Abstract
Aim
The features and outcomes of sepsis‐associated acute kidney injury (SA‐AKI) may be affected by chronic kidney disease (CKD). Accordingly, we aimed to compare SA‐AKI in patients with or without CKD.
Methods
Retrospective cohort study in 12 intensive care units (ICU). We studied the prevalence, patient characteristics, timing, trajectory, treatment and outcomes of SA‐AKI with and without CKD.
Results
Of 84 240 admissions, 7255 (8.6%) involved patients with CKD. SA‐AKI was more common in patients with CKD (21% vs 14%; p < .001). CKD patients were older (70 vs. 60 years; p < .001), had a higher median Charlson co‐morbidity index (5 vs. 3; p < .001) and acute physiology and chronic health evaluation (APACHE) III score (78 vs. 60; p < .001) and were more likely to receive renal replacement therapy (RRT) (25% vs. 17%; p < .001). They had less complete return to baseline function at ICU discharge (48% vs. 60%; p < .001), higher major adverse kidney events at day 30 (MAKE‐30) (38% vs. 27%; p < .001), and higher hospital and 90‐day mortality (21% vs. 13%; p < .001, and 27% vs. 16%; p < .001, respectively). After adjustment for patient characteristics and severity of illness, however, CKD was not an independent risk factor for increased 90‐day mortality (OR 0.88; 95% CI 0.76–1.02; p = .08) or MAKE‐30 (OR 0.98; 95% CI 0.80–1.09; p = .4).
Conclusion
SA‐AKI is more common in patients with CKD. Such patients are older, more co‐morbid, have higher disease severity, receive different ICU therapies and have different trajectories of renal recovery and greater unadjusted mortality. However, after adjustment day‐90 mortality and MAKE‐30 risk were not increased by CKD.
Keywords: acute kidney injury, chronic kidney disease, critical care, sepsis, sepsis‐associated acute kidney injury
Summary at a Glance
It was previously unknown how sepsis‐associated acute kidney injury affects patients with chronic kidney disease. In a study involving a large group of critically ill patients, it was found that those with CKD were more likely to develop the condition, were older and had more health problems, received different treatments, and had a higher unadjusted mortality rate. However, after adjusting for other factors, there was no significant increase in mortality.
1. INTRODUCTION
The epidemiology of sepsis‐associated acute kidney injury (SA‐AKI) in the intensive care unit (ICU) according to the Acute Disease Quality Initiative (ADQI) definition 1 has been recently described. 2 , 3 Such study demonstrated that SA‐AKI is increasing, occurs in one in every six patients and is associated with significant morbidity and mortality risk. 3
Debate surrounding the assessment of SA‐AKI, however, has emphasized the need to understand its interaction with chronic kidney disease (CKD). 2 , 4 , 5 This is because current SA‐AKI research has not included data on CKD status and because CKD is likely to be a major modifier of the incidence, trajectory, treatment and outcomes of SA‐AKI.
In this regard, in the general ICU population, the prevalence of CKD has been increasing 6 and CKD is associated with a higher risk of acute kidney injury (AKI). 7 Moreover, AKI in the presence of CKD, has been associated with higher rates of death in the ICU, as well as an increased risk of long‐term dependence on renal replacement therapy (RRT). 7 , 8 , 9 Furthermore, a recent post hoc analysis of the STARRT‐AKI trial demonstrated that allocation to accelerated RRT initiation resulted in a three‐fold increase in the risk of long‐term RRT dependence at 90 days in patients with CKD. 10
Given the above consideration, we obtained CKD status for a cohort of critically ill patients. We hypothesized that patients with CKD would have a higher incidence of SA‐AKI and that their trajectory, treatments and outcomes would differ from those of SA‐AKI patients without CKD.
2. METHODS
2.1. Study design
We conducted a multicentre, retrospective cohort study of granular, routinely collected, electronic medical record (EMR)‐based clinical data.
2.2. Study sites
The study was conducted in 12 closed‐model, mixed (medical and surgical) ICUs in Queensland, Australia, which included five tertiary, three outer metropolitan, and four regional ICUs. The study evaluated all adult patients admitted between January 1, 2015, and December 31, 2021, except for patients with advanced CKD requiring chronic dialysis, patients admitted with palliative intent, and patients transferred from another participating ICU.
