Abstract
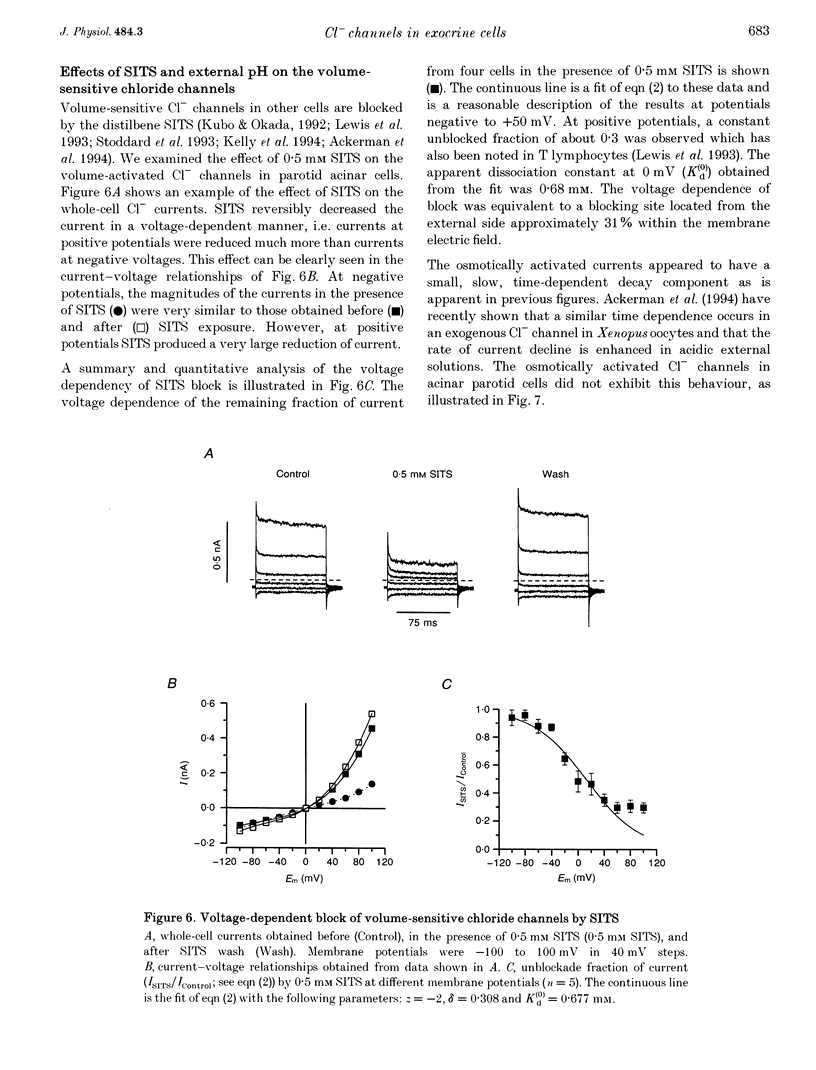
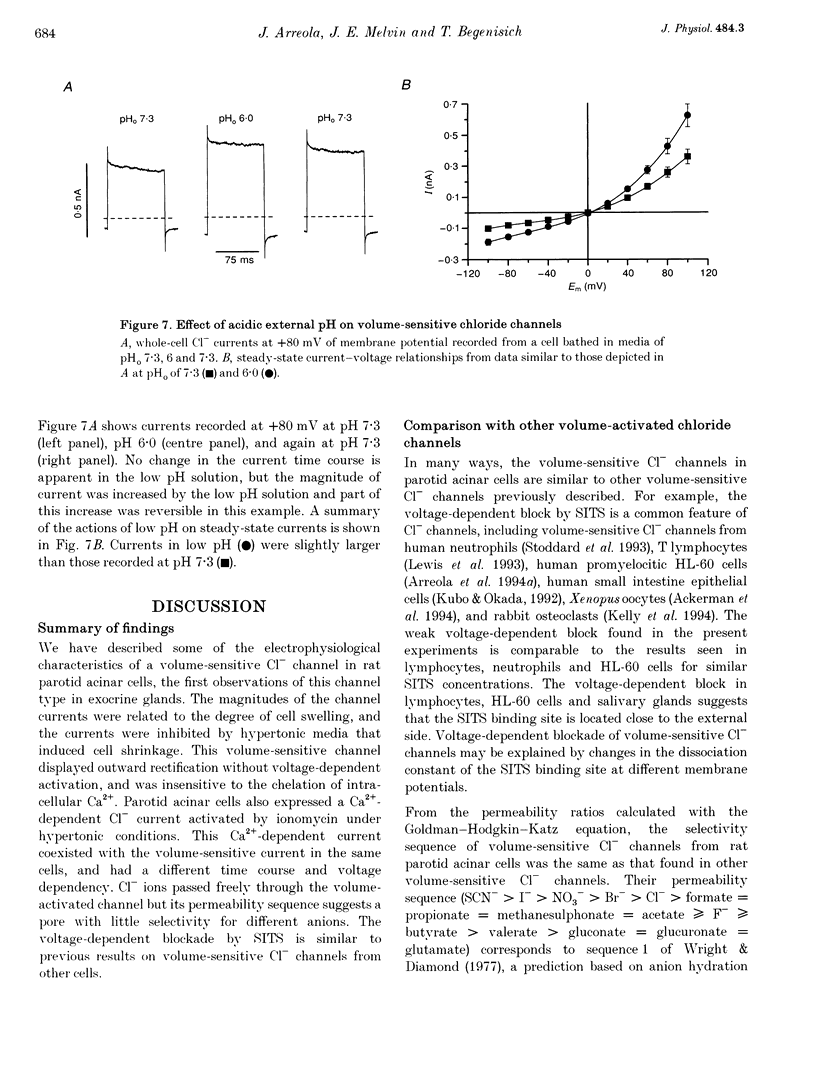
1. Rat parotid acinar cells undergo a regulatory volume decrease in response to hypotonically induced cell swelling that is sensitive to K+ and Cl- gradients. To investigate the potential mechanisms involved, the whole-cell patch-clamp technique was used to characterize a volume-sensitive Cl- channel in rat parotid acinar cells. 2. Exposure of cells to a hyposmotic gradient induced large Cl- currents that exhibited outward rectification and were not affected by membrane potential or the absence of intracellular Ca2+. Low external pH increased the currents at all potentials without affecting current kinetics. These currents were nearly abolished when the cells were in hypertonic conditions. This decrease in the current amplitude was correlated with a decrease in the cell size. 3. The volume-sensitive currents displayed little or no time dependence, whereas Ca(2+)-activated Cl- channels, present in the same cells, displayed slow activation kinetics and large, time-dependent tail currents upon repolarization to the holding potential. 4. The reversal potential of the osmotically activated channels was close to the predicted chloride equilibrium potential and was sensitive to the physiological extracellular Cl- concentration ([Cl-]o). The relationship between reversal potential and [Cl-]o was fitted to a modified Nernst equation with a slope of 51 mV per decade, consistent with a Cl- selective conductance. 5. The anion permeability sequence of the channel, obtained from the shifts of the reversal potentials of the volume-sensitive Cl- current, was: SCN- > I- > NO-3 > Br- > Cl- > formate > propionate = methanesulphonate = acetate > or = F- > or = butyrate > valerate > gluconate = glucuronate = glutamate. 6. The current through the volume-sensitive channels was inhibited by the Cl- channel blocker SITS (4-acetamido-4'-isothiocyanatostilbene-2,2'-disulphonic acid) in a voltage-dependent manner. 7. We conclude that rat parotid acinar cells express an outwardly rectifying Cl- current that can be activated by swelling under hypotonic conditions. This Cl- conductance may be an element of the cellular mechanisms of volume regulation in exocrine glands.
