2017;238:94–105. doi: 10.1016/j.vetpar.2017.04.007. doi: 10.1016/j.vetpar.2017.04.007. Available from: [DOI] [PubMed] [Google Scholar]
- 14.Beltrami EM, Williams IT, Shapiro CN, Chamberland ME. Risk and management of blood-borne infections in health care workers. Clin Microbiol Rev. 2000;13:385–407. doi: 10.1128/cmr.13.3.385-407.2000. doi: 10.1128/cmr.13.3.385-407.2000. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas' disease in the United States. Clin Microbiol Rev. 2011;24:655–81. doi: 10.1128/cmr.00005-11. doi: 10.1128/cmr.00005-11. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biagetti M, Cuccioloni M, Bonfili L, Cecarini V, Sebastiani C, Curcio L, et al. Chimeric DNA/LNA-based biosensor for the rapid detection of African Swine Fever virus. Talanta. 2018;184:35–41. doi: 10.1016/j.talanta.2018.02.095. doi: 10.1016/j.talanta.2018.02.095. Available from: [DOI] [PubMed] [Google Scholar]
- 17.Call DR, Borucki MK, Loge FJ. Detection of bacterial pathogens in environmental samples using DNA microarrays. J Microbiol Methods. 2003;53:235–43. doi: 10.1016/s0167-7012(03)00027-7. doi: 10.1016/s0167-7012(03)00027-7. Available from: [DOI] [PubMed] [Google Scholar]
- 18.Camejo Leonor M, Mendez MD. Treasure Island (FL): StatPearls Publ; 2024. Rubella. StatPearls [Internet] [PubMed] [Google Scholar]
- 19.Cavalcanti IT, Guedes MIF, Sotomayor MDPT, Yamanaka H, Dutra RF. A label-free immunosensor based on recordable compact disk chip for early diagnostic of the Dengue Virus Infection. Biochem Eng J. 2012;67:225–30. doi: 10.1016/j.bej.2012.06.016. doi: 10.1016/j.bej.2012.06.016. Available from: [DOI] [Google Scholar]
- 20.Chaudhary VS, Kumar D, Kumar S. Gold-immobilized photonic crystal fiber-based SPR biosensor for detection of malaria disease in human body. IEEE Sens J. 2021;21:17800–7. doi: 10.1109/jsen.2021.3085829. doi: 10.1109/jsen.2021.3085829. Available from: [DOI] [Google Scholar]
- 21.Chen J, Chen D, Xie Y, Yuan T, Chen X. Progress of microfluidics for biology and medicine. Nano-Micro Lett. 2013;5(1):66–80. doi: 10.1007/bf03354852. doi: 10.1007/bf03354852. Available from: [DOI] [Google Scholar]
- 22.Chen S, Wang Y, Choi S. Applications and technology of electronic nose for clinical diagnosis. Open J Appl Biosens. 2013;2(2):39–50. doi: 10.4236/ojab.2013.22005. doi: 10.4236/ojab.2013.22005. Available from: [DOI] [Google Scholar]
- 23.Chen Y, Qian C, Liu C, Shen H, Wang Z, Ping J, et al. Nucleic acid amplification free biosensors for pathogen detection. Biosens Bioelectron. 2020;153:112049. doi: 10.1016/j.bios.2020.112049. doi: 10.1016/j.bios.2020.112049. Available from: [DOI] [PubMed] [Google Scholar]
- 24.Chen YT, Liao YY, Chen CC, Hsiao HH, Huang JJ. Surface plasmons coupled two-dimensional photonic crystal biosensors for Epstein-Barr virus protein detection. Sens Actuators B Chem. 2019;291:81–8. doi: 10.1016/j.snb.2019.04.059. doi: 10.1016/j.snb.2019.04.059. Available from: [DOI] [Google Scholar]
- 25.Chen Z, Liu Q, Liu JQ, Xu BL, Lv S, Xia S, et al. Tick-borne pathogens and associated co-infections in ticks collected from domestic animals in Central China. Parasit Vectors. 2014;7(1):237. doi: 10.1186/1756-3305-7-237. doi: 10.1186/1756-3305-7-237. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho IH, Kim DH, Park S. Electrochemical biosensors: Perspective on functional nanomaterials for on-site analysis. Biomater Res. 2020;24(1):22. doi: 10.1186/s40824-019-0181-y. doi: 10.1186/s40824-019-0181-y. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chuang CS, Wu CY, Juan PH, Hou NC, Fan YJ, Wei PK, et al. lmp1gene detection using a capped gold nanowire array surface plasmon resonance sensor in a microfluidic chip. Analyst. 2020;145(1):52–60. doi: 10.1039/c9an01419e. doi: 10.1039/c9an01419e. Available from: [DOI] [PubMed] [Google Scholar]
- 28.Cleveland JL, Gray SK, Harte JA, Robison VA, Moorman AC, Gooch BF. Transmission of blood-borne pathogens in US dental health care settings. J Am Dent Assoc. 2016;147:729–738. doi: 10.1016/j.adaj.2016.03.020. doi: 10.1016/j.adaj.2016.03.020. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Contini C, Seraceni S, Cultrera R, Incorvaia C, Sebastiani A, Picot S. Evaluation of a real-time PCR-based assay using the LightCycler system for detection of Toxoplasma gondii bradyzoite genes in blood specimens from patients with toxoplasmic retinochoroiditis. Int J Parasitol. 2005;35:275–283. doi: 10.1016/j.ijpara.2004.11.016. doi: 10.1016/j.ijpara.2004.11.016. Available from: [DOI] [PubMed] [Google Scholar]
- 30.Cordeiro TAR, Gonçalves MVC, Franco DL, Reis AB, Martins HR, Ferreira LF. Label-free electrochemical impedance immunosensor based on modified screen-printed gold electrodes for the diagnosis of canine visceral leishmaniasis. Talanta. 2019;195:327–332. doi: 10.1016/j.talanta.2018.11.087. doi: 10.1016/j.talanta.2018.11.087. Available from: [DOI] [PubMed] [Google Scholar]
- 31.Cox A, Tilley A, McOdimba F, Fyfe J, Eisler M, Hide G, et al. A PCR based assay for detection and differentiation of African trypanosome species in blood. Exp Parasitol. 2005;111(1):24–29. doi: 10.1016/j.exppara.2005.03.014. doi: 10.1016/j.exppara.2005.03.014. Available from: [DOI] [PubMed] [Google Scholar]
- 32.Damborský P, Švitel J, Katrlík J. Optical biosensors. Essays Biochem. 2016;60(1):91–100. doi: 10.1042/EBc20150010. doi: 10.1042/EBc20150010. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Damhorst GL, Murtagh M, Rodriguez WR, Bashir R. Microfluidics and nanotechnology for detection of global infectious diseases. Proc IEEE. 2015;103:150–160. doi: 10.1109/jproc.2014.2385078. doi: 10.1109/jproc.2014.2385078. Available from: [DOI] [Google Scholar]
- 34.Darwish NT, Alrawi AH, Sekaran SD, Alias Y, Khor SM. Electrochemical immunosensor based on antibody-nanoparticle hybrid for specific detection of the dengue virus NS1 Biomarker. J Electrochem Soc. 2016;163(3):B19–B25. doi: 10.1149/2.0471603jes. doi: 10.1149/2.0471603jes. Available from: [DOI] [Google Scholar]
