Abstract
Alzheimer’s disease is the most general type of cognitive impairments. Until recently, strategies that prevent its clinical progression have remained more elusive. Consequently, research direction should be for finding effective neuroprotective agents. It has been suggested oxidative stress, mitochondrial injury, and inflammation level might lead to brain cell death in many neurological disorders. Therefore, several autophagy-targeted bioactive compounds may be promising candidate therapeutics for the prevention of brain cell damage. Interestingly, some risk genes to Alzheimer’s disease are expressed within brain cells, which may be linked to cholesterol metabolism, lipid transport, endocytosis, exocytosis and/or caveolae formation, suggesting that caveolae may be a fruitful therapeutic target to improve cognitive impairments. This review would highlight the latest advances in therapeutic technologies to improve the treatment of Alzheimer’s disease. In particular, a paradigm that serotonin and N-methyl-d-aspartate (NMDA) receptors agonist/antagonist within caveolae structure might possibly improve the cognitive impairment. Consequently, cellular membrane biophysics should improve our understanding of the pathology of the cognitive dysfunction associated with Alzheimer’s disease. Here, this research direction for the purpose of therapy may open the potential to move a clinical care toward disease-modifying treatment strategies with certain benefits for patients
Keywords: Caveolae, caveolin, serotonin, glucagon-like peptide-1, NMDA, autophagy, mitophagy, cognitive impairment, Alzheimer’s disease
Introduction
Neurodegenerative diseases may be characterized by persistent behavioral and cognitive impairment, which may include memory loss and/or apathy [1]. Alzheimer’s disease is the most representative type of neurodegenerative disease characterized by deficit of cognition and/or alteration of behavior. Despite the huge efforts against Alzheimer’s disease, there has been yet no successful treatment for this disease [2]. Alzheimer’s disease is considered as one of the most difficult medical problems with large financial and/or social costs. The histopathology of Alzheimer’s disease is mostly defined by the accumulation of amyloid-β plaques and the development of neurofibrillary tangles in brains [3]. Extracellular plaques in the brain of Alzheimer’s disease are mostly formed by amyloid-β aggregation, which is supposed to be a crucial stage in the pathogenesis of this disease [4]. In general, some developments of novel therapeutics in neurodegenerative diseases may be targeting molecular pathologies in the neurodegeneration. Additionally, recent treatments for behavioral symptoms may include several serotonin reuptake inhibitors (SSRI) approved for behavioral disorders including Alzheimer’s disease [5]. However, only about a third of clinical patients may act in response to such medications even in the precision medicine [6]. Apparently, there is a necessity to find out new interventions to lessen the pathology of cognitive impairment with Alzheimer’s disease. Accumulating evidences may suggest that the serotonin (5HT) receptor family could act for potential new targets for the treatment of behavioral dysfunctions associated with Alzheimer’s disease, in which some types of G protein-coupled receptor (GPCR) may principally be coupling to the relevant signal transduction pathway [6]. For example, the 5HT2A receptor is a subtype of serotonin receptor belonging to the serotonin receptor family [7], which may generally expresses throughout the central nervous system (CNS) with various brain functions including thermoregulation, appetite, and cognition control [8]. In addition, the dysfunction of the neurotransmission associated with glutamatergic N-methyl-D-aspartate (NMDA) receptor is also well-known to direct to behavioral changes and/or cognitive impairment in Alzheimer’s disease [9]. The NMDA receptor is a type of glutamatergic ion channel. Stimulatingly, the NMDA-induced motor depression effect may be due to the protective mechanism to evade the NMDA-induced excito-toxic effect to cells [10]. In fact, a neuroprotective agent memantine is a kind of the NMDA antagonist [11]. Memantine could temporarily improve the cognitive function [12,13]. The identification for the more efficient disease-modifying treatment for Alzheimer’s disease has been required, which could also target the early stage of the diseases [13,14]. Interestingly, it has been shown that gut microbial tryptophan metabolites may be negatively correlated with several markers of Alzheimer’s disease [15]. The gut microbiota metabolism could also influence on the signaling of serotonin and/or orexin receptors [16]. Remarkably, some efficacious drugs against Alzheimer’s disease may target important brain receptors including for serotonin and orexin signaling pathways involved in memory and/or learning [17]. For example, an orexin could partially rescue the cognitive impairment induced by the NMDA receptor antagonist in female mice [18]. In addition, it has also been revealed that endogenous orexins could modify hippocampal NMDA receptor function, suggesting that they may influence neuron plasticity and/or memory performance [19]. Remarkably, several neurotransmitters including serotonin and/or orexin could be regulated by several dietary nutrients involved in their metabolic pathways [20]. In these ways, therefore, the intricate metabolic pathway from dietary nutrients might have highlighted its implications for the treatment of neurodegenerative diseases including Alzheimer’s disease (Figure 1).
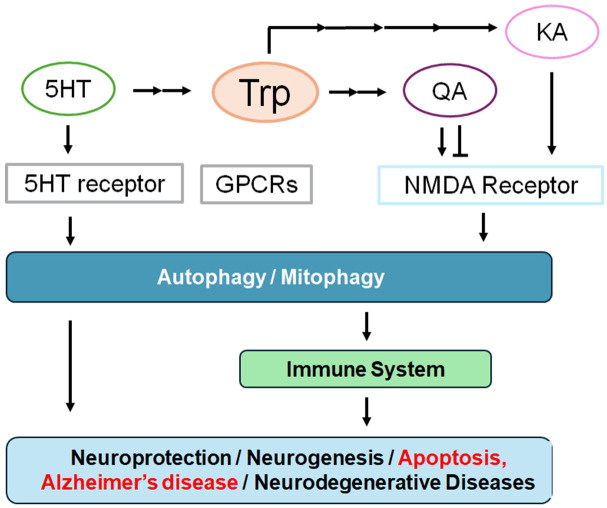
Figure 1.
Schematic diagram for some biological effects of tryptophan (Trp) metabolites. In particular, kynurenic acid (KA), quinolinic acid (QA) and serotonin (5HT) may be involved in the N-methyl-D-aspartate (NMDA) receptor, 5-hydroxytryptamine (5-HT, serotonin) receptor, and G protein-coupled receptors (GPCRs) signaling, which might eventually result in the pathogenesis of Alzheimer’s disease via the regulation of autophagy. The arrowhead means stimulation and/or augmentation whereas hammerhead represents inhibition. Some critical events have been omitted for clarity.
Tryptophan metabolite involved in the pathogenesis of Alzheimer’s disease
The tryptophan metabolic pathway may involve complex interactions between host cellular and bacteria processes, producing bioactive compounds such as serotonin (5-hydroxytryptamine; 5-HT) and kynurenine derivatives. Dysregulation of this pathway may be implicated in various diseases including Alzheimer’s disease, Parkinson’s disease, and/or multiple sclerosis [21]. In addition, this pathway has been also associated with stroke and impaired cognitive functioning [22]. Therefore, aberrant tryptophan metabolism and metabolic intermediates of tryptophan have accumulated substantial attention. For example, serotonin is mediated by tryptophan hydroxylase, which may be a precursor of melatonin [23]. Interestingly, pathophysiological roles of this metabolic pathway have also been recognized to immune tolerance in the placenta and fetal protection from maternal immune elimination [24]. In entero-chromaffin cells of gut, dietary tryptophan is converted by the tryptophan hydroxylase 1 to 5-hydroxytryptophan, which is subsequently changed into serotonin by aromatic amino acid decarboxylase [25]. Serotonin could influence on gut immune cells activity [26,27]. In addition, it has been shown that increased gut serotonin may also hamper autophagy with enhanced colitis susceptibility [28]. Therefore, serotonin could be a therapeutic target for gut inflammatory disorders with dysregulated autophagy [28]. Some of the serotonin receptors have been shown to belong to the G protein-coupled receptor (GPCR) family, by which serotonin could regulate intracellular signaling processes with arrangements of G proteins constituted of Gα, Gβ, and Gγ molucules [29]. Some of the GPCR agonists have been revealed possibly to have promising activities on the brain with neurological deficits via the phosphoinositide-3 kinase (PI3K)/AKT/mTOR signaling pathway [30]. One serotonin receptor, the 5-HT1A receptor is broadly expressed in the brain, predominantly being found in regions such as the hippocampus, nasal septum, and cortical periphery [31]. Changes in the expression of hippocampal 5-HT1A receptor during the prodromal stages of Alzheimer’s disease have inspired discussions [32]. Interestingly, it has been suggested that the 5HT receptor family, especially the 5HT-2b receptor, may represent potential new targets for treatment of behavioral dysfunctions associated with Alzheimer’s disease [6,33]. In addition, the levels of the 5HT-2b receptor may be increased in the brain of Alzheimer’s disease rather than that of age-matched controls, and 5HT-2b receptor inhibition with a selective antagonist could prevent amyloid-β and tau-based dysfunction in mouse models [34].