Full text
PDF


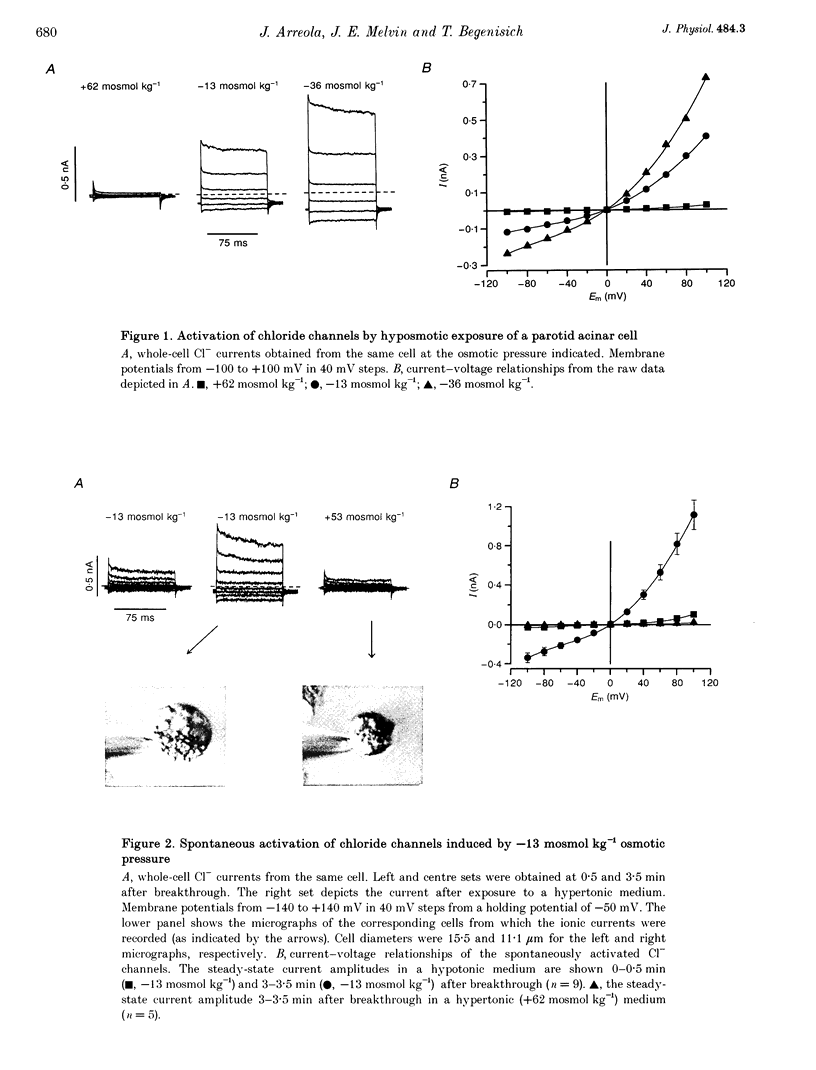
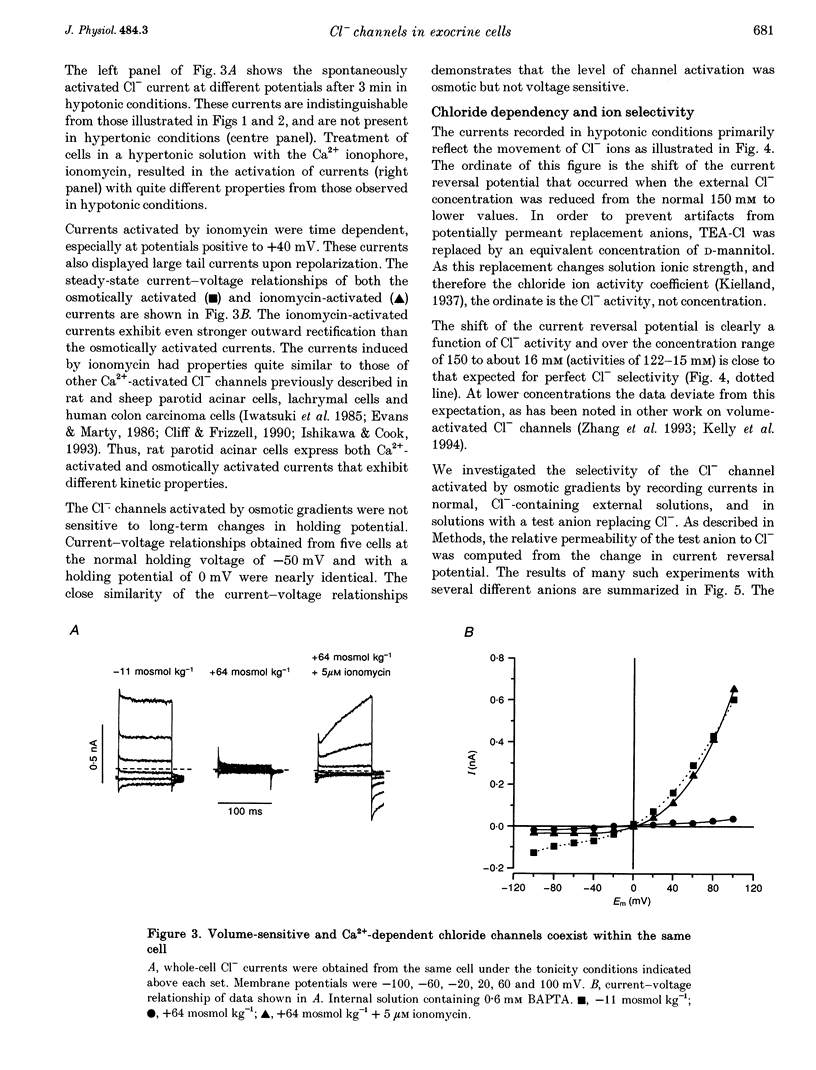
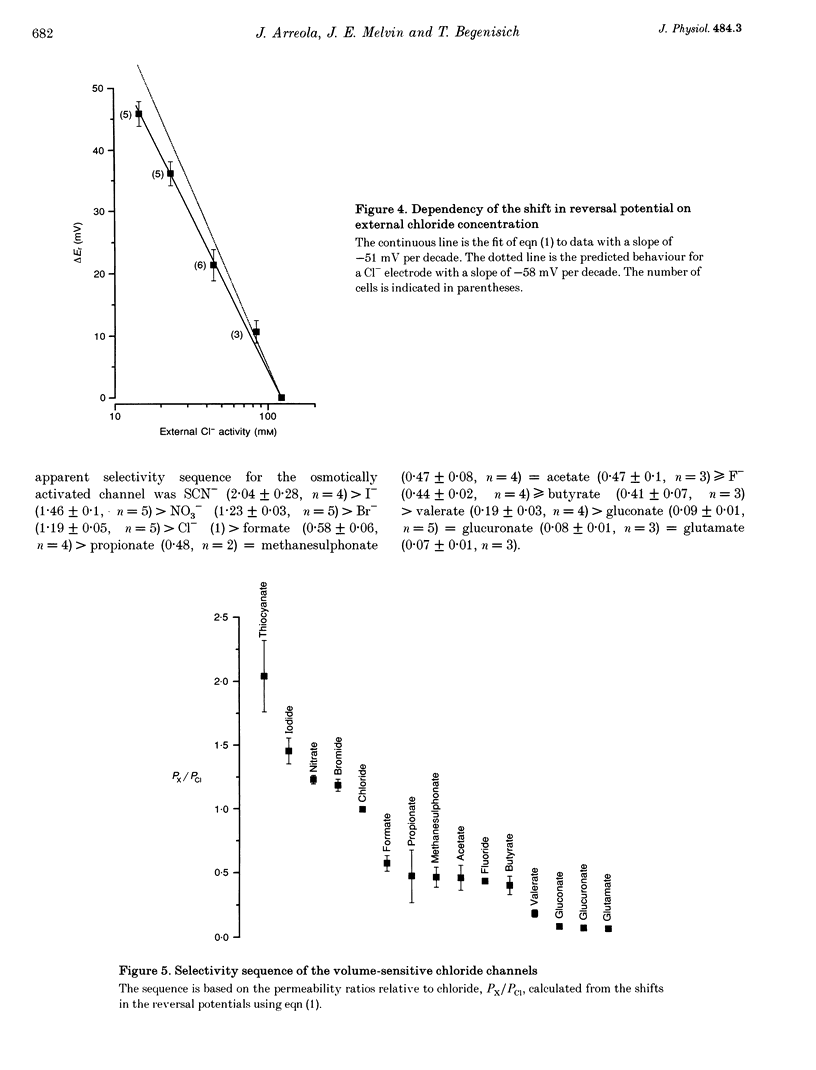



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman M. J., Wickman K. D., Clapham D. E. Hypotonicity activates a native chloride current in Xenopus oocytes. J Gen Physiol. 1994 Feb;103(2):153–179. doi: 10.1085/jgp.103.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum B. J., Ambudkar I. S., Helman J., Horn V. J., Melvin J. E., Mertz L. M., Turner R. J. Dispersed salivary gland acinar cell preparations for use in studies of neuroreceptor-coupled secretory events. Methods Enzymol. 1990;192:26–37. doi: 10.1016/0076-6879(90)92061-h. [DOI] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff W. H., Frizzell R. A. Separate Cl- conductances activated by cAMP and Ca2+ in Cl(-)-secreting epithelial cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4956–4960. doi: 10.1073/pnas.87.13.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroshenko P., Neher E. Volume-sensitive chloride conductance in bovine chromaffin cell membrane. J Physiol. 1992 Apr;449:197–218. doi: 10.1113/jphysiol.1992.sp019082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz M., Valverde M. A., Higgins C. F., Rucăreanu C., Sepúlveda F. V. Volume-activated chloride channels in HeLa cells are blocked by verapamil and dideoxyforskolin. Pflugers Arch. 1993 Jan;422(4):347–353. doi: 10.1007/BF00374290. [DOI] [PubMed] [Google Scholar]
- Foskett J. K., Melvin J. E. Activation of salivary secretion: coupling of cell volume and [Ca2+]i in single cells. Science. 1989 Jun 30;244(4912):1582–1585. doi: 10.1126/science.2500708. [DOI] [PubMed] [Google Scholar]