- 35.de la Rica R, Stevens MM. Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat Nanotechnol. 2012;7:821–824. doi: 10.1038/nnano.2012.186. doi: 10.1038/nnano.2012.186. Available from: [DOI] [PubMed] [Google Scholar]
- 36.Defaye B, Moutailler S, Pasqualini V, Quilichini Y. Distribution of tick-borne pathogens in domestic animals and their ticks in the countries of the Mediterranean Basin between 2000 and 2021: A systematic review. Microorganisms. 2022;10(6):1236. doi: 10.3390/microorganisms10061236. doi: 10.3390/microorganisms10061236. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denault D, Gardner H. Treasure Island (FL): StatPearls Publ; 2022. OSHA bloodborne pathogen standards. StatPearls [Internet] [PubMed] [Google Scholar]
- 38.Deuffic-Burban S, Delarocque-Astagneau E, Abiteboul D, Bouvet E, Yazdanpanah Y. Blood-borne viruses in health care workers: Prevention and Management. J Clin Virol. 2011;52(1):4–10. doi: 10.1016/j.jcv.2011.05.016. doi: 10.1016/j.jcv.2011.05.016. Available from: [DOI] [PubMed] [Google Scholar]
- 39.Devi S, Sharma N, Ahmed T, Huma ZI, Kour S, Sahoo B, et al. Aptamer-based diagnostic and therapeutic approaches in animals: Current potential and challenges. Saudi J Biol Sci. 2021;28:5081–5093. doi: 10.1016/j.sjbs.2021.05.031. doi: 10.1016/j.sjbs.2021.05.031. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diekema DJ, Hsueh PR, Mendes RE, Pfaller MA, Rolston KV, Sader HS, et al. The microbiology of bloodstream infection: 20-year trends from the SENTRY Antimicrobial Surveillance Program. Antimicrob Agents Chemother. 2019;63(7):e00355–e00319. doi: 10.1128/AAC.00355-19. doi: 10.1128/AAC.00355-19. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong J, Olano JP, McBride JW, Walker DH. Emerging pathogens: Challenges and successes of molecular diagnostics. J Mol Diagn. 2008;10:185–197. doi: 10.2353/jmoldx.2008.070063. doi: 10.2353/jmoldx.2008.070063. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duncan R, Kourout M, Grigorenko E, Fisher C, Dong M. Advances in multiplex nucleic acid diagnostics for blood-borne pathogens: Promises and pitfalls. Expert Rev Mol Diagn. 2016;16(1):83–95. doi: 10.1586/14737159.2016.1112272. doi: 10.1586/14737159.2016.1112272. Available from: [DOI] [PubMed] [Google Scholar]
- 43.Dunn JJ, Baldanti F, Puchhammer-Stockl E, Panning M, Perez O, Harvala H. Measles is back – considerations for laboratory diagnosis. J Clin Virol. 2020;128:104430. doi: 10.1016/j.jcv.2020.104430.. doi: 10.1016/j.jcv.2020.104430.. Available from: [DOI] [PubMed] [Google Scholar]
- 44.EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2015. EFSA J. 2016;14(12):4634. doi: 10.2903/j.efsa.2016.4634. doi: 10.2903/j.efsa.2016.4634. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.EFSA (European Food Safety Authority) Scientific Committee, Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen HK, More S, et al. Guidance on the risk assessment of substances present in food intended for infants below 16 weeks of age. EFSA J. 2017;15(5):4849. doi: 10.2903/j.efsa.2017.4849. doi: 10.2903/j.efsa.2017.4849. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eksin E, Erdem A. Recent progress on optical biosensors developed for nucleic acid detection related to infectious viral diseases. Micromachines. 2023;14(2):295. doi: 10.3390/mi14020295. doi: 10.3390/mi14020295. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Dakhly KM, Arafa WM, Soliman S, Abdel-Fatah OR, Wahba AA, Esteve-Gasent MD, et al. Molecular detection, phylogenetic analysis, and genetic diversity of Theileria annulata, Babesia Bigemina, and Anaplasma marginale in cattle in three districts of Egypt. Acta Parasitol. 2020;65:620–627. doi: 10.2478/s11686-020-00189-z. doi: 10.2478/s11686-020-00189-z. Available from: [DOI] [PubMed] [Google Scholar]
- 48.Faria AM, Mazon T. Early diagnosis of Zika infection using a ZnO nanostructures-based rapid electrochemical biosensor. Talanta. 2019;203:153–160. doi: 10.1016/j.talanta.2019.04.080. doi: 10.1016/j.talanta.2019.04.080. Available from: [DOI] [PubMed] [Google Scholar]
- 49.Figueroa-Miranda G, Wu C, Zhang Y, Nörbel L, Lo Y, Tanner JA, et al. Polyethylene glycol-mediated blocking and monolayer morphology of an electrochemical aptasensor for malaria biomarker detection in human serum. Bioelectrochemistry. 2020;136:107589. doi: 10.1016/j.bioelechem.2020.107589. doi: 10.1016/j.bioelechem.2020.107589. Available from: [DOI] [PubMed] [Google Scholar]
- 50.Fischer C, Torres MC, Patel P, Moreira-Soto A, Gould EA, Charrel RN, et al. Lineage-specific real-time RT-PCR for yellow fever virus outbreak surveillance, Brazil. Emerg Infect Dis. 2017;23:1867–1871. doi: 10.3201/eid2311.171131. doi: 10.3201/eid2311.171131. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Foudeh AM, Fatanat Didar T, Veres T, Tabrizian M. Microfluidic designs and techniques using lab-on-a-chip devices for pathogen detection for point-of-care diagnostics. Lab Chip. 2012;12:3249–3266. doi: 10.1039/c2lc40630f. doi: 10.1039/c2lc40630f. Available from: [DOI] [PubMed] [Google Scholar]
- 52.Fronczek CF. Lab-on-a-chip biosensors for the rapid detection of pathogens in clinical and field samples. University of Arizona, Thesis. 2013. Available from: https://repository.arizona.edu/handle/10150/311459. [Google Scholar]
- 53.Gil Rosa B, Akingbade OE, Guo X, Gonzalez-Macia L, Crone MA, Cameron LP, et al. Multiplexed immunosensors for point-of-care diagnostic applications. Biosens Bioelectron. 2022;203:114050. doi: 10.1016/j.bios.2022.114050.. doi: 10.1016/j.bios.2022.114050.. Available from: [DOI] [PubMed] [Google Scholar]
- 54.Giri S, Kindo AJ. A review of candida species causing blood stream infection. Indian J Med Microbiol. 2012;30:270–278. doi: 10.4103/0255-0857.99484. doi: 10.4103/0255-0857.99484. Available from: [DOI] [PubMed] [Google Scholar]