Stimulatingly, it has been shown that the NMDA receptor and GPCR activation may be involved in establishing synaptic value and/or neuron excitability in hippocampus [35]. In particular, the kynurenine pathway could account for the metabolism of tryptophan, which may include both an agonist (quinolinic acid) and an antagonist (kynurenic acid) at NMDA receptors [36]. As an important consequence of inflammation may be the enhanced breakdown of tryptophan to kynurenine, it is crucial for the NMDA receptor within a neuron and/or brain to change the metabolism of kynurenine into several neuroactive metabolites including the neurotoxic agonist quinolinic acid or the neuroprotective antagonist kynurenic acid [37]. While the CNS and immune system have been considered as independent units, it is understandable that some immune cells could influence the CNS, and that the activity of CNS could also affect the immune system [38]. For example, the kynurenic acid is well-known as an anti-inflammatory, neuroprotective, and/or anti-epileptic molecule that can inhibit ionotropic NMDA receptors, which exhibits the high affinity toward the glycine site of the receptor in addition to the G protein-coupled orphan receptor 35 (GPR35) [39]. Correspondingly, the insufficiency of kynurenic acid might contribute to the development of various brain disorders and/or immune related cardiovascular diseases [40]. Actually, NMDA receptors with kynurenic acid may play an indispensable role for synaptic plasticity in the CNS [41]. However, excess-activation of NMDA receptors might promote the elevation of intracellular Ca2+ levels leading to neuronal cell damage/death with synaptic dysfunction [42]. Likewise, the elevation of quinolinate may also direct to neuronal cell damage/death with synaptic dysfunction through the NMDA receptor-facilitated excitotoxicity [43]. Interestingly, it has been revealed that NMDA receptors signaling pathway may be involved in the regulation of autophagy, which could reduce the neuronal excitotoxic cell damage/death [44]. Therefore, the dysfunction of NMDA receptors may be linked to neurodegenerative disorders including Alzheimer’s disease [45]. Indeed, synaptic NMDA receptors may be essential for mediating neuronal healthy cell survival, synaptic plasticity, long-term potentiation, learning, and/or memory [46]. As such, therapeutic implications of aberrant tryptophan metabolism targeting the NMDA receptors have been suggested to be therapeutically relevant and useful in addressing various neurodegenerative disorders. This concept would eventually provide the framework to begin to identify compounds that can selectively target the downstream metabolism of tryptophan, thereby serving as a panel of immune modulatory agents that may be applied in neurodegenerative diseases.
Caveolae with NMDA receptors involved in Alzheimer’s disease
Cholesterol is one of the essential molecules in cellular physiology by reason of its involvement in important biochemical processes. For instance, cholesterol forms caveolae that facilitate the localization of significant molecules including several receptors and downstream signal transduction enzymes in cells. In addition, cholesterol also plays an important function in the plasticity of synapse in CNS [47]. Remarkably, the CNS is different from the other peripheral cells/organs in terms of certain cholesterol metabolism [48]. A decrease in cholesterol level could result in neural cell apoptosis via the impairment of the survival signaling in the brain [48]. The depletion of cholesterol from the plasma membrane within some neurons might also lead to the total loss of caveolae formation, which may drastically impair their functional reactions [49]. Some risk genes to Alzheimer’s disease are commonly expressed in brain neurons/cells, which may be linked to cholesterol metabolism and/or caveolae formation [50,51]. Caveolae are around 100-nm flagon-shaped invaginations of the plasma membrane with the existence of several caveolin proteins [52]. Caveolae may also have various signaling molecules including transmembrane receptors through their association with caveolin proteins, which have been thought to act as vital centers for supporting intracellular signaling [53] (Figure 2). Therefore, caveolae might also be involved in the synapse formation and/or its plasticity in CNS. For instance, neuron-targeted caveolin-1 may improve the NMDA receptor-mediated signaling, which might enhance the growth of dendritic cells in CNS [54]. Consequently, these biological alterations in hippocampal cells may be related with the modification in hippocampal roles such as memory and/or learning [55]. In addition, a study has revealed that caveolin-1 could interact with various glutamate receptors in hippocampus, sustaining their expression [56]. Caveolin-1 can also regulate the transport of glutamate receptors to the plasma-membrane, which may be responsible for the synaptic excursion in the development of long-term potentiation (LTP) [57]. In relation to this, caveolin-1 may be involved in the endocytosis of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, another type of glutamatergic ion channel, in hippocampus, which can reverse the glutamate receptors-associated long-term depression (LTD) [58]. Actually, overexpression of caveolin-1 might encourage the endocytosis of AMPA receptor from caveolae-like structures in neuronal cells, which may support the caveolin-1 connected receptor trafficking [59]. Interestingly, it has been exhibited that the AMPA receptor trafficking could amend the pathology of various behavioral disorders [58,60]. Therefore, caveolin-1 might be significant for the intensification of glutamate receptors-related function such as memory and/or learning in CNS.
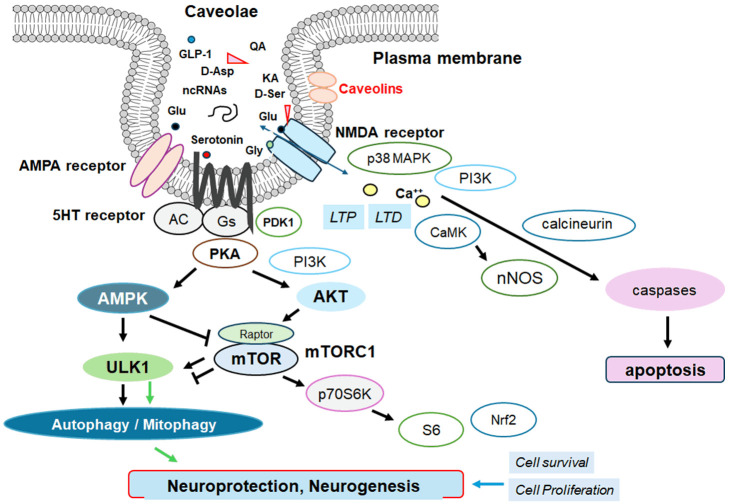
Figure 2.
An illustrative representation of caveolae associated with caveolins, in which various signaling molecules including serotonin and NMDA receptors may work with. Several modulator molecules linked to these receptors for their downstream pathways are also demonstrated. For example, NMDA receptors may require the binding of several amino acid molecules of glycine, glutamate, and/or aspartate, which could modulate the receptor function. The stimulation of serotonin (5HT) receptor may result in the stimulation of adenylyl cyclase (AC) that transforms adenosine triphosphate (ATP) into 3-5-cyclic adenosine monophosphate (cAMP), which may then stimulate the cAMP-dependent protein kinase (PKA) as well as the following AMPK for the modification of autophagy/mitophagy. Glutamate is to the glutamate-binding site and glycine is to the glycine-binding site in the NMDA receptor subunit within caveolae. The stimulation of NMDA receptors may allow Ca2+ entry into the cytoplasm of neurons, which may contribute to long-term potentiation (LTP) or long-term depression (LTD) as well as to a phase of excitotoxicity and/or apoptosis. Note that some critical pathways for the development of various disease-related signaling have been omitted for clarity. Arrowhead means stimulation whereas hammerhead represents inhibition. Abbreviation: GLP-1, glucagon-like peptide-1; AMPK, adenosine monophosphate-activated protein kinase; mTOR, mammalian/mechanistic target of rapamycin; PI3K, phosphoinositide-3 kinase; PKA, protein kinase A.
The key protein of caveolae is the caveolin-1, a 23 kDa of membrane protein with a hairpin-like structure, ubiquitously expressed in various cells, while caveolin-3 is predominantly expressed in muscle cells [61]. Both amino- and carboxy-terminal domains of caveolins are grasped in the cytoplasm, whereas the central hairpin loop is within transmembrane region. The juxtamembrane region in the amino-terminus of caveolin-1 through the hetero-oligomeric complex with caveolin-2 might specifically work as a scaffold platform, connecting with various signaling molecules such as G-proteins [62,63]. Notably, it has been shown that the deficiency of caveolin-1 could abrogate the serotonin-induced contraction of extrapulmonary airways, suggesting that caveolin-1 is indispensable for the serotonergic action [64]. The serotonin receptor mainly belong to the GPCR family [65]. Interestingly, it has been revealed that caveolin-1 could support structural plasticity of neuronal cells as well as neurogenesis in brain, suggesting that caveolae could be an effective therapeutic target against several neurodegenerative diseases including Alzheimer’s disease [66,67]. Amazingly, the alteration of caveolin-1 could trigger memory deficits that may look like a behavioral phenotype of Alzheimer’s disease [68]. Moreover, it has been reported that caveolin-1 may relate with the pathological amyloid precursor protein of Alzheimer’s disease [69]. Some roles of caveolae could influence the neuro-pathological situation of Alzheimer’s disease, possibly via the oxidative stress-associated mechanism during the neurodegeneration [70,71]. Therefore, it has been revealed that caveolin-1 may be a candidate with anti-oxidative and/or neuroprotective properties against neurodegenerative diseases [72]. Further studies are required to evaluate the comprehensive consequence of caveolae-impairment for the development of neurodegenerative diseases.