2.3. Data sources
Data was collected from all centres using eCritical MetaVision™ (iMDsoft, Boston, MA, USA) clinical information systems 11 , 12 , 13 , 14 and the ANZICS CORE Adult Patient Database (APD). 15 , 16 , 17 , 18 The data included daily laboratory reports, medications, microbiology, haemodynamics, fluid balance, patient demographics, diagnoses, severity of illness and outcomes. Primary and secondary diagnoses, from the International Classification of Diseases 10 Australia Modification (ICD‐10‐AM) codes, as well as mortality data, were collected from the Queensland Hospital Admitted Patient Data Collection (QHAPDC) 19 , 20 , 21 and Queensland births, deaths and marriage registry, 22 , 23 respectively. As previously published, the amount of missing data for key variables in the dataset was very low. 3 Admission diagnoses were categorized to optimize data accuracy and interpretability (Table S1). The Charlson‐defined co‐morbidities and index were calculated from the ICD‐10 codes (Table S2). 24 , 25 The conversion of vasopressor dosage to norepinephrine equivalent and calculation of vasoactive inotrope score was carried out as per protocol (Tables S3 and S4). 26
2.4. Identification of CKD
Utilising previously published methods, patients with CKD were identified from their ICD‐10‐AM codes. 6 , 8 , 27 The ICD‐10‐AM codes used to identify patients with CKD are provided in Table S2.
2.5. Identification of sepsis‐associated kidney injury and pre‐admission creatinine
Patients with SA‐AKI were identified according to the ADQI definition 1 as previously published. 3 AKI was defined according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria using daily serum creatinine and hourly urine output data. 28 Imputation of hourly urine output was performed to manage absent urine output measurements.
As pre‐admission serum creatinine was not available in the data set, we estimated baseline serum creatinine using a method previously validated in hospitalized Australian patients with and without CKD where pre‐admission creatinine was available for such validation. 29 , 30 This method utilised the Chronic Kidney Disease–Epidemiology Collaboration (CKD‐EPI) equation and assumed a baseline estimated glomerular filtration rate (eGFR) of 40 mL/min/1.73m2 and 75 mL/min/1.73m2 for patients with and without CKD, respectively. 29 To ensure robustness, we further performed sensitivity analysis and compared incidence and outcomes in patients with CKD according to different methods used to estimate baseline serum creatinine (Table S5). Using ADQI criteria, SA‐AKI was diagnosed if AKI occurred between day 1 and 7 after sepsis diagnosis. 1
2.6. Outcomes
The primary outcome was SA‐AKI incidence in patients with and without CKD. The secondary outcome was the timing of SA‐AKI to ICU admission. The additional secondary outcomes include renal outcomes such as AKI severity, renal recovery status at ICU discharge, and major adverse kidney events at 30 days (MAKE‐30) and non‐renal outcomes such ICU and hospital length of stay, and ICU, hospital and 90‐day mortality. Renal outcome definitions used in this study are provided in Table S6.
2.7. Statistical analysis
Descriptive statistics were expressed as frequencies and proportions for categorical variables and medians with interquartile ranges (IQR) for continuous variables. Fisher's exact test was used to compare categorical variables. The Wilcoxon rank sum test was used to compare continuous variables. A mixed‐effect logistic regression model was developed, including hospitals as a random effect, to examine factors associated with 90‐day mortality and MAKE‐30. The variables used for analysis were determined a priori and reflected the clinical utility of available data. The results of the multivariable analysis were reported as odds ratios (OR) with 95% confidence intervals (95% CI). Given the large data set and multiple comparisons, a two‐sided p‐value of <0.01 was chosen to indicate statistical significance. 31 Statistical analyses were performed using R v.4.0.3.
2.8. Ethical considerations
This study was approved by the Metro South Hospital and Health Service Human Research Ethics Committee (HREC/2022/QMS/82024) with an individual waiver of consent granted.