- 55.Grabias B, Essuman E, Quakyi IA, Kumar S. Sensitive real-time PCR detection of Plasmodium falciparum parasites in whole blood by erythrocyte membrane protein 1 gene amplification. Malar J. 2019;18(1):288. doi: 10.1186/s12936-019-2743-9. doi: 10.1186/s12936-019-2743-9. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gray ER, Turbé V, Lawson VE, Page RH, Cook ZC, Bridget Ferns R, et al. Ultra-rapid, sensitive and specific digital diagnosis of HIV with a dual-channel SAW biosensor in a pilot clinical study. NPJ Digit Med. 2018;1(1):30. doi: 10.1038/s41746-018-0041-5. doi: 10.1038/s41746-018-0041-5. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grigorenko E, Fisher C, Patel S, Winkelman V, Williamson P, Chancey C, et al. Highly multiplex real-time PCR-based screening for blood-borne pathogens on an OpenArray platform. J Mol Diagn. 2017;19:549–560. doi: 10.1016/j.jmoldx.2017.03.004. doi: 10.1016/j.jmoldx.2017.03.004. Available from: [DOI] [PubMed] [Google Scholar]
- 58.Guatelli JC, Gingeras TR, Richman DD. Nucleic acid amplification in vitro: Detection of sequences with low copy numbers and application to diagnosis of human immunodeficiency virus type 1 infection. Clin Microbiol Rev. 1989;2:217–226. doi: 10.1128/cmr.2.2.217. doi: 10.1128/cmr.2.2.217. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hailemariam Z, Krücken J, Baumann M, Ahmed JS, Clausen P-H, Nijhof AM. Molecular detection of tick-borne pathogens in cattle from southwestern Ethiopia. PLoS One. 2017;12(11):e0188248. doi: 10.1371/journal.pone.0188248. doi: 10.1371/journal.pone.0188248. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hemben A, Ashley J, Tothill I. Development of an immunosensor for pfhrp 2 as a biomarker for malaria detection. Biosensors (Basel) 2017;7(4):28. doi: 10.3390/bios7030028. doi: 10.3390/bios7030028. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hill DE, Dubey JP. Toxoplasma gondii as a parasite in food: Analysis and control. In: Thakur S, Kniel KE, editors. Preharvest food safety. Chichester: Wiley; 2018. pp. 227–247. Available from: [DOI] [Google Scholar]
- 62.Hsia CC, Chizhikov VE, Yang AX, Selvapandiyan A, Hewlett I, Duncan R, et al. Microarray multiplex assay for the simultaneous detection and discrimination of hepatitis B, hepatitis C, and human immunodeficiency type-1 viruses in human blood samples. Biochem Biophys Res Commun. 20078;356:1017–23. doi: 10.1016/j.bbrc.2007.03.087. doi: 10.1016/j.bbrc.2007.03.087. Available from: [DOI] [PubMed] [Google Scholar]
- 63.Hsieh H-Y, Luo J-X, Shen Y-H, Lo S-C, Hsu Y-C, Tahara H, et al. A nanofluidic preconcentrator integrated with an aluminum-based nanoplasmonic sensor for Epstein-Barr Virus Detection. Sens Actuators B Chem. 2022;355:131327. doi: 10.1016/j.snb.2021.131327. doi: 10.1016/j.snb.2021.131327. Available from: [DOI] [Google Scholar]
- 64.Huang H-K, Huang N-T. A microfluidic Microwell device integrating surface-enhanced Raman scattering for rapid antibiotic susceptibility test of blood-borne pathogen. 2019 IEEE 14th International Conference on Nano/Micro Engineered and Molecular Systems (NEMS); 2019. Available from: [DOI] [Google Scholar]
- 65.Huang R, Xi Z, Deng Y, He N. Fluorescence based aptasensors for the determination of hepatitis B virus E antigen. Sci Rep. 2016;6:31103. doi: 10.1038/srep31103. doi: 10.1038/srep31103. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hunter S. The Five W's of Bloodborne Pathogens: Who, What, When, Where, and Why. Murray, KY: Murray State University; 2017. (Integrated Studies, no. 70). Available from: https://digitalcommons.murraystate.edu/bis437/70. [Google Scholar]
- 67.Ikeda M, Yamaguchi N, Nasu M. Rapid on-chip flow cytometric detection of listeria monocytogenes in milk. J Health Sci. 2009;55:851–6. doi: 10.1248/jhs.55.851. doi: 10.1248/jhs.55.851. Available from: [DOI] [Google Scholar]
- 68.Jain U, Shakya S, Saxena K. Nano-biosensing devices detecting biomarkers of communicable and non-communicable diseases of animals. In: Pudake RN, Jain U, Kole C, editors. Biosensors in agriculture: recent trends and future perspectives. Cham: Springer; 2021. pp. 415–434. Available from: [DOI] [Google Scholar]
- 69.Janik-Karpinska E, Ceremuga M, Niemcewicz M, Podogrocki M, Stela M, Cichon N, et al. Immunosensors - the future of pathogen real-time detection. Sensors (Basel) 2022;22(24):9757. doi: 10.3390/s22249757.. doi: 10.3390/s22249757.. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jankowska KI, Nagarkatti R, Acharyya N, Dahiya N, Stewart CF, Macpherson RW, et al. Complete inactivation of blood borne pathogen Trypanosoma cruzi in stored human platelet concentrates and plasma treated with 405 nm violet-blue light. Front Med. 2020;7:617373. doi: 10.3389/fmed.2020.617373. doi: 10.3389/fmed.2020.617373. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kabir MA, Zilouchian H, Younas MA, Asghar W. Dengue detection: advances in diagnostic tools from conventional technology to point of care. Biosensors (Basel) 2021;11(7):206. doi: 10.3390/bios11070206.. doi: 10.3390/bios11070206.. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kashish, Soni DK, Mishra SK, Prakash R, Dubey SK. Label-free impedimetric detection of listeria monocytogenes based on poly-5-carboxy indole modified ssDNA probe. J Biotechnol. 2015;200:70–6. doi: 10.1016/j.jbiotec.2015.02.025. doi: 10.1016/j.jbiotec.2015.02.025. Available from: [DOI] [PubMed] [Google Scholar]
- 73.Kermode M, Jolley D, Langkham B, Thomas MS, Crofts N. Occupational exposure to blood and risk of bloodborne virus infection among health care workers in rural North Indian Health Care Settings. Am J Infect Control. 2005;33(1):34–41. doi: 10.1016/j.ajic.2004.07.015. doi: 10.1016/j.ajic.2004.07.015. Available from: [DOI] [PubMed] [Google Scholar]
- 74.Kham-Kjing N, Ngo-Giang-Huong N, Tragoolpua K, Khamduang W, Hongjaisee S. Highly specific and rapid detection of hepatitis C virus using RT-LAMP-coupled CRISPR-Cas12 assay. Diagnostics (Basel) 2022;12(7):1524. doi: 10.3390/diagnostics12071524.. doi: 10.3390/diagnostics12071524.. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kheiri F, Sabzi RE, Jannatdoust E, Shojaeefar E, Sedghi H. A novel amperometric immunosensor based on acetone-extracted propolis for the detection of the HIV-1 P24 antigen. Biosens Bioelectron. 2011;26:4457–63. doi: 10.1016/j.bios.2011.05.002. doi: 10.1016/j.bios.2011.05.002. Available from: [DOI] [PubMed] [Google Scholar]