Autophagy and mitophagy involved in the pathogenesis of Alzheimer’s disease
It is of importance to find positive interventions that could prevent neurodegenerative diseases including Alzheimer’s disease. In this regard, it has been revealed that the dysregulation of autophagy may be a significant pathophysiological feature of Alzheimer’s disease, as the hyper-activation of mTOR in patients with Alzheimer’s disease may spoil the autophagy, providing to the exacerbation of Alzheimer’s disease with the increase of neurofibrillary tangles [73]. Interestingly, it has been revealed that orexin may participate in the modulation of autophagy via the mTOR-p70S6K signaling pathway [74]. Orexin has neuroprotective properties for several neurons [75]. In a neuropathology, there is also degeneration of orexinergic neurons [75]. In oxygen and glucose deprivation, orexin may exert its neuroprotective role by regulating autophagy [76]. In addition, an autophagy receptor, p62 molecule, is well-known to be associated with the pathology of Alzheimer’s disease [77]. An impairment of mitophagy with increased levels of impaired mitochondria has been shown to lead to the neuro-degeneration [78]. Therefore, corrected autophagy/mitophagy with the decreased mTOR activity could improve the pathology of Alzheimer’s disease [79]. Interestingly, a promising easy-use candidate substance for the modulation of autophagy may be the disaccharide trehalose, which has been revealed the effectiveness for inhibiting neurodegeneration in experimental animal models of Alzheimer’s disease [80]. Trehalose, a natural compound, could also stimulate autophagy improving disease phenotype in multiple models of neurodegenerative diseases [81]. In Alzheimer’s disease, trehalose could inhibit the accumulation of the amyloid-β by encouraging autophagy, which might clinically retain a substantial therapeutic activity against Alzheimer’s disease [82]. Auto-phagosomal activation with the treatment of trehalose could also remove the cytotoxic matters including hyper-phosphorylated tau protein and/or impaired mitochondria [83,84]. Remarkably, the chemical modification of autophagy/mitophagy could impede the neuroprotective effect of trehalose [85]. It has been shown that the dose-dependent effect of autophagy with trehalose in the hippocampus or frontal cortex could improve the behavioral disturbances of model animals with Alzheimer’s disease [86]. Now, autophagy/mitophagy has been accepted as a key player in the pathology of various neurodegenerative diseases.
In general, autophagy/mitophagy might be developed to clear misfolded proteins and/or damaged mitochondria thereby supporting cellular survival [87], which is predominantly regulated by the adenosine monophosphate-activated protein kinase (AMPK) and mammalian/mechanistic target of rapamycin (mTOR) signaling cascade [88]. Interestingly, the activation of GLP-1 receptor could accelerate the autophagy [89]. And, the GLP-1 receptor is also a member of GPCR family. Interventions of GLP-1 mediated modulation of autophagy/mitophagy have been applied in the clinical practice to decrease the incidence of type 2 diabetes mellitus [90]. It is known that amyloid-β could act together with the neuronal NMDA receptor, resulting in the excessive entry of calcium thereby causing excitotoxicity induced-cell death/damage for neurons [91]. Memantine, an approved drug usually prescribed for patients with Alzheimer’s disease, is a non-competitive inhibitor for the NMDA receptor, which could work in the appropriate regulation of calcium entry [92]. Additionally, memantine has been shown to stimulate autophagy, which may reduce the amyloid-β improving the survival of neuronal cells [93,94]. Another calcium channel-blocking non-competitive NMDA receptor antagonist might also exhibit to slowdown the pathology of cognitive decline [95]. The 3,4-methylenedioxymethamphetamine (MDMA) is a widespread recreational drug, which may eventually lead to serotonergic neurotoxicity and/or psychiatric disorders. The MDMA induced neurotoxicity may involve autophagy induction in serotonergic neurons [96]. While several studies have explored the involvement of autophagy in synaptic plasticity, the early steps of auto-phagosomes may remain unknown. Interestingly, it has been revealed that inhibition of mTOR coupled with the NMDA receptor activation could modulate the autophagy via the association of Rab11 with Atg9A [97]. Synaptic plasticity is a process that shapes neuronal connections during neurodevelopment, in which autophagosomes might appear in response to the plasticity-inducing stimuli.
Tryptophan metabolic pathway, autophagy, lifestyle, diet, and Gut-Brain axis for the therapeutic implication and clinical translation
The microbiota-gut-brain axis may be a complex cooperating network between the gut and brain involved in neurodegeneration (Figure 3). Gut microbial metabolites may regulate the communication between astrocytes and microglia in the CNS. As shown here, one of the potential links in bilateral gut-brain communication may be the tryptophan metabolism. In addition, the microbiota-originated tryptophan and its metabolites could move in the CNS to manipulate microglial activation, and the activated microglia may consequently influence brain functions [98]. In fact, several metabolites of tryptophan are associated with different neurodegenerative/neurological disorders including Alzheimer’s disease [99]. Alterations in the tryptophan metabolic pathway may also be involved in the pathogenesis of Parkinson’s disease with potential therapeutic implication [100]. High efficacy with less adverse events would be critical applied for the clinical translation. An excito-toxicity brought from stimulated NMDA receptors has directed to the finding of several molecules that could be employed to treat neurodegenerative diseases by hindering the NMDA receptor activation. In general, NMDA receptors are activated by glutamate and glycine, in which glycine acts as a co-agonist at the glycine modulatory site on the NMDA receptor. Therefore, inhibition of glycine transporter might improve the hypofunction of NMDA receptor by elevating the concentration of glycine in the synaptic split [101]. It is hypothesised that an appropriate increase in NMDA receptor signaling could lead to an increase in synaptic plasticity, which should improve cognitive function and/or memory. Remarkably, it has been shown that some NMDA receptors are expressed in astrocytes [102]. Astrocytes may play a crucial role in the regulation of synaptic strength, synaptic plasticity, and/or memory. As glycine and/or D-serine are co-agonists for the NMDA receptor regulating the strength and direction of synaptic plasticity, activity-dependent D-serine release mediated by astrocytes may therefore be a candidate for mediating between LTD and LTP during learning [103]. Additionally, the amino acid racemases, which can lead to the synthesis of D-amino acids from L-amino acids, have been broadly identified in gut microbiota species [104]. Furthermore, it has been shown that both GLP-1 and GLP-1 receptor proteins are also expressed in neurons as well as astrocytes and/or microglia in normal mice, which could promote neuroprotection in autoimmune encephalomyelitis [105]. Interestingly, importance of GLP-1 production in the gut-brain disorders such as Parkinson’s disease has been suggested for the neuroprotective strategies [106]. Dietary polyphenols such as anthocyanins could also improve the cognition, learning, and/or memory by modifying autophagy [107]. It has been shown that mild fasting might stimulate autophagy for the beneficial effects in the brain [108]. Recent studies have suggested that a form of intermittent fasting involving daily caloric intake within a limited time window may have a promise in the treatment of neurodegenerative diseases, which might improve brain cognitive function, mitochondrial function, up-regulate autophagy, reduce oxidative stress [109]. Similarly, it has been shown that a diet restricted to the circadian rhythm may induce increased expression of brain-derived neurotrophic factor (BDNF) in the forebrain region, regulating autophagy and increasing synaptic plasticity, thus enhancing cognitive function [110].
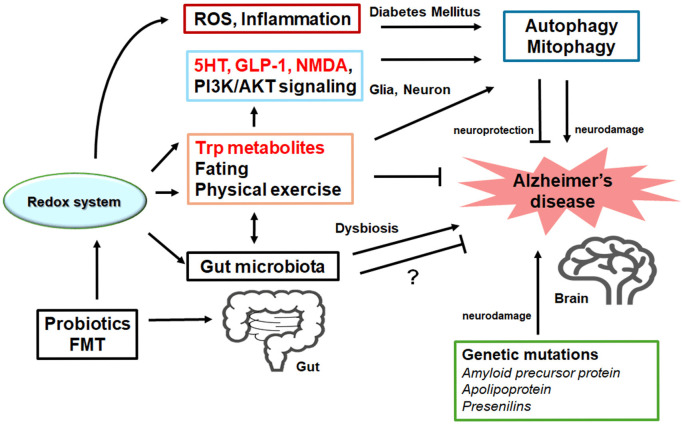
Figure 3.