3. RESULTS
3.1. Incidence of SA‐AKI
Between January 1, 2015, and December 31, 2021, there were 89 184 adult admissions to the participating ICUs for which data linkage was complete. After the exclusion of patients admitted with palliative intent (543; 0.6%), transferred from another ICU (2541; 2.8%), and history of advanced CKD requiring chronic dialysis (1942; 2.2%), 84 240 patients remained. Pre‐morbid CKD was identified in 7255 (8.6%) of patients. Among study patients, SA‐AKI was more common (p < .001) in patients with CKD (1492; 21.0%) than in patients without CKD (10 546; 13.7%).
3.2. Patient characteristics
The key characteristics of SA‐AKI patients with and without CKD are displayed in Table 1. Patients with CKD were older and had a higher median Charlson co‐morbidity index (CCI) than those without CKD. Moreover, they had higher rates of co‐morbidities related to cardiovascular diseases, renal diseases, diabetes and moderate–severe liver disease, but were less likely to have been diagnosed with a gastrointestinal or respiratory‐related illness during admission. Both groups had similar admission circumstances, although the CKD group had slightly higher rates of post‐rapid response admissions and readmissions.
TABLE 1.
Baseline characteristics of SA‐AKI patients according to CKD status.
| Chronic kidney disease | |||
|---|---|---|---|
| Variable | Yes, N = 1492 1 | No, N = 10027 1 | p value 2 |
| Age (years) | 70 (60, 77) | 60 (46, 71) | <.001 |
| Female | 564 (38%) | 4421 (44%) | <.001 |
| Body mass index | 29.3 (26.1, 35.5) | 27.8 (24.9, 33.8) | <.001 |
| Charlson co‐morbidity index* | 5 (3, 6) | 3 (1, 5) | <.001 |
| Co‐morbidities | |||
| Ischaemic heart disease | 190 (13%) | 750 (7.5%) | <.001 |
| Congestive heart failure | 484 (32%) | 1445 (14%) | <.001 |
| Peripheral vascular disease | 83 (5.6%) | 428 (4.3%) | .026 |
| Cerebrovascular Disease | 84 (5.6%) | 828 (8.3%) | <.001 |
| Chronic pulmonary disease | 246 (16%) | 1508 (15%) | .15 |
| Mild liver disease | 68 (4.6%) | 483 (4.8%) | .7 |
| Moderate–severe liver disease | 87 (5.8%) | 421 (4.2%) | .006 |
| Diabetes | 300 (20%) | 959 (9.6%) | <.001 |
| Diabetes with complications | 798 (53%) | 1498 (15%) | <.001 |
| Localised Cancer | 132 (8.8%) | 1008 (10%) | .15 |
| Metastatic cancer | 44 (2.9%) | 357 (3.6%) | .26 |
| APACHE diagnosis group | |||
| Cardiovascular | 239 (16%) | 1212 (12%) | |
| Gastrointestinal | 201 (13%) | 1592 (16%) | |
| Genitourinary | 145 (9.7%) | 385 (3.8%) | |
| Haematological | 8 (0.5%) | 70 (0.7%) | |
| Metabolic | 78 (5.2%) | 1314 (13%) | |
| Neurological | 51 (3.4%) | 1214 (12%) | |
| Other | 52 (3.5%) | 307 (3.1%) | |
| Respiratory | 197 (13%) | 1871 (19%) | |
| Sepsis | 520 (35%) | 2036 (20%) | |
| Trauma | 1 (<0.1%) | 26 (0.3%) | |
| Admission circumstances | |||
| Post elective surgery | 4 (0.3%) | 53 (0.5%) | .24 |
| Post rapid response | 371 (25%) | 1792 (18%) | <.001 |
| Post cardiac arrest | 1 (<0.1%) | 15 (0.1%) | .71 |
| Readmission | 136 (9.1%) | 698 (7.0%) | .004 |
| LOS in hospital before ICU (hours) | 11.8 (4.9, 66.0) | 7.8 (2.9, 35.3) | <.001 |
| Severity of illness | |||
| APACHE 2 score | 24 (19, 29) | 18 (13, 24) | <.001 |
| APACHE 3 score | 78 (65, 93) | 60 (44, 79) | <.001 |
| APACHE 3 risk of death | 0.29 (0.16, 0.52) | 0.12 (0.04, 0.31) | <.001 |