- 76.Khristunova E, Dorozhko E, Korotkova E, Kratochvil B, Vyskocil V, Barek J. Label-free electrochemical biosensors for the determination of Flaviviruses: Dengue, Zika, and Japanese Encephalitis. Sensors. 2020;20(16):4600. doi: 10.3390/s20164600. doi: 10.3390/s20164600. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klarkowski D, O’Brien DP, Shanks L, Singh KP. Causes of false-positive HIV rapid diagnostic test results. Expert Rev Anti Infect Ther. 2013;12(1):49–62. doi: 10.1586/14787210.2014.866516. doi: 10.1586/14787210.2014.866516. Available from: [DOI] [PubMed] [Google Scholar]
- 78.Kong M, Li Z, Wu J, Hu J, Sheng Y, Wu D, et al. A wearable microfluidic device for rapid detection of HIV-1 DNA using recombinase polymerase amplification. Talanta. 2019;205:120155. doi: 10.1016/j.talanta.2019.120155. doi: 10.1016/j.talanta.2019.120155. Available from: [DOI] [PubMed] [Google Scholar]
- 79.Konstantinou GN. Enzyme-linked immunosorbent assay (ELISA) Methods Mol Biol. 2017;1592:79–94. doi: 10.1007/978-1-4939-6925-8_7. doi: 10.1007/978-1-4939-6925-8_7. Available from: [DOI] [PubMed] [Google Scholar]
- 80.Kumar H, Bhardwaj K, Kaur T, Nepovimova E, Kuča K, Kumar V, et al. Detection of bacterial pathogens and antibiotic residues in chicken meat: a review. Foods. 2020;9(10):1504. doi: 10.3390/foods9101504. doi: 10.3390/foods9101504. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lanphear BP. Trends and patterns in the transmission of bloodborne pathogens to health care workers. Epidemiol Rev. 1994;16:437–50. doi: 10.1093/oxfordjournals.epirev.a036162. doi: 10.1093/oxfordjournals.epirev.a036162. Available from: [DOI] [PubMed] [Google Scholar]
- 82.Lawal JR, Ibrahim UI, Biu AA, Musa HI. Molecular detection of avian Haemosporidian parasites in village chickens (Gallus gallus domesticus) in Gombe State, Nigeria. J Vet Med Animal Sci. 2022;5(1):1095. [Google Scholar]
- 83.Lazcka O, Campo FJ, Muñoz FX. Pathogen detection: A perspective of traditional methods and biosensors. Biosens Bioelectron. 2007;22:1205–17. doi: 10.1016/j.bios.2006.06.036. doi: 10.1016/j.bios.2006.06.036. Available from: [DOI] [PubMed] [Google Scholar]
- 84.Lee JH, Kim BC, Oh BK, Choi JW. Highly sensitive localized surface plasmon resonance immunosensor for label-free detection of HIV-1. Nanomed Nanotechnol Biol Med. 2013;9:1018–26. doi: 10.1016/j.nano.2013.03.005. doi: 10.1016/j.nano.2013.03.005. Available from: [DOI] [PubMed] [Google Scholar]
- 85.Lee S, Kim YS, Jo M, Jin M, Lee D, Kim S. Chip-based detection of hepatitis C virus using RNA aptamers that specifically bind to HCV core antigen. Biochem Biophys Res Commun. 2007;358(1):47–52. doi: 10.1016/j.bbrc.2007.04.057. doi: 10.1016/j.bbrc.2007.04.057. Available from: [DOI] [PubMed] [Google Scholar]
- 86.Letowski J, Brousseau R, Masson L. Designing better probes: Effect of probe size, mismatch position and number on hybridization in DNA oligonucleotide microarrays. J Microbiol Methods. 2004;57:269–278. doi: 10.1016/j.mimet.2004.02.002. doi: 10.1016/j.mimet.2004.02.002. Available from: [DOI] [PubMed] [Google Scholar]
- 87.Li B, Yu Q, Duan Y. Fluorescent labels in biosensors for pathogen detection. Crit Rev Biotechnol. 2013;35(1):82–93. doi: 10.3109/07388551.2013.804487. doi: 10.3109/07388551.2013.804487. Available from: [DOI] [PubMed] [Google Scholar]
- 88.Li HY, Jia WN, Li XY, Zhang L, Liu C, Wu J, et al. Advances in detection of infectious agents by Aptamer-based technologies. Emerg Microbes Infect. 2020;9:1671–1681. doi: 10.1080/22221751.2020.1792352. doi: 10.1080/22221751.2020.1792352. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li J, Jin X, Feng M, Huang S, Feng J. Ultrasensitive and highly selective electrochemical biosensor for HIV gene detection based on amino-reduced graphene oxide and β-cyclodextrin modified glassy carbon electrode. Int J Electrochem Sci. 2020;15:2727–2738. doi: 10.20964/2020.03.62. doi: 10.20964/2020.03.62. Available from: [DOI] [Google Scholar]
- 90.Li S, Liu Y, Wang Y, Wang M, Liu C, Wang Y, et al. Rapid detection of Brucella spp. and elimination of carryover using multiple cross displacement amplification coupled with nanoparticles-based lateral flow biosensor. Front Cell Infect Microbiol. 2019;9:78. doi: 10.3389/fcimb.2019.00078. doi: 10.3389/fcimb.2019.00078. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin C, Li Y, Peng Y, Zhao S, Xu M, Zhang L, et al. Recent development of surface-enhanced Raman scattering for Biosensing. J Nanobiotechnology. 2023;21(1):90. doi: 10.1186/s12951-023-01890-7. doi: 10.1186/s12951-023-01890-7. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin S, Yang S. Molecular methods for pathogen detection in blood. Lancet. 2010;375(9710):178–179. doi: 10.1016/s0140-6736(09)61791-8. doi: 10.1016/s0140-6736(09)61791-8. Available from: [DOI] [PubMed] [Google Scholar]
- 93.Liu L, Han Z, An F, Gong X, Zhao C, Zheng W, et al. Aptamer-based biosensors for the diagnosis of sepsis. J Nanobiotechnology. 2021;19(1):49. doi: 10.1186/s12951-021-00959-5. doi: 10.1186/s12951-021-00959-5. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu Y, Huang CZ. One-step conjugation chemistry of DNA with highly scattered silver nanoparticles for sandwich detection of DNA. Analyst. 2012;137:3434–3436. doi: 10.1039/c2an35167f. doi: 10.1039/c2an35167f. Available from: [DOI] [PubMed] [Google Scholar]
- 95.Lo Y, Cheung YW, Wang L, Lee M, Figueroa-Miranda G, Liang S, et al. An electrochemical aptamer-based biosensor targeting plasmodium falciparum histidine-rich protein II for malaria diagnosis. Biosens Bioelectron. 2021;192:113472. doi: 10.1016/j.bios.2021.113472. doi: 10.1016/j.bios.2021.113472. Available from: [DOI] [PubMed] [Google Scholar]
- 96.Lu X, Dong X, Zhang K, Han X, Fang X, Zhang Y, et al. A gold nanorods-based fluorescent biosensor for the detection of hepatitis B virus DNA based on fluorescence resonance energy transfer. Analyst. 2013;138:642–650. doi: 10.1039/c2an36099c. doi: 10.1039/c2an36099c. Available from: [DOI] [PubMed] [Google Scholar]