Possible tactics for the neuroprotection and/or against the pathology of Alzheimer’s disease have been shown. One ideal approach might be to utilize agonists of serotonin (5HT) and GLP-1 receptors and/or antagonists of NMDA receptors with the use of tryptophan (Trp) metabolites to pause the neuron-damaging process for the neuroprotection. Probiotics and/or fecal microbiota transplantation (FMT) might contribute to the alteration of gut microbiota, which could also be advantageous for the treatment of Alzheimer’s disease. Note that several important activities such as inflammatory reaction, autophagy initiation/regulation, and reactive oxygen species (ROS) production have been omitted for clarity. Stimulatory effects are indicated with arrows; inhibitory effects with a line ending with hammerhead. “?” means for author speculation.
Among the pharmacological NMDA receptor-inhibitors, memantine has been permitted for the clinical treatment of Alzheimer’s disease [111]. Memantine, an NMDA receptor channel blocker, could prevent glutamine hyperactivity in Alzheimer’s disease by modulating autophagy [92,112], which has been used for the treatment of cognitive dysfunctions by acting as a channel blocker [113]. Excess activation of superfluous NMDA receptors could cause the death of glial cells/neurons that is considered as a causative factor in the development of Alzheimer’s disease, which could be partially prevented by memantine [114]. Another receptor antagonist ifenprodil has been found to be a potent negative modulator for NMDA receptors [115]. Combination memantine with ifenprodil or a selective serotonin reuptake inhibitor (SSRI) has been significantly more efficient in amending cognitive consequences of Alzheimer’s disease [115,116]. Interestingly, it has been shown that one of the SSRI, citalopram, could activate autophay/mitophagy in patients with Alzheimer’s disease [117]. GLP-1 receptor agonists, presently encouraged for type 2 diabetes and/or obesity, may evoke original mechanisms to prevent neurotoxicity associated with Alzheimer’s disease. One of the GLP-1 receptor agonists, semaglutide, has been investigated for the reduction of neuroinflammation as well as amyloid-β and/or cortical tau protein in the brain with Alzheimer’s disease [118]. Exenatide, another GLP-1 receptor agonist may be an obtainable therapeutic agent used in the treatment of diabetes or obesity, which might have additional effects on cognitive function in human patients [119]. Interestingly, the impact of GLP-1 from dipeptidyl peptidase-4 inhibitors has also been evaluated on cognitive function in type 2 diabetes mellitus [120]. Since diet with natural products involved in the GLP-1 signaling may be believed safe for long-term use, they could also be a promising therapeutic approach against Alzheimer’s disease [30,120].
Future perspectives
Tryptophan is an indispensable amino acid used for protein synthesis, and it is metabolized into several biologically active compounds including kynurenines, melatonin, and serotonin [121]. Increasing evidence suggests that changes in the composition of the gut microbiota may affect the tryptophan metabolism, which may in turn impact several brain functions [122] (Figure 3). Especially, dietary alterations for the tryptophan pathway might be of potential value as comparatively safe, easy, and real therapeutic approach to improve certain neurodegenerative diseases [123]. Cognitive impairment is a central feature of neurodegenerative diseases such as Alzheimer’s disease as well as of psychiatric disorders such as schizophrenia [124,125]. Interestingly, both schizophrenia and Alzheimer’s disease may be characterized by abnormalities in glutamatergic pathways associated to NMDA receptor dysfunction [126]. In addition, it has been revealed that the NMDA receptor antagonism with the GLP-1 receptor agonism may effectively reverse the hyper-glycaemia, dyslipidaemia and/or obesity in animal models of metabolic disease [127]. Probably, the ideal approach might be to utilize agonists of serotonin receptors and antagonists of NMDA receptors to halt the neuron-damaging process for the neuroprotection, as both GLP-1 receptor and certain serotonin receptor may be comparable each other in the same GPCR family [29,128].
Currently, this approach for the treatment of cognitive dysfunctions does have only its limitation due to detrimental adverse effects. However, many competitive or noncompetitive agonists/antagonists of GLP-1 receptors or NMDA receptors might have been manifested by undesirable adverse effects, which may be connected to the receptors-binding affinity and/or allosteric modulation of receptors function. At present, cognitive disorders and/or dementia are notorious owing to several limitations of therapy. As a result, a more detailed exploration of the structure and function of these receptors would have led to the development of some selective modulators with satisfactory side-effect profiles. Again, there is an urgent necessity for diagnostics and/or therapeutic options against cognitive dysfunctions with Alzheimer’s disease. New treatment strategies targeting depression are also urgently required.
Conclusions
Caveolae with serotonin and NMDA receptors are structural cholesterol-rich microdomains involved in active intracellular signal transduction for the survival of brain cells, which could also be involved in a broad range of neurodegenerative diseases including Alzheimer’s disease. Noteworthy function of those structures have been identified on several cell types in the CNS including astrocytes, microglia, and/or neurons. Built on the accessible evidence of caveolae with serotonin and NMDA receptors, this structure with the adjustment of autophagy could be a favorable treatment tactic of Alzheimer’s disease.
Disclosure of conflict of interest
None.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AMPK
adenosine monophosphate-activated protein kinase
- BDNF
brain-derived neurotrophic factor
- CNS
central nervous system
- GLP-1
glucagon-like peptide-1
- GPCR
G protein-coupled receptor
- GPR35
G protein-coupled orphan receptor 35
- 5-HT
5-hydroxytryptamine, serotonin
- LTD
long-term depression
- LTP
long-term potentiation
- mTOR
mammalian/mechanistic target of rapamycin
- MDMA
3,4-methylenedioxymethamphetamine
- NMDA
N-methyl-d-aspartate
- PI3K
phosphoinositide-3 kinase
- ROS
reactive oxygen species
- SSRI
selective serotonin reuptake inhibitor
References
- 1.Aboukhatwa M, Dosanjh L, Luo Y. Antidepressants are a rational complementary therapy for the treatment of Alzheimer’s disease. Mol Neurodegener. 2010;5:10. doi: 10.1186/1750-1326-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brambilla D. Drug discovery, development and delivery in Alzheimer’s disease. Pharm Res. 2017;35:3. doi: 10.1007/s11095-017-2329-6. [DOI] [PubMed] [Google Scholar]
- 3.Vijayraghavan S, Major AJ, Everling S. Muscarinic M1 receptor overstimulation disrupts working memory activity for rules in primate prefrontal cortex. Neuron. 2018;98:1256–1268. e1254. doi: 10.1016/j.neuron.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 4.Parhizkar S, Arzberger T, Brendel M, Kleinberger G, Deussing M, Focke C, Nuscher B, Xiong M, Ghasemigharagoz A, Katzmarski N, Krasemann S, Lichtenthaler SF, Müller SA, Colombo A, Monasor LS, Tahirovic S, Herms J, Willem M, Pettkus N, Butovsky O, Bartenstein P, Edbauer D, Rominger A, Ertürk A, Grathwohl SA, Neher JJ, Holtzman DM, Meyer-Luehmann M, Haass C. Loss of TREM2 function increases amyloid seeding but reduces plaque-associated ApoE. Nat Neurosci. 2019;22:191–204. doi: 10.1038/s41593-018-0296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mdawar B, Ghossoub E, Khoury R. Selective serotonin reuptake inhibitors and Alzheimer’s disease. Neural Regen Res. 2020;15:41–46. doi: 10.4103/1673-5374.264445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Popova NK, Tsybko AS, Naumenko VS. The implication of 5-HT receptor family members in aggression, depression and suicide: similarity and difference. Int J Mol Sci. 2022;23:8814. doi: 10.3390/ijms23158814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lippold K, Dewey W. The role of 5-HT2a/2c receptors in nociception and opioid antinociception: a review of the preclinical literature. Curr Treat Options Psychiatry. 2017;4:210–220. [Google Scholar]