- Foskett J. K., Wong M. M., Sue-A-Quan G., Robertson M. A. Isosmotic modulation of cell volume and intracellular ion activities during stimulation of single exocrine cells. J Exp Zool. 1994 Feb 1;268(2):104–110. doi: 10.1002/jez.1402680206. [DOI] [PubMed] [Google Scholar]
- Franciolini F., Nonner W. Anion and cation permeability of a chloride channel in rat hippocampal neurons. J Gen Physiol. 1987 Oct;90(4):453–478. doi: 10.1085/jgp.90.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Toyama K., Hayashi H. Mechanisms of anion and cation permeations in the resting membrane of a barnacle muscle fiber. J Gen Physiol. 1971 Apr;57(4):408–434. doi: 10.1085/jgp.57.4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halm D. R., Frizzell R. A. Anion permeation in an apical membrane chloride channel of a secretory epithelial cell. J Gen Physiol. 1992 Mar;99(3):339–366. doi: 10.1085/jgp.99.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O., Lambert I. H. Volume-induced increase of K+ and Cl- permeabilities in Ehrlich ascites tumor cells. Role of internal Ca2+. J Membr Biol. 1984;78(3):211–222. doi: 10.1007/BF01925969. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol Rev. 1989 Apr;69(2):315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Cook D. I. A Ca(2+)-activated Cl- current in sheep parotid secretory cells. J Membr Biol. 1993 Sep;135(3):261–271. doi: 10.1007/BF00211098. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Maruyama Y., Matsumoto O., Nishiyama A. Activation of Ca2+-dependent Cl- and K+ conductances in rat and mouse parotid acinar cells. Jpn J Physiol. 1985;35(6):933–944. doi: 10.2170/jjphysiol.35.933. [DOI] [PubMed] [Google Scholar]