- 97.Ludlam CA, Powderly WG, Bozzette S, Diamond M, Koerper MA, Kulkarni R, et al. Clinical perspectives of emerging pathogens in bleeding disorders. Lancet. 2006;367(9506):252–261. doi: 10.1016/s0140-6736(06)68036-7. doi: 10.1016/s0140-6736(06)68036-7. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luz JGG, Souto DEP, Machado-Assis GF, de Lana M, Kubota LT, Luz RCS, et al. Development and evaluation of a SPR-based immunosensor for detection of anti-Trypanosoma cruzi antibodies in human serum. Sens Actuators B Chem. 2015;212:287–296. doi: 10.1016/j.snb.2015.01.135. doi: 10.1016/j.snb.2015.01.135. Available from: [DOI] [Google Scholar]
- 99.Maddali H, Miles CE, Kohn J, O’Carroll DM. Optical biosensors for virus detection: Prospects for SARS‐COV‐2/Covid-19. ChemBioChem. 2020;22:1176–89. doi: 10.1002/cbic.202000744. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mahato K, Kumar S, Srivastava A, Maurya PK, Singh R, Chandra P. Electrochemical immunosensors: Fundamentals and applications in clinical diagnostics. In: Vashist SK, Luong JHT, editors. Handbook of immunoassay technologies. Approaches, performances, and applications. Amsterdam: Elsevier; 2018. pp. 359–414. Available from: [DOI] [Google Scholar]
- 101.Manessis G, Frant M, Wozniakowski G, Nannucci L, Benedetti M, Denes L, et al. Point-of-care and label-free detection of porcine reproductive and respiratory syndrome and swine influenza viruses using a microfluidic device with photonic integrated circuits. Viruses. 2022;14(5):988. doi: 10.3390/v14050988. doi: 10.3390/v14050988. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mansuy JM, Lhomme S, Cazabat M, Pasquier C, Martin-Blondel G, Izopet J. Detection of Zika, dengue and chikungunya viruses using single-reaction multiplex real-time RT-PCR. Diagn Microbiol Infect Dis. 2018;92:284–7. doi: 10.1016/j.diagmicrobio.2018.06.019. doi: 10.1016/j.diagmicrobio.2018.06.019. Available from: [DOI] [PubMed] [Google Scholar]
- 103.Martins BR, Barbosa YO, Andrade CM, Pereira LQ, Simão GF, de Oliveira CJ, et al. Development of an electrochemical immunosensor for specific detection of visceral leishmaniasis using gold-modified screen-printed carbon electrodes. Biosensors. 2020;10(8):81. doi: 10.3390/bios10080081. doi: 10.3390/bios10080081. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Medyantseva EP, Khaldeeva EV, Glushko NI, Budnikov HC. Amperometric enzyme immunosensor for the determination of the antigen of the pathogenic fungi Trichophyton rubrum. Anal Chim Acta. 2000;411(1–2):13–8. doi: 10.1016/s0003-2670(99)00889-2. doi: 10.1016/s0003-2670(99)00889-2. Available from: [DOI] [Google Scholar]
- 105.Miao G, Zhang L, Zhang J, Ge S, Xia N, Qian S, et al. Free convective PCR: From principle study to commercial applications - A critical review. Anal Chim Acta. 2020;1108:177–97. doi: 10.1016/j.aca.2020.01.069. doi: 10.1016/j.aca.2020.01.069. Available from: [DOI] [PubMed] [Google Scholar]
- 106.Ming K, Kim J, Biondi MJ, Syed A, Chen K, Lam A, et al. Integrated quantum dot barcode smartphone optical device for wireless multiplexed diagnosis of infected patients. ACS Nano. 2015;9:3060–74. doi: 10.1021/nn5072792. doi: 10.1021/nn5072792. Available from: [DOI] [PubMed] [Google Scholar]
- 107.Mohammed AS, Balapure A, Khaja MN, Ganesan R, Dutta JR. Naked-eye colorimetric detection of HCV RNA mediated by a 5′ UTR-targeted antisense oligonucleotide and plasmonic gold nanoparticles. Analyst. 2021;146:1569–78. doi: 10.1039/d0an02481c. doi: 10.1039/d0an02481c. Available from: [DOI] [PubMed] [Google Scholar]
- 108.Mohmad A, Chandra D, Saravanan BC, H V M, O R VK, Fular A, et al. Development of a recombinant TASP-based dot-Elisa for detection of theileria annulata infection in cattle. Ticks Tick-Borne Dis. 2018;9:1416–20. doi: 10.1016/j.ttbdis.2018.06.016. doi: 10.1016/j.ttbdis.2018.06.016. Available from: [DOI] [PubMed] [Google Scholar]
- 109.Mohsin DH, Mashkour MS, Fatemi F. Design of aptamer-based sensing platform using gold nanoparticles functionalized reduced graphene oxide for ultrasensitive detection of hepatitis B virus. Chem Papers. 2020;75:279–95. doi: 10.1007/s11696-020-01292-1. doi: 10.1007/s11696-020-01292-1. Available from: [DOI] [Google Scholar]
- 110.Mutangadura GB. World Health Report 2002: Reducing risks, promoting healthy life: World Health Organization, Geneva, 2002, 250 pages, US$ 13.50, ISBN 9-2415-6207-2. Agric Econ. 2004;30:170–2. doi: 10.1016/j.agecon.2003.11.006. doi: 10.1016/j.agecon.2003.11.006. Available from: [DOI] [Google Scholar]
- 111.Nagarkatti R, de Araujo FF, Gupta C, Debrabant A. Aptamer based, non-PCR, non-serological detection of chagas disease biomarkers in Trypanosoma cruzi infected mice. PLoS Negl Trop Dis. 2014;8(1):e2650. doi: 10.1371/journal.pntd.0002650. doi: 10.1371/journal.pntd.0002650. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Noedl H, Yingyuen K, Laoboonchai A, Fukuda M, Sirichaisinthop J, Miller RS. Sensitivity and specificity of an antigen detection ELISA for malaria diagnosis. Am J Trop Med Hyg. 2006;75:1205–1208. doi: 10.4269/ajtmh.2006.75.1205. doi: 10.4269/ajtmh.2006.75.1205. Available from: [DOI] [PubMed] [Google Scholar]
- 113.Pan D, Wang W, Cheng T. Current methods for the detection of antibodies of Varicella-Zoster Virus: a review. Microorganisms. 2023;11(2):519. doi: 10.3390/microorganisms11020519.. doi: 10.3390/microorganisms11020519.. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pardo M, Sberveglieri G. 13th International Symposium on Olfaction and Electronic Nose. Sens Actuators B Chem. 2010;146:419. doi: 10.1016/j.snb.2010.02.033. doi: 10.1016/j.snb.2010.02.033. Available from: [DOI] [Google Scholar]
- 115.Park KS. Nucleic acid aptamer-based methods for diagnosis of infections. Biosens Bioelectron. 2018;102:179–88. doi: 10.1016/j.bios.2017.11.028. doi: 10.1016/j.bios.2017.11.028. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Patel S, Cassidy SR. Diagnosis and monitoring of HIV (including resistance testing. Medicine. 2018;46:283–286. doi: 10.1016/j.mpmed.2018.02.007.. doi: 10.1016/j.mpmed.2018.02.007.. Available from: [DOI] [Google Scholar]