- 8.Zhang G, Stackman RW Jr. The role of serotonin 5-HT2A receptors in memory and cognition. Front Pharmacol. 2015;6:225. doi: 10.3389/fphar.2015.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin CH, Lane HY. The role of N-methyl-D-aspartate receptor neurotransmission and precision medicine in behavioral and psychological symptoms of dementia. Front Pharmacol. 2019;10:540. doi: 10.3389/fphar.2019.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baeta-Corral R, De la Fuente M, Giménez-Llort L. Sex-dependent worsening of NMDA-induced responses, anxiety, hypercortisolemia, and organometry of early peripheral immunoendocrine impairment in adult 3xTg-AD mice and their long-lasting ontogenic modulation by neonatal handling. Behav Brain Res. 2023;438:114189. doi: 10.1016/j.bbr.2022.114189. [DOI] [PubMed] [Google Scholar]
- 11.Gao DP, Weng QY, Zhang YY, Ou YX, Niu YF, Lou Q, Xie DL, Cai Y, Yang JH. Memantine alleviates cognitive impairment and hippocampal morphology injury in a mouse model of chronic alcohol exposure. Pharmacol Biochem Behav. 2024;20:173827. doi: 10.1016/j.pbb.2024.173827. [DOI] [PubMed] [Google Scholar]
- 12.Whitfield T, Chouliaras L, Morrell R, Rubio D, Radford D, Marchant NL, Walker Z. The criteria used to rule out mild cognitive impairment impact dementia incidence rates in subjective cognitive decline. Alzheimers Res Ther. 2024;16:142. doi: 10.1186/s13195-024-01516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Ogunniyi A, Orgeta V, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. 2020;396:413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Self WK, Holtzman DM. Emerging diagnostics and therapeutics for Alzheimer disease. Nat Med. 2023;29:2187–2199. doi: 10.1038/s41591-023-02505-2. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Cai Y, Guan T, Zhang Y, Huang K, Zhang Z, Cao W, Guan X. Quinic acid alleviates high-fat diet-induced neuroinflammation by inhibiting DR3/IKK/NF-kappaB signaling via gut microbial tryptophan metabolites. Gut Microbes. 2024;16:2374608. doi: 10.1080/19490976.2024.2374608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu H, Wang G, Zhang J, Lu B, Li D, Chen J. Inhalation of diesel exhaust particulate matter accelerates weight gain via regulation of hypothalamic appetite-related genes and gut microbiota metabolism. J Hazard Mater. 2024;466:133570. doi: 10.1016/j.jhazmat.2024.133570. [DOI] [PubMed] [Google Scholar]
- 17.Chyr J, Gong H, Zhou X. DOTA: deep learning optimal transport approach to advance drug repositioning for Alzheimer’s disease. Biomolecules. 2022;12:196. doi: 10.3390/biom12020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durairaja A, Pandey S, Kahl E, Fendt M. Nasal administration of orexin A partially rescues dizocilpine-induced cognitive impairments in female C57BL/6 J mice. Behav Brain Res. 2023;450:114491. doi: 10.1016/j.bbr.2023.114491. [DOI] [PubMed] [Google Scholar]
- 19.Perin M, Longordo F, Massonnet C, Welker E, Lüthi A. Diurnal inhibition of NMDA-EPSCs at rat hippocampal mossy fibre synapses through orexin-2 receptors. J Physiol. 2014;592:4277–4295. doi: 10.1113/jphysiol.2014.272757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Innocenti A, Lentini G, Rapacchietta S, Cinnirella P, Elia M, Ferri R, Bruni O. The role of supplements and over-the-counter products to improve sleep in children: a systematic review. Int J Mol Sci. 2023;24:7821. doi: 10.3390/ijms24097821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyamoto K, Sujino T, Kanai T. The tryptophan metabolic pathway of the microbiome and host cells in health and disease. Int Immunol. 2024:dxae035. doi: 10.1093/intimm/dxae035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakker L, Ramakers IHGB, J P M Eussen S, Choe K, van den Hove DLA, Kenis G, Rutten BPF, van Oostenbrugge RJ, Staals J, Ulvik A, Ueland PM, Verhey FRJ, Köhler S. The role of the kynurenine pathway in cognitive functioning after stroke: a prospective clinical study. J Neurol Sci. 2023;454:120819. doi: 10.1016/j.jns.2023.120819. [DOI] [PubMed] [Google Scholar]
- 23.Taleb S. Tryptophan dietary impacts gut barrier and metabolic diseases. Front Immunol. 2019;10:2113. doi: 10.3389/fimmu.2019.02113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 25.Grondin JA, Khan WI. Emerging roles of gut serotonin in regulation of immune response, microbiota composition and intestinal inflammation. J Can Assoc Gastroenterol. 2023;7:88–96. doi: 10.1093/jcag/gwad020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan WI, Ghia JE. Gut hormones: emerging role in immune activation and inflammation. Clin Exp Immunol. 2010;161:19–27. doi: 10.1111/j.1365-2249.2010.04150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nat Rev Microbiol. 2012;10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- 28.Haq S, Wang H, Grondin J, Banskota S, Marshall JK, Khan II, Chauhan U, Cote F, Kwon YH, Philpott D, Brumell JH, Surette M, Steinberg GR, Khan WI. Disruption of autophagy by increased 5-HT alters gut microbiota and enhances susceptibility to experimental colitis and Crohn’s disease. Sci Adv. 2021;7:eabi6442. doi: 10.1126/sciadv.abi6442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright SC, Avet C, Gaitonde SA, Muneta-Arrate I, Le Gouill C, Hogue M, Breton B, Koutsilieri S, Diez-Alarcia R, Héroux M, Lauschke VM, Bouvier M. Conformation- and activation-based BRET sensors differentially report on GPCR-G protein coupling. Sci Signal. 2024;17:eadi4747. doi: 10.1126/scisignal.adi4747. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda Y, Nagase N, Tsuji A, Kitagishi Y, Matsuda S. Neuroprotection by dipeptidyl-peptidase-4 inhibitors and glucagon-like peptide-1 analogs via the modulation of AKT-signaling pathway in Alzheimer’s disease. World J Biol Chem. 2021;12:104–113. doi: 10.4331/wjbc.v12.i6.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto K, Tsuji M, Oguchi T, Momma Y, Ohashi H, Ito N, Nohara T, Nakanishi T, Ishida A, Hosonuma M, Nishikawa T, Murakami H, Kiuchi Y. Comparison of protective effects of antidepressants mediated by serotonin receptor in abeta-oligomer-induced neurotoxicity. Biomedicines. 2024;12:1158. doi: 10.3390/biomedicines12061158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verdurand M, Zimmer L. Hippocampal 5-HT1A receptor expression changes in prodromal stages of Alzheimer’s disease: beneficial or deleterious? Neuropharmacology. 2017;123:446–454. doi: 10.1016/j.neuropharm.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 33.Anzalone M, Karam SA, Briting SRR, Petersen S, Thomsen MB, Babcock AA, Landau AM, Finsen B, Metaxas A. Serotonin-2B receptor (5-HT(2B)R) expression and binding in the brain of APP(swe)/PS1(dE9) transgenic mice and in Alzheimer’s disease brain tissue. Neurosci Lett. 2024;17:138013. doi: 10.1016/j.neulet.2024.138013. [DOI] [PubMed] [Google Scholar]
- 34.Acquarone E, Argyrousi EK, Arancio O, Watterson DM, Roy SM. The 5HT2b receptor in Alzheimer’s disease: increased levels in patient brains and antagonist attenuation of amyloid and tau induced dysfunction. J Alzheimers Dis. 2024;98:1349–1360. doi: 10.3233/JAD-240063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanhoose AM, Ritchie MD, Winder DG. Regulation of cAMP levels in area CA1 of hippocampus by Gi/o-coupled receptors is stimulus dependent in mice. Neurosci Lett. 2004;370:80–83. doi: 10.1016/j.neulet.2004.07.093. [DOI] [PubMed] [Google Scholar]
- 36.Stone TW. Kynurenic acid antagonists and kynurenine pathway inhibitors. Expert Opin Investig Drugs. 2001;10:633–645. doi: 10.1517/13543784.10.4.633. [DOI] [PubMed] [Google Scholar]
- 37.Meier TB, Savitz J. The kynurenine pathway in traumatic brain injury: implications for psychiatric outcomes. Biol Psychiatry. 2022;91:449–458. doi: 10.1016/j.biopsych.2021.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stone TW, Williams RO. Tryptophan metabolism as a ‘reflex’ feature of neuroimmune communication: sensor and effector functions for the indoleamine-2, 3-dioxygenase kynurenine pathway. J Neurochem. 2024;168:3333–3357. doi: 10.1111/jnc.16015. [DOI] [PubMed] [Google Scholar]