- Kelly M. E., Dixon S. J., Sims S. M. Outwardly rectifying chloride current in rabbit osteoclasts is activated by hyposmotic stimulation. J Physiol. 1994 Mar 15;475(3):377–389. doi: 10.1113/jphysiol.1994.sp020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotera T., Brown P. D. Calcium-dependent chloride current activated by hyposmotic stress in rat lacrimal acinar cells. J Membr Biol. 1993 May;134(1):67–74. doi: 10.1007/BF00233476. [DOI] [PubMed] [Google Scholar]
- Kubo M., Okada Y. Volume-regulatory Cl- channel currents in cultured human epithelial cells. J Physiol. 1992 Oct;456:351–371. doi: 10.1113/jphysiol.1992.sp019340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. S., Ross P. E., Cahalan M. D. Chloride channels activated by osmotic stress in T lymphocytes. J Gen Physiol. 1993 Jun;101(6):801–826. doi: 10.1085/jgp.101.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J. R., Cassity N., Reed P. Effects of isoproterenol on Cl transport in rat submandibular salivary-gland acini. Arch Oral Biol. 1988;33(7):505–509. doi: 10.1016/0003-9969(88)90032-5. [DOI] [PubMed] [Google Scholar]
- Nakahari T., Murakami M., Yoshida H., Miyamoto M., Sohma Y., Imai Y. Decrease in rat submandibular acinar cell volume during ACh stimulation. Am J Physiol. 1990 Jun;258(6 Pt 1):G878–G886. doi: 10.1152/ajpgi.1990.258.6.G878. [DOI] [PubMed] [Google Scholar]
- Nauntofte B. Regulation of electrolyte and fluid secretion in salivary acinar cells. Am J Physiol. 1992 Dec;263(6 Pt 1):G823–G837. doi: 10.1152/ajpgi.1992.263.6.G823. [DOI] [PubMed] [Google Scholar]
- Nilius B., Oike M., Zahradnik I., Droogmans G. Activation of a Cl- current by hypotonic volume increase in human endothelial cells. J Gen Physiol. 1994 May;103(5):787–805. doi: 10.1085/jgp.103.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulais M., Turner R. J. Beta-adrenergic upregulation of the Na(+)-K(+)-2Cl- cotransporter in rat parotid acinar cells. J Clin Invest. 