- 117.Pejcic B, Marco RD, Parkinson G. The role of biosensors in the detection of emerging infectious diseases. Analyst. 2006;131:1079–1090. doi: 10.1039/b603402k. doi: 10.1039/b603402k. Available from: [DOI] [PubMed] [Google Scholar]
- 118.Phillips EK, Simwale OJ, Chung MJ, Parker G, Perry J, Jagger JC. Risk of bloodborne pathogen exposure among Zambian healthcare workers. J Infect Public Health. 2012;5:244–9. doi: 10.1016/j.jiph.2012.02.005. doi: 10.1016/j.jiph.2012.02.005. Available from: [DOI] [PubMed] [Google Scholar]
- 119.Pingle MR, Granger K, Feinberg P, Shatsky R, Sterling B, Rundell M, et al. Multiplexed identification of blood-borne bacterial pathogens by use of a novel 16S rrna gene PCR-ligase detection reaction-capillary electrophoresis assay. J Clin Microbiol. 2007;45:1927–35. doi: 10.1128/jcm.00226-07. doi: 10.1128/jcm.00226-07. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Piriya VS A, Joseph P, Daniel SCGK, Lakshmanan S, Kinoshita T, Muthusamy S. Colorimetric sensors for rapid detection of various analytes. Mater Sci Eng C. 2017;78:1231–45. doi: 10.1016/j.msec.2017.05.018. doi: 10.1016/j.msec.2017.05.018. Available from: [DOI] [PubMed] [Google Scholar]
- 121.Pirozzolo JJ, LeMay DC. Blood-borne infections. Clin Sports Med. 2007;26:425–31. doi: 10.1016/j.csm.2007.04.010. doi: 10.1016/j.csm.2007.04.010. Available from: [DOI] [PubMed] [Google Scholar]
- 122.Pripuzova N, Wang R, Tsai S, Li B, Hung GC, Ptak RG, et al. Development of real-time PCR array for simultaneous detection of eight human blood-borne viral pathogens. PLoS ONE. 2012;7(8):e43246. doi: 10.1371/journal.pone.0043246. doi: 10.1371/journal.pone.0043246. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Puri M, Kaur Brar H, Madan E, Srinivasan R, Rawat K, Gorthi SS, et al. Rapid diagnosis of Plasmodium falciparum malaria using a point-of-care loop-mediated isothermal amplification device. Front Cell Infect Microbiol. 2022;12:961832. doi: 10.3389/fcimb.2022.961832.. doi: 10.3389/fcimb.2022.961832.. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rahi A, Sattarahmady N, Heli H. An ultrasensitive electrochemical Genosensor for Brucella based on palladium nanoparticles. Anal Biochem. 2016;510:11–7. doi: 10.1016/j.ab.2016.07.012. doi: 10.1016/j.ab.2016.07.012. Available from: [DOI] [PubMed] [Google Scholar]
- 125.Rahmati Z, Roushani M, Hosseini H. Three-dimensional NICO2O4 nanowires encapsulated in nitrogen-doped carbon networks as a high-performance aptamer stabilizer for impedimetric ultrasensitive detection of hepatitis C virus core antigen. Surfaces Interfaces. 2021;22:100813. doi: 10.1016/j.surfin.2020.100813. doi: 10.1016/j.surfin.2020.100813. Available from: [DOI] [Google Scholar]
- 126.Saadat S, Mardaneh J, Ahouran M, MohammadzadehA, Ardebili A, Yousefi M, et al. Diagnosis of cattle brucellosis by PCR and serological methods: Comparison of diagnostic tests. Biomed Pharmacol J. 2017;10:881–8. doi: 10.13005/bpj/1181. doi: 10.13005/bpj/1181. Available from: [DOI] [Google Scholar]
- 127.Saleh M, El-Matbouli M. Rapid detection of Cyprinid herpesvirus-3 (cyhv-3) using a gold nanoparticle-based hybridization assay. J Virol Methods. 2015;217:50–4. doi: 10.1016/j.jviromet.2015.02.021. doi: 10.1016/j.jviromet.2015.02.021. Available from: [DOI] [PubMed] [Google Scholar]
- 128.Santos PS, Nascimento R, Rodrigues LP, Santos FA, Faria PC, Martins JR, et al. Functional epitope core motif of the Anaplasma marginale major surface protein 1A and its incorporation onto Bioelectrodes for antibody detection. PLoS ONE. 2012;7(3):e33045. doi: 10.1371/journal.pone.0033045. doi: 10.1371/journal.pone.0033045. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schijman AG, Bisio M, Orellana L, Sued M, Duffy T, Mejia Jaramillo AM, et al. International Study to evaluate PCR methods for detection of Trypanosoma cruzi DNA in blood samples from Chagas disease patients. PLoS Negl Trop Dis. 2011;5(1):e931. doi: 10.1371/journal.pntd.0000931. doi: 10.1371/journal.pntd.0000931. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sharma MK, Agarwal GS, Rao VK, Upadhyay S, Merwyn S, Gopalan N, et al. Amperometric immunosensor based on gold nanoparticles/alumina sol–gel modified screen-printed electrodes for antibodies to Plasmodium falciparum histidine rich protein-2. Analyst. 2010;135:608–614. doi: 10.1039/b918880k. doi: 10.1039/b918880k. Available from: [DOI] [PubMed] [Google Scholar]
- 131.Sharma P, Batheja G, Verma HN, Seth P. Single tube multiplex PCR, liquid hybridization, and Elisa (Multiplex Nat-ELISA) for rapid detection of HIV, HBV, and HCV in the window period. 06 June 2022, PREPRINT (Version 1) available at Research Square. Available from: [DOI]
- 132.Sharma TK, Shukla R. Nucleic acid aptamers as an emerging diagnostic tool for animal pathogens. Adv Anim Vet Sci. 2014;2(1):50–55. doi: 10.14737/journal.aavs/2014.2.1.50.55. doi: 10.14737/journal.aavs/2014.2.1.50.55. Available from: [DOI] [Google Scholar]
- 133.Shourian M, Ghourchian H, Boutorabi M. Ultra-sensitive immunosensor for detection of hepatitis B surface antigen using multi-functionalized gold nanoparticles. Anal Chim Acta. 2015;895:1–11. doi: 10.1016/j.aca.2015.07.013. doi: 10.1016/j.aca.2015.07.013. Available from: [DOI] [PubMed] [Google Scholar]
- 134.Silva M, Wilkowsky S, DE Echaide ST, Farber M, Oliva A. Development of an immunosensor for the diagnosis of bovine anaplasmosis. Ann N Y Acad Sci. 2006;1081:379–381. doi: 10.1196/annals.1373.056. doi: 10.1196/annals.1373.056. Available from: [DOI] [PubMed] [Google Scholar]
- 135.Silva MG, Helali S, Esseghaier C, Suarez CE, Oliva A, Abdelghani A. An impedance spectroscopy method for the detection and evaluation of Babesia Bovis antibodies in cattle. Sens Actuators B Chem. 2008;135:206–13. doi: 10.1016/j.snb.2008.08.019. doi: 10.1016/j.snb.2008.08.019. Available from: [DOI] [Google Scholar]