- 39.Ostapiuk A, Urbanska EM. Kynurenic acid in neurodegenerative disorders-unique neuroprotection or double-edged sword? CNS Neurosci Ther. 2022;28:19–35. doi: 10.1111/cns.13768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka M, Toldi J, Vécsei L. Exploring the etiological links behind neurodegenerative diseases: inflammatory cytokines and bioactive kynurenines. Int J Mol Sci. 2020;21:2431. doi: 10.3390/ijms21072431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist--a review of preclinical data. Neuropharmacol. 1999;38:735–767. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Li P, Feng J, Wu M. Dysfunction of NMDA receptors in Alzheimer’s disease. Neurol Sci. 2016;37:1039–1047. doi: 10.1007/s10072-016-2546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hama-Tomioka K, Kinoshita H, Nakahata K, Kondo T, Azma T, Kawahito S, Hatakeyama N, Matsuda N. Roles of neuronal nitric oxide synthase, oxidative stress, and propofol in N-methyl-D-aspartate-induced dilatation of cerebral arterioles. Br J Anaesth. 2012;108:21–29. doi: 10.1093/bja/aer368. [DOI] [PubMed] [Google Scholar]
- 44.Davis SE, Roth JR, Aljabi Q, Hakim AR, Savell KE, Day JJ, Arrant AE. Delivering progranulin to neuronal lysosomes protects against excitotoxicity. J Biol Chem. 2021;297:100993. doi: 10.1016/j.jbc.2021.100993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar A. NMDA receptor function during senescence: Implication on cognitive performance. Front Neurosci. 2015;9:1–15. doi: 10.3389/fnins.2015.00473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abraham WC, Jones OD, Glanzman DL. Is plasticity of synapses the mechanism of long-term memory storage? NPJ Sci Learn. 2019;4:9. doi: 10.1038/s41539-019-0048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mauch DH, Nägler K, Schumacher S, Göritz C, Müller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 48.Liu T, Li Y, Yang B, Wang H, Lu C, Chang AK, Huang X, Zhang X, Lu Z, Lu X, Gao B. Suppression of neuronal cholesterol biosynthesis impairs brain functions through insulin-like growth factor I-Akt signaling. Int J Biol Sci. 2021;17:3702–3716. doi: 10.7150/ijbs.63512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lentini D, Guzzi F, Pimpinelli F, Zaninetti R, Cassetti A, Coco S, Maggi R, Parenti M. Polarization of caveolins and caveolae during migration of immortalized neurons. J Neurochem. 2008;104:514–523. doi: 10.1111/j.1471-4159.2007.05005.x. [DOI] [PubMed] [Google Scholar]
- 50.Shin JW, Lee JC. Roles of microglial membranes in Alzheimer’s disease. Curr Top Membr. 2020;86:301–314. doi: 10.1016/bs.ctm.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mattera V, Occhiuzzi F, Correale J, Pasquini JM. Remyelinating effect driven by transferrin-loaded extracellular vesicles. Glia. 2024;72:338–361. doi: 10.1002/glia.24478. [DOI] [PubMed] [Google Scholar]
- 52.Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 53.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–194. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 54.Head BP, Hu Y, Finley JC, Saldana MD, Bonds JA, Miyanohara A, Niesman IR, Ali SS, Murray F, Insel PA, Roth DM, Patel HH, Patel PM. Neuron-targeted caveolin-1 protein enhances signaling and promotes arborization of primary neurons. J Biol Chem. 2011;286:33310–33321. doi: 10.1074/jbc.M111.255976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mandyam CD, Schilling JM, Cui W, Egawa J, Niesman IR, Kellerhals SE, Staples MC, Busija AR, Risbrough VB, Posadas E, Grogman GC, Chang JW, Roth DM, Patel PM, Patel HH, Head BP. Neuron-targeted caveolin-1 improves molecular signaling, plasticity, and behavior dependent on the hippocampus in adult and aged mice. Biol Psychiatry. 2017;81:101–110. doi: 10.1016/j.biopsych.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Francesconi A, Kumari R, Zukin RS. Regulation of group I metabotropic glutamate receptor trafficking and signaling by the caveolar/lipid raft pathway. J Neurosci. 2009;29:3590–3602. doi: 10.1523/JNEUROSCI.5824-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Q, Yang L, Zhang K, Guo YY, Liu SB, Wu YM, Li XQ, Song Q, Zhuo M, Zhao MG. Increased coupling of caveolin-1 and estrogen receptor alpha contributes to the fragile X syndrome. Ann Neurol. 2015;77:618–636. doi: 10.1002/ana.24358. [DOI] [PubMed] [Google Scholar]
- 58.Luo L, Yang L, Zhang K, Zhou SM, Wang Y, Yang LK, Feng B, Liu SB, Wu YM, Zhao MG, Yang Q. Caveolin-1-mediated cholesterol accumulation contributes to exaggerated mGluR-dependent long-term depression and impaired cognition in Fmr1 knockout mice. Mol Neurobiol. 2023;60:3379–3395. doi: 10.1007/s12035-023-03269-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gaudreault SB, Chabot C, Gratton JP, Poirier J. The caveolin scaffolding domain modifies 2-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor binding properties by inhibiting phospholipase A2 activity. J Biol Chem. 2004;279:356–362. doi: 10.1074/jbc.M304777200. [DOI] [PubMed] [Google Scholar]
- 60.Ramsakha N, Ojha P, Pal S, Routh S, Citri A, Bhattacharyya S. A vital role for PICK1 in the differential regulation of metabotropic glutamate receptor internalization and synaptic AMPA receptor endocytosis. J Biol Chem. 2023;299:104837. doi: 10.1016/j.jbc.2023.104837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams TM, Lisanti MP. The caveolin proteins. Genome Biol. 2004;5:214. doi: 10.1186/gb-2004-5-3-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hill MM, Bastiani M, Luetterforst R, Kirkham M, Kirkham A, Nixon SJ, Walser P, Abankwa D, Oorschot VM, Martin S, Hancock JF, Parton RG. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 2008;132:113–124. doi: 10.1016/j.cell.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carman CV, Lisanti MP, Benovic JL. Regulation of G protein-coupled receptor kinases by caveolin. J Biol Chem. 1999;274:8858–8864. doi: 10.1074/jbc.274.13.8858. [DOI] [PubMed] [Google Scholar]
- 64.Keshavarz M, Schwarz H, Hartmann P, Wiegand S, Skill M, Althaus M, Kummer W, Krasteva-Christ G. Caveolin-1: functional insights into its role in muscarine- and serotonin-induced smooth muscle constriction in murine airways. Front Physiol. 2017;8:295. doi: 10.3389/fphys.2017.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sarkar P, Mozumder S, Bej A, Mukherjee S, Sengupta J, Chattopadhyay A. Structure, dynamics and lipid interactions of serotonin receptors: excitements and challenges. Biophys Rev. 2020;13:101–122. doi: 10.1007/s12551-020-00772-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang W, Li Y, Li Y, Wang Q. Caveolin-1, a novel player in cognitive decline. Neurosci Biobehav Rev. 2021;129:95–106. doi: 10.1016/j.neubiorev.2021.06.044. [DOI] [PubMed] [Google Scholar]
- 67.Wu J, Zhou SL, Pi LH, Shi XJ, Ma LR, Chen Z, Qu ML, Li X, Nie SD, Liao DF, Pei JJ, Wang S. High glucose induces formation of tau hyperphosphorylation via Cav-1-mTOR pathway: a potential molecular mechanism for diabetes-induced cognitive dysfunction. Oncotarget. 2017;8:40843–40856. doi: 10.18632/oncotarget.17257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gupta A, Sharma A, Kumar A, Goyal R. Alteration in memory cognition due to activation of caveolin-1 and oxidative damage in a model of dementia of Alzheimer’s type. Indian J Pharmacol. 2019;51:173–180. doi: 10.4103/ijp.IJP_81_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ikezu T, Trapp BD, Song KS, Schlegel A, Lisanti MP, Okamoto T. Caveolae, plasma membrane microdomains for alpha-secretase-mediated processing of the amyloid precursor protein. J Biol Chem. 1998;273:10485–10495. doi: 10.1074/jbc.273.17.10485. [DOI] [PubMed] [Google Scholar]
- 70.Sharma S, Singh M, Sharma PL. Ameliorative effect of daidzein: a caveolin-1 inhibitor in vascular endothelium dysfunction induced by ovariectomy. Indian J Exp Biol. 2012;50:28–34. [PubMed] [Google Scholar]