1992 Apr;89(4):1142–1147. doi: 10.1172/JCI115695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P. E., Garber S. S., Cahalan M. D. Membrane chloride conductance and capacitance in Jurkat T lymphocytes during osmotic swelling. Biophys J. 1994 Jan;66(1):169–178. doi: 10.1016/S0006-3495(94)80754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkadi B., Parker J. C. Activation of ion transport pathways by changes in cell volume. Biochim Biophys Acta. 1991 Dec 12;1071(4):407–427. doi: 10.1016/0304-4157(91)90005-h. [DOI] [PubMed] [Google Scholar]
- Shigetomi T., Hayashi T., Ueda M., Kaneda T., Tokuno H., Takai A., Tomita T. Effects of Ca2+ removal and of tetraethylammonium on membrane currents induced by carbachol in isolated cells from the rat parotid gland. Pflugers Arch. 1991 Oct;419(3-4):332–337. doi: 10.1007/BF00371115. [DOI] [PubMed] [Google Scholar]
- Stoddard J. S., Steinbach J. H., Simchowitz L. Whole cell Cl- currents in human neutrophils induced by cell swelling. Am J Physiol. 1993 Jul;265(1 Pt 1):C156–C165. doi: 10.1152/ajpcell.1993.265.1.C156. [DOI] [PubMed] [Google Scholar]
- Thiemann A., Gründer S., Pusch M., Jentsch T. J. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature. 1992 Mar 5;356(6364):57–60. doi: 10.1038/356057a0. [DOI] [PubMed] [Google Scholar]
- Wright E. M., Diamond J. M. Anion selectivity in biological systems. Physiol Rev. 1977 Jan;57(1):109–156. doi: 10.1152/physrev.1977.57.1.109. [DOI] [PubMed] [Google Scholar]
- Zhang J., Rasmusson R. L., Hall S. K., Lieberman M. A chloride current associated with swelling of cultured chick heart cells. J Physiol. 1993 Dec;472:801–820. doi: 10.1113/jphysiol.1993.sp019974. [DOI] [PMC free article] [PubMed] [Google Scholar]