- 136.Silva SJRD, Pardee K, Pena L. Loop-Mediated Isothermal Amplification (LAMP) for the diagnosis of Zika virus: a review. Viruses. 2019;12(1):19. doi: 10.3390/v12010019.. doi: 10.3390/v12010019.. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sin ML, Mach KE, Wong PK, Liao JC. Advances and challenges in biosensor-based diagnosis of infectious diseases. Expert Rev Mol Diagn. 2014;14:225–44. doi: 10.1586/14737159.2014.888313. doi: 10.1586/14737159.2014.888313. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Singh A, Sharma A, Ahmed A, Sundramoorthy AK, Furukawa H, Arya S, et al. Recent advances in electrochemical biosensors: Applications, challenges, and future scope. Biosensors. 2021;11(9):336. doi: 10.3390/bios11090336. doi: 10.3390/bios11090336. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Singh R, Mukherjee MD, Sumana G, Gupta RK, Sood S, Malhotra BD. Biosensors for pathogen detection: A smart approach towards clinical diagnosis. Sens Actuators B Chem. 2014;197:385–404. doi: 10.1016/j.snb.2014.03.005. doi: 10.1016/j.snb.2014.03.005. Available from: [DOI] [Google Scholar]
- 140.Singhal C, Bruno JG, Kaushal A, Sharma TK. Recent advances and a roadmap to aptamer-based sensors for bloodstream infections. ACS Appl Bio Mater. 2021;4:3962–84. doi: 10.1021/acsabm.0c01358. doi: 10.1021/acsabm.0c01358. Available from: [DOI] [PubMed] [Google Scholar]
- 141.Smalls S, Fischbach FT. A manual of laboratory diagnostic tests. Am J Nurs. 1982;82(2):334. doi: 10.2307/3463097. doi: 10.2307/3463097. Available from: [DOI] [Google Scholar]
- 142.Song J, Mauk MG, Hackett BA, Cherry S, Bau HH, Liu C. Instrument-free point-of-care molecular detection of zika virus. Anal Chem. 2016;88:7289–94. doi: 10.1021/acs.analchem.6b01632. doi: 10.1021/acs.analchem.6b01632. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Spatola Rossi C, Coulon F, Ma S, Zhang YS, Yang Z. Microfluidics for rapid detection of live pathogens. Adv Funct Mater. 2023;33:2212081. doi: 10.1002/adfm.202212081. doi: 10.1002/adfm.202212081. Available from: [DOI] [Google Scholar]
- 144.Spiess B, Seifarth W, Hummel M, Frank O, Fabarius A, Zheng C, et al. DNA microarray-based detection and identification of fungal pathogens in clinical samples from neutropenic patients. J Clin Microbiol. 2007;45:3743–53. doi: 10.1128/jcm.00942-07. doi: 10.1128/jcm.00942-07. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tan J, Xu J. Applications of electronic nose (E-nose) and electronic tongue (e-tongue) in food quality-related properties determination: a review. Artif Intell Agric. 2020;4:104–15. doi: 10.1016/j.aiia.2020.06.003. doi: 10.1016/j.aiia.2020.06.003. Available from: [DOI] [Google Scholar]
- 146.Tang XL, Hua Y, Guan Q, Yuan CH. Improved detection of deeply invasive candidiasis with DNA aptamers specific binding to (1→3)-β-D-glucans from Candida albicans. Eur J Clin Microbiol Infect Dis. 2016;35:587–95. doi: 10.1007/s10096-015-2574-8. doi: 10.1007/s10096-015-2574-8. Available from: [DOI] [PubMed] [Google Scholar]
- 147.Taylor D, Durigon M, Davis H, Archibald C, Konrad B, Coombs D, et al. Probability of a false-negative HIV antibody test result during the window period: A tool for pre- and post-test counselling. Int J STD AIDS. 2014;26:215–24. doi: 10.1177/0956462414542987. doi: 10.1177/0956462414542987. Available from: [DOI] [PubMed] [Google Scholar]
- 148.Tsegay G, Tesfagaber W, Zhu Y, He Y, Wang W, Zhang Z, et al. Novel P22-monoclonal antibody based blocking Elisa for the detection of African swine fever virus antibodies in serum. Biosaf Health. 2022;4:234–43. doi: 10.1016/j.bsheal.2022.04.002. doi: 10.1016/j.bsheal.2022.04.002. Available from: [DOI] [Google Scholar]
- 149.Turner A, Magan N. Electronic noses and disease diagnostics. Nat Rev Microbiol. 2004;2:161–6. doi: 10.1038/nrmicro823. doi: 10.1038/nrmicro823. Available from: [DOI] [PubMed] [Google Scholar]
- 150.Udonsom R, Mahittikorn A, Jirapattharasate C. Molecular detection and genetic diversity of tick-borne pathogens in goats from the southern part of Thailand. Pathogens. 2022;11(4):477. doi: 10.3390/pathogens11040477. doi: 10.3390/pathogens11040477. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vázquez-Guardado A, Mehta F, Jimenez B, Biswas A, Baksh A, Lee S, et al. DNA-modified plasmonic sensor for the direct detection of virus biomarkers from the blood. Nano Lett. 2021;21:7505–11. doi: 10.1021/acs.nanolett.1c01609. doi: 10.1021/acs.nanolett.1c01609. Available from: [DOI] [PubMed] [Google Scholar]
- 152.Vidic J, Manzano M, Chang CM, Jaffrezic-Renault N. Advanced biosensors for detection of pathogens related to livestock and poultry. Vet Res. 2017;48(1):11. doi: 10.1186/s13567-017-0418-5. doi: 10.1186/s13567-017-0418-5. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.WHO, World Health Organization. Geneva: WHO; 2017. WHO polio laboratory manual/supplement 1: An alternative test algorithm for poliovirus isolation and characterization. Available from: https://polioeradication.org/wp-content/uploads/2017/05/NewAlgorithmForPoliovirusIsolationSupplement1.pdf. [Google Scholar]
- 154.Wilson AD, Baietto M. Advances in electronic-nose technologies developed for biomedical applications. Sensors. 2011;11:1105–76. doi: 10.3390/s110101105. doi: 10.3390/s110101105. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wu J, Mukama O, Wu W, Li Z, Habimana JD, Zhang Y, et al. A CRISPR/CAS12A based universal lateral flow biosensor for the sensitive and specific detection of African swine-fever viruses in whole blood. Biosensors. 2020;10(12):203. doi: 10.3390/bios10120203. doi: 10.3390/bios10120203. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xeroulis G, Inaba K, Stewart TC, Lannigan R, Gray D, Malthaner R, et al. Human immunodeficiency virus, hepatitis B, and hepatitis C seroprevalence in a Canadian trauma population. J Trauma. 2005;59:105–8. doi: 10.1097/01.ta.0000171464.51584.f5. doi: 10.1097/01.ta.0000171464.51584.f5. Available from: [DOI] [PubMed] [Google Scholar]
- 157.Xiang A, Wei F, Lei X, Liu Y, Liu Y, Guo Y. A simple and rapid capillary chemiluminescence immunoassay for quantitatively detecting human serum HBsAg. Eur J Clin Microbiol Infect Dis. 2013;32:1557–64. doi: 10.1007/s10096-013-1910-0. doi: 10.1007/s10096-013-1910-0. Available from: [DOI] [PubMed] [Google Scholar]