- 71.Ajmani P, Yadav HN, Singh M, Sharma PL. Possible involvement of caveolin in attenuation of cardioprotective effect of ischemic preconditioning in diabetic rat heart. BMC Cardiovasc Disord. 2011;11:43. doi: 10.1186/1471-2261-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang W, Li Y, He S, Jiang T, Wang N, Du M, Cheng B, Gao W, Li Y, Wang Q. Caveolin-1 alleviates diabetes-associated cognitive dysfunction through modulating neuronal ferroptosis-mediated mitochondrial homeostasis. Antioxid Redox Signal. 2022;37:867–886. doi: 10.1089/ars.2021.0233. [DOI] [PubMed] [Google Scholar]
- 73.Perluigi M, Di Domenico F, Barone E, Butterfield DA. mTOR in Alzheimer disease and its earlier stages: links to oxidative damage in the progression of this dementing disorder. Free Radic Biol Med. 2021;169:382–396. doi: 10.1016/j.freeradbiomed.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo X, Wen J, Gao Q, Zhao Y, Zhao Y, Wang C, Xu N, Shao Y, Chang X. Orexin-A/OX1R is involved in regulation of autophagy to promote cortisol secretion in adrenocortical cell. Biochim Biophys Acta Mol Basis Dis. 2024;1870:166844. doi: 10.1016/j.bbadis.2023.166844. [DOI] [PubMed] [Google Scholar]
- 75.Alrouji M, Al-Kuraishy HM, Al-Gareeb AI, Zaafar D, Batiha GE. Orexin pathway in Parkinson’s disease: a review. Mol Biol Rep. 2023;50:6107–6120. doi: 10.1007/s11033-023-08459-5. [DOI] [PubMed] [Google Scholar]
- 76.Xu D, Kong T, Zhang S, Cheng B, Chen J, Wang C. Orexin-A protects against cerebral ischemia-reperfusion injury by inhibiting excessive autophagy through OX1R-mediated MAPK/ERK/mTOR pathway. Cell Signal. 2021;79:109839. doi: 10.1016/j.cellsig.2020.109839. [DOI] [PubMed] [Google Scholar]
- 77.Su H, Wang X. Autophagy and p62 in cardiac protein quality control. Autophagy. 2011;7:1382–1383. doi: 10.4161/auto.7.11.17339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mary A, Eysert F, Checler F, Chami M. Mitophagy in Alzheimer’s disease: molecular defects and therapeutic approaches. Mol Psychiatry. 2023;28:202–216. doi: 10.1038/s41380-022-01631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Babygirija R, Sonsalla MM, Mill J, James I, Han JH, Green CL, Calubag MF, Wade G, Tobon A, Michael J, Trautman MM, Matoska R, Yeh CY, Grunow I, Pak HH, Rigby MJ, Baldwin DA, Niemi NM, Denu JM, Puglielli L, Simcox J, Lamming DW. Protein restriction slows the development and progression of pathology in a mouse model of Alzheimer’s disease. Nat Commun. 2024;15:5217. doi: 10.1038/s41467-024-49589-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yap KH, Azmin S, Makpol S, Damanhuri HA, Mustapha M, Hamzah JC, Ibrahim NM. Profiling neuroprotective potential of trehalose in animal models of neurodegenerative diseases: a systematic review. Neural Regen Res. 2023;18:1179–1185. doi: 10.4103/1673-5374.360164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rusmini P, Cortese K, Crippa V, Cristofani R, Cicardi ME, Ferrari V, Vezzoli G, Tedesco B, Meroni M, Messi E, Piccolella M, Galbiati M, Garrè M, Morelli E, Vaccari T, Poletti A. Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration. Autophagy. 2019;15:631–651. doi: 10.1080/15548627.2018.1535292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khalifeh M, Read MI, Barreto GE, Sahebkar A. Trehalose against Alzheimer’s disease: insights into a potential therapy. Bioessays. 2020;42:e1900195. doi: 10.1002/bies.201900195. [DOI] [PubMed] [Google Scholar]
- 83.Liu R, Barkhordarian H, Emadi S, Park CB, Sierks MR. Trehalose differentially inhibits aggregation and neurotoxicity of beta-amyloid 40 and 42. Neurobiol Dis. 2005;20:74–81. doi: 10.1016/j.nbd.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 84.Tien NT, Karaca I, Tamboli IY, Walter J. Trehalose alters subcellular trafficking and the metabolism of the alzheimer-associated amyloid precursor protein. J Biol Chem. 2016;291:10528–10540. doi: 10.1074/jbc.M116.719286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pupyshev AB, Belichenko VM, Tenditnik MV, Bashirzade AA, Dubrovina NI, Ovsyukova MV, Akopyan AA, Fedoseeva LA, Korolenko TA, Amstislavskaya TG, Tikhonova MA. Combined induction of mTOR-dependent and mTOR-independent pathways of autophagy activation as an experimental therapy for Alzheimer’s disease-like pathology in a mouse model. Pharmacol Biochem Behav. 2022;217:173406. doi: 10.1016/j.pbb.2022.173406. [DOI] [PubMed] [Google Scholar]
- 86.Pupyshev AB, Akopyan AA, Tenditnik MV, Ovsyukova MV, Dubrovina NI, Belichenko VM, Korolenko TA, Zozulya SA, Klyushnik TP, Tikhonova MA. Alimentary treatment with trehalose in a pharmacological model of Alzheimer’s disease in mice: effects of different dosages and treatment regimens. Pharmaceutics. 2024;16:813. doi: 10.3390/pharmaceutics16060813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pi H, Li M, Tian L, Yang Z, Yu Z, Zhou Z. Enhancing lysosomal biogenesis and autophagic flux by activating the transcription factor EB protects against cadmium-induced neurotoxicity. Sci Rep. 2017;7:43466. doi: 10.1038/srep43466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Querfurth H, Lee HK. Mammalian/mechanistic target of rapamycin (mTOR) complexes in neurodegeneration. Mol Neurodegener. 2021;16:44. doi: 10.1186/s13024-021-00428-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Złotek M, Kurowska A, Herbet M, Piątkowska-Chmiel I. GLP-1 analogs, SGLT-2, and DPP-4 inhibitors: a triad of Hope for Alzheimer’s disease therapy. Biomedicine. 2023;11:3035. doi: 10.3390/biomedicines11113035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xia W, Yu H, Wen P. Meta-analysis on GLP-1 mediated modulation of autophagy in islet beta-cells: prospectus for improved wound healing in type 2 diabetes. Int Wound J. 2024;21:e14841. doi: 10.1111/iwj.14841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO, Lin Y, Bennett MV, Yuste R, Castillo PE, Zukin RS. Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci. 2006;9:501–510. doi: 10.1038/nn1664. [DOI] [PubMed] [Google Scholar]
- 93.Wang Y, Jiang B, Luo W. Memantine ameliorates oxaliplatin-induced neurotoxicity via mitochondrial protection. Bioengineered. 2022;13:6688–6697. doi: 10.1080/21655979.2022.2026553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee JR, Jeong KW. N-retinylidene-N-retinylethanolamine degradation in human retinal pigment epithelial cells via memantine- and ifenprodil-mediated autophagy. Korean J Physiol Pharmacol. 2023;27:449–456. doi: 10.4196/kjpp.2023.27.5.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Johnson JW, Kotermanski SE. Mechanism of action of memantine. Curr Opin Pharmacol. 2006;6:61–67. doi: 10.1016/j.coph.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 96.Mercer LD, Higgins GC, Lau CL, Lawrence AJ, Beart PM. MDMA-induced neurotoxicity of serotonin neurons involves autophagy and rilmenidine is protective against its pathobiology. Neurochem Int. 2017;105:80–90. doi: 10.1016/j.neuint.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 97.Janusz-Kaminska A, Brzozowska A, Tempes A, Urbanska M, Blazejczyk M, Miłek J, Kuzniewska B, Zeng J, Wesławski J, Kisielewska K, Bassell GJ, Jaworski J. Rab11 regulates autophagy at dendritic spines in an mTOR- and NMDA-dependent manner. Mol Biol Cell. 2024;35:ar43. doi: 10.1091/mbc.E23-02-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xie L, Wu Q, Li K, Khan MAS, Zhang A, Sinha B, Li S, Chang SL, Brody DL, Grinstaff MW, Zhou S, Alterovitz G, Liu P, Wang X. Tryptophan metabolism in Alzheimer’s disease with the involvement of microglia and astrocyte crosstalk and gut-brain axis. Aging Dis. 2024;15:2168–2190. doi: 10.14336/AD.2024.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jankech T, Gerhardtova I, Majerova P, Piestansky J, Fialova L, Jampilek J, Kovac A. A novel RP-UHPLC-MS/MS approach for the determination of tryptophan metabolites derivatized with 2-bromo-4’-nitroacetophenone. Biomedicines. 2024;12:1003. doi: 10.3390/biomedicines12051003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bohár Z, Toldi J, Fülöp F, Vécsei L. Changing the face of kynurenines and neurotoxicity: therapeutic considerations. Int J Mol Sci. 2015;16:9772–9793. doi: 10.3390/ijms16059772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hashimoto K. Glycine transport inhibitors for the treatment of schizophrenia. Open Med Chem J. 2010;4:10–19. doi: 10.2174/1874104501004010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kosenkov AM, Maiorov SA, Gaidin SG. Astrocytic NMDA receptors. Biochemistry (Mosc) 2024;89:1045–1060. doi: 10.1134/S0006297924060063. [DOI] [PubMed] [Google Scholar]
- 103.Squadrani L, Wert-Carvajal C, Müller-Komorowska D, Bohmbach K, Henneberger C, Verzelli P, Tchumatchenko T. Astrocytes enhance plasticity response during reversal learning. Commun Biol. 2024;7:852. doi: 10.1038/s42003-024-06540-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Taniguchi K, Sawamura H, Ikeda Y, Tsuji A, Kitagishi Y, Matsuda S. D-amino acids as a biomarker in schizophrenia. Diseases. 2022;10:9. doi: 10.3390/diseases10010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee CH, Jeon SJ, Cho KS, Moon E, Sapkota A, Jun HS, Ryu JH, Choi JW. Activation of glucagon-like peptide-1 receptor promotes neuroprotection in experimental autoimmune encephalomyelitis by reducing neuroinflammatory responses. Mol Neurobiol. 2018;55:3007–3020. doi: 10.1007/s12035-017-0550-2. [DOI] [PubMed] [Google Scholar]
- 106.Wu H, Wei J, Zhao X, Liu Y, Chen Z, Wei K, Lu J, Chen W, Jiang M, Li S, Chen T. Neuroprotective effects of an engineered Escherichia coli Nissle 1917 on Parkinson’s disease in mice by delivering GLP-1 and modulating gut microbiota. Bioeng Transl Med. 2022;8:e10351. doi: 10.1002/btm2.10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rosli H, Shahar S, Rajab NF, Che Din N, Haron H. The effects of polyphenols-rich tropical fruit juice on cognitive function and metabolomics profile - a randomized controlled trial in middle-aged women. Nutr Neurosci. 2022;25:1577–1593. doi: 10.1080/1028415X.2021.1880312. [DOI] [PubMed] [Google Scholar]
- 108.Martinez-Lopez N, Tarabra E, Toledo M, Garcia-Macia M, Sahu S, Coletto L, Batista-Gonzalez A, Barzilai N, Pessin JE, Schwartz GJ, Kersten S, Singh R. System-wide benefits of intermeal fasting by autophagy. Cell Metab. 2017;26:856–871. doi: 10.1016/j.cmet.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wells RG, Neilson LE, McHill AW, Hiller AL. Dietary fasting and time-restricted eating in Huntington’s disease: therapeutic potential and underlying mechanisms. Transl Neurodegener. 2024;13:17. doi: 10.1186/s40035-024-00406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hu X, Peng J, Tang W, Xia Y, Song P. A circadian rhythm-restricted diet regulates autophagy to improve cognitive function and prolong lifespan. Biosci Trends. 2023;17:356–368. doi: 10.5582/bst.2023.01221. [DOI] [PubMed] [Google Scholar]
- 111.Kim AY, Al Jerdi S, MacDonald R, Triggle CR. Alzheimer’s disease and its treatment-yesterday, today, and tomorrow. Front Pharmacol. 2024;15:1399121. doi: 10.3389/fphar.2024.1399121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Companys-Alemany J, Turcu AL, Schneider M, Müller CE, Vázquez S, Griñán-Ferré C, Pallàs M. NMDA receptor antagonists reduce amyloid-β deposition by modulating calpain-1 signaling and autophagy, rescuing cognitive impairment in 5XFAD mice. Cell Mol Life Sci. 2022;79:408. doi: 10.1007/s00018-022-04438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Song X, Jensen MØ, Jogini V, Stein RA, Lee CH, Mchaourab HS, Shaw DE, Gouaux E. Mechanism of NMDA receptor channel block by MK-801 and memantine. Nature. 2018;556:515–519. doi: 10.1038/s41586-018-0039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang R, Reddy PH. Role of glutamate and NMDA receptors in Alzheimer’s disease. J Alzheimer’s Dis. 2017;57:1041–1048. doi: 10.3233/JAD-160763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Harris LD, Regan MC, Myers SJ, Nocilla KA, Akins NS, Tahirovic YA, Wilson LJ, Dingledine R, Furukawa H, Traynelis SF, Liotta DC. Novel GluN2B-selective NMDA receptor negative allosteric modulator possesses intrinsic analgesic properties and enhances analgesia of morphine in a rodent tail flick pain model. ACS Chem Neurosci. 2023;14:917–935. doi: 10.1021/acschemneuro.2c00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lavretsky H, Laird KT, Krause-Sorio B, Heimberg BF, Yeargin J, Grzenda A, Wu P, Thana-Udom K, Ercoli LM, Siddarth P. A randomized double-blind placebo-controlled trial of combined escitalopram and memantine for older adults with major depression and subjective memory complaints. Am J Geriatr Psychiatry. 2020;28:178–190. doi: 10.1016/j.jagp.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Reddy AP, Sawant N, Morton H, Kshirsagar S, Bunquin LE, Yin X, Reddy PH. Selective serotonin reuptake inhibitor citalopram ameliorates cognitive decline and protects against amyloid beta-induced mitochondrial dynamics, biogenesis, autophagy, mitophagy and synaptic toxicities in a mouse model of Alzheimer’s disease. Hum Mol Genet. 2021;30:789–810. doi: 10.1093/hmg/ddab091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Koychev I, Adler AI, Edison P, Tom B, Milton JE, Butchart J, Hampshire A, Marshall C, Coulthard E, Zetterberg H, Hellyer P, Cormack F, Underwood BR, Mummery CJ, Holman RR. Protocol for a double-blind placebo-controlled randomised controlled trial assessing the impact of oral semaglutide in amyloid positivity (ISAP) in community dwelling UK adults. BMJ Open. 2024;14:e081401. doi: 10.1136/bmjopen-2023-081401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Carr RD, Larsen MO, Jelic K, Lindgren O, Vikman J, Holst JJ, Deacon CF, Ahrén B. Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. J Clin Endocrinol Metab. 2010;95:872–878. doi: 10.1210/jc.2009-2054. [DOI] [PubMed] [Google Scholar]
- 120.Meng J, Yan R, Zhang C, Bai X, Yang X, Yang Y, Feng T, Liu X. Dipeptidyl peptidase-4 inhibitors alleviate cognitive dysfunction in type 2 diabetes mellitus. Lipids Health Dis. 2023;22:219. doi: 10.1186/s12944-023-01985-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Planells-Cárcel A, Kazakova J, Pérez C, Gonzalez-Ramirez M, Garcia-Parrilla MC, Guillamón JM. A consortium of different Saccharomyces species enhances the content of bioactive tryptophan-derived compounds in wine fermentations. Int J Food Microbiol. 2024;416:110681. doi: 10.1016/j.ijfoodmicro.2024.110681. [DOI] [PubMed] [Google Scholar]
- 122.Davidson M, Rashidi N, Nurgali K, Apostolopoulos V. The role of tryptophan metabolites in neuropsychiatric disorders. Int J Mol Sci. 2022;23:9968. doi: 10.3390/ijms23179968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chojnacki C, Popławski T, Chojnacki J, Fila M, Konrad P, Blasiak J. Tryptophan intake and metabolism in older adults with mood disorders. Nutrients. 2020;12:3183. doi: 10.3390/nu12103183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schaefer J, Giangrande E, Weinberger DR, Dickinson D. The global cognitive impairment in schizophrenia: consistent over decades and around the world. Schizophr Res. 2013;150:42–50. doi: 10.1016/j.schres.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hu NW, Ondrejcak T, Rowan MJ. Glutamate receptors in preclinical research on Alzheimer’s disease: update on recent advances. Pharmacol Biochem Behav. 2012;100:855–862. doi: 10.1016/j.pbb.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 126.Lin CH, Lane HY, Tsai GE. Glutamate signaling in the pathophysiology and therapy of schizophrenia. Pharmacol Biochem Behav. 2012;100:665–677. doi: 10.1016/j.pbb.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 127.Petersen J, Ludwig MQ, Juozaityte V, Ranea-Robles P, Svendsen C, Hwang E, Kristensen AW, Fadahunsi N, Lund J, Breum AW, Mathiesen CV, Sachs L, Moreno-Justicia R, Rohlfs R, Ford JC, Douros JD, Finan B, Portillo B, Grose K, Petersen JE, Trauelsen M, Feuchtinger A, DiMarchi RD, Schwartz TW, Deshmukh AS, Thomsen MB, Kohlmeier KA, Williams KW, Pers TH, Frølund B, Strømgaard K, Klein AB, Clemmensen C. GLP-1 directed NMDA receptor antagonism for obesity treatment. Nature. 2024;629:1133–1141. doi: 10.1038/s41586-024-07419-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lund ML, Egerod KL, Engelstoft MS, Dmytriyeva O, Theodorsson E, Patel BA, Schwartz TW. Enterochromaffin 5-HT cells - a major target for GLP-1 and gut microbial metabolites. Mol Metab. 2018;11:70–83. doi: 10.1016/j.molmet.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]