- 158.Xie C, Chen S, Zhang L, He X, Ma Y, Wu H, et al. Multiplex detection of blood-borne pathogens on a self-driven microfluidic chip using loop-mediated isothermal amplification. Anal Bioanal Chem. 2021;413:2923–2931. doi: 10.1007/s00216-021-03224-8.. doi: 10.1007/s00216-021-03224-8.. Available from: [DOI] [PubMed] [Google Scholar]
- 159.Xie K, Chen H, Peng B, Jin Z, Xiao W, Zhang Z, et al. On-site determination of classical swine fever virus (CSFV) by a fluorescent microsphere-based lateral flow immunoassay strip (FM-lfias) based on monoclonal antibodies. Anal Lett. 2020;54:2347–62. doi: 10.1080/00032719.2020.1860998. doi: 10.1080/00032719.2020.1860998. Available from: [DOI] [Google Scholar]
- 160.Xing G, Zhang W, Li N, Pu Q, Lin J-M. Recent progress on microfluidic biosensors for rapid detection of pathogenic bacteria. Chin Chem Lett. 2022;33:1743–51. doi: 10.1016/j.cclet.2021.08.073. doi: 10.1016/j.cclet.2021.08.073. Available from: [DOI] [Google Scholar]
- 161.Xu H, Lin G, Chen R, Cai Z, Sun Y, Zhang X, et al. CRISPR/Cas12b assisted loop-mediated isothermal amplification for easy, rapid and sensitive quantification of chronic HBV DNA in one-pot. Anal Chim Acta. 2024;1310:342702. doi: 10.1016/j.aca.2024.342702.. doi: 10.1016/j.aca.2024.342702.. Available from: [DOI] [PubMed] [Google Scholar]
- 162.Yang H, Guo Y, Li S, Lan G, Jiang Q, Yang X, et al. Magnetic beads-based chemiluminescent assay for ultrasensitive detection of pseudorabies virus. J Nanosci Nanotechnol. 2014;14:3337–42. doi: 10.1166/jnn.2014.8254. doi: 10.1166/jnn.2014.8254. Available from: [DOI] [PubMed] [Google Scholar]
- 163.Yang S, Rothman RE. PCR-based diagnostics for infectious diseases: Uses, limitations, and future applications in acute-care settings. Lancet Infect Dis. 2004;4:337–48. doi: 10.1016/s1473-3099(04)01044-8. doi: 10.1016/s1473-3099(04)01044-8. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yang Z, Xu G, Reboud J, Ali SA, Kaur G, McGiven J. Rapid veterinary diagnosis of bovine reproductive infectious diseases from semen using paper-origami DNA microfluidics. ACS Sens. 2018;3:403–9. doi: 10.1021/acssensors.7b00825. doi: 10.1021/acssensors.7b00825. Available from: [DOI] [PubMed] [Google Scholar]
- 165.Ye X, Li L, Li J, Wu X, Fang X, Kong J. Microfluidic-CFPA chip for the point-of-care detection of African swine fever virus with a median time to threshold in about 10 min. ACS Sens. 2019;4:3066–71. doi: 10.1021/acssensors.9b01731. doi: 10.1021/acssensors.9b01731. Available from: [DOI] [PubMed] [Google Scholar]
- 166.Ye Z, Liu Y, Li Q. Recent progress in Smart Electronic Nose Technologies enabled with machine learning methods. Sensors. 2021;21(22):7620. doi: 10.3390/s21227620. doi: 10.3390/s21227620. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yue F, Li F, Kong Q, Guo Y, Sun X. Recent advances in aptamer-based sensors for aminoglycoside antibiotics detection and their applications. Sci Total Environ. 2021;762:143129. doi: 10.1016/j.scitotenv.2020.143129. doi: 10.1016/j.scitotenv.2020.143129. Available from: [DOI] [PubMed] [Google Scholar]
- 168.Zadran A, Ho AV, Zadran L, entura Curiel IJ, Pham TT, et al. Optimizing public health preparedness for highly infectious diseases in Central Vietnam. Diagnostics. 2022;12(9):2047. doi: 10.3390/diagnostics12092047. doi: 10.3390/diagnostics12092047. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Zhang H, Xu T, Li CW, Yang M. A microfluidic device with microbead array for sensitive virus detection and genotyping using quantum dots as fluorescence labels. Biosens Bioelectron. 2010;25:2402–7. doi: 10.1016/j.bios.2010.02.032. doi: 10.1016/j.bios.2010.02.032. Available from: [DOI] [PubMed] [Google Scholar]
- 170.Zhang WW, Ghosh AK, Mohamath R, Whittle J, Picone A, Lypaczewski P, et al. Development of a sandwich ELISA to detect Leishmania 40S ribosomal protein S12 antigen from blood samples of visceral leishmaniasis patients. BMC Infect Dis. 2018;18(1):500. doi: 10.1186/s12879-018-3420-2. doi: 10.1186/s12879-018-3420-2. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhao F, Niu L, Yan L, Nong J, Wang C, Wang J, et al. Establishment and application of multiple cross displacement amplification coupled with lateral flow biosensor (MCDA-LFB) for visual and rapid detection of Candida albicans in clinical samples. Front Cell Infect Microbiol. 2019;9:102. doi: 10.3389/fcimb.2019.00102.. doi: 10.3389/fcimb.2019.00102.. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zheng L, Jia L, Li B, Situ B, Liu Q, Wang Q, et al. A sandwich HIV P24 amperometric immunosensor based on a direct gold electroplating-modified electrode. Molecules. 2012;17:5988–6000. doi: 10.3390/molecules17055988. doi: 10.3390/molecules17055988. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhi X, Deng M, Yang H, Gao G, Wang K, Fu H, et al. A novel HBV genotypes detecting system combined with microfluidic chip, loop-mediated isothermal amplification and GMR Sensors. Biosens Bioelectron. 2014;54:372–7. doi: 10.1016/j.bios.2013.11.025. doi: 10.1016/j.bios.2013.11.025. Available from: [DOI] [PubMed] [Google Scholar]
- 174.Zhou J, Gan N, Li T, Hu F, Li X, Wang L, et al. A cost-effective sandwich electrochemiluminescence immunosensor for ultrasensitive detection of HIV-1 antibody using magnetic molecularly imprinted polymers as capture probes. Biosens Bioelectron. 2014;54:199–206. doi: 10.1016/j.bios.2013.10.044. doi: 10.1016/j.bios.2013.10.044. Available from: [DOI] [PubMed] [Google Scholar]
- 175.Zhou W, Jimmy Huang PJ, Ding J, Liu J. Aptamer-based biosensors for biomedical diagnostics. Analyst. 2014;139:2627–2640. doi: 10.1039/c4an00132j. doi: 10.1039/c4an00132j. Available from: [DOI] [PubMed] [Google Scholar]
- 176.Zhu Q, Shibata T, Kabashima T, Kai M. Inhibition of HIV-1 protease expression in T cells owing to DNA aptamer-mediated specific delivery of Sirna. Eur J Med Chem. 2012;56:396–9. doi: 10.1016/j.ejmech.2012.07.045. doi: 10.1016/j.ejmech.2012.07.045. Available from: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data related to this study has been included in the manuscript.