| Day of admission | |||
| Any ventilation | 818 (55%) | 5822 (58%) | .02 |
| Invasive ventilation | 770 (52%) | 5547 (55%) | .007 |
| Non‐invasive ventilation | 48 (3.2%) | 275 (2.7%) | .31 |
| Maximum creatinine (μmol/L) | 195 (106, 302) | 123 (75.8, 201) | <.001 |
| AKI stage | .046 | ||
| 0 | 667 (45%) | 4815 (48%) | |
| 1 | 364 (24%) | 2443 (24%) | |
| 2 | 224 (15%) | 1344 (13%) | |
| 3 | 237 (16%) | 1425 (14%) | |
| RRT | 182 (12%) | 787 (7.8%) | <.001 |
| Vasopressors | 952 (64%) | 6053 (61%) | .018 |
| NEE score (μg/kg/min) | 0.07 (0.02, 0.16) | 0.06 (0.02, 0.14) | .098 |
| Mean MAP (mmHg) | 74.7 (69.8, 82.6) | 75.8 (70.5, 83.9) | .001 |
| Maximum SOFA score | 7 (5, 9) | 6 (4, 9) | <.001 |
| Maximum lactate (mmol/L) | 2.4 (1.5, 4.6) | 2.3 (1.4, 4.2) | .11 |
| ICU level | <.001 | ||
| Tertiary | 880 (59%) | 5133 (52%) | |
| Outer metropolitan | 316 (21%) | 2072 (21%) | |
| Regional | 291 (20%) | 2760 (28%) | |
| Source of hospital admission | .013 | ||
| Home | 1124 (75%) | 7291 (73%) | |
| Other hospital | 350 (23%) | 2661 (27%) | |
| Low acuity facility | 3 (0.2%) | 21 (0.2%) | |
| High care facility | 15 (1.0%) | 54 (0.5%) | |
| Source of ICU admission | <.001 | ||
| Emergency department | 599 (40%) | 4323 (43%) | |
| Operating theatre | 315 (21%) | 2489 (25%) | |
| Ward | 508 (34%) | 2396 (24%) | |
| Other hospital | 70 (4.7%) | 819 (8.2%) | |
| Treatment goals on admission | <.001 | ||
| Full active treatment | 1216 (82%) | 9138 (91%) | |
| Missing | 0 (0%) | 1 (<0.1%) | |
| Treatment limitation order | 276 (18%) | 888 (8.9%) | |
Abbreviations: AKI, acute kidney injury; APACHE, acute physiology and chronic health evaluation; ICU, intensive care unit; LOS, length of stay; NEE, norepinephrine equivalent; RRT, renal replacement therapy; SA‐AKI, sepsis‐associated acute kidney injury; SOFA, sequential organ failure assessment.
Median (IQR) or frequency (%).
Wilcoxon rank sum test; Pearson's chi‐squared test; Fisher's exact test.
Charlson co‐morbidity index calculation excluded chronic kidney disease component.
Upon admission, patients with CKD exhibited more severe illness as indicated by higher acute physiology and chronic health evaluation (APACHE) scores and SOFA scores. Additionally, there was no difference in the administration of vasopressors or ventilation by CKD status. On the day of admission to the ICU, patients with CKD had significantly higher rates of RRT.
3.3. Timing and characteristics of SA‐AKI diagnosis
As shown in Table S7, the median ICU Day for the diagnosis of sepsis was Day 1 (IQR 1–1) in both CKD and non‐CKD patients. For both groups, the diagnosis of sepsis and AKI essentially occurred simultaneously. At diagnosis of SA‐AKI, patients with CKD had more advanced AKI as evidenced by higher stages of AKI and higher rates of RRT. Furthermore, they were more likely to be diagnosed with AKI by both urine output and creatinine.
3.4. Trajectory of SA‐AKI
We examined the trajectory of SA‐AKI patients over 14 days from ICU admission. The mean daily serum creatinine for patients with a history of CKD was >250 μmol/L, rapidly declined to <200 μmol/L over 4 days then steadily decreased to <175 μmol/L by day 14 of ICU. In patients without CKD, the mean serum creatinine remained relatively constant between 140 and 160 μmol/L (Figure 1).
FIGURE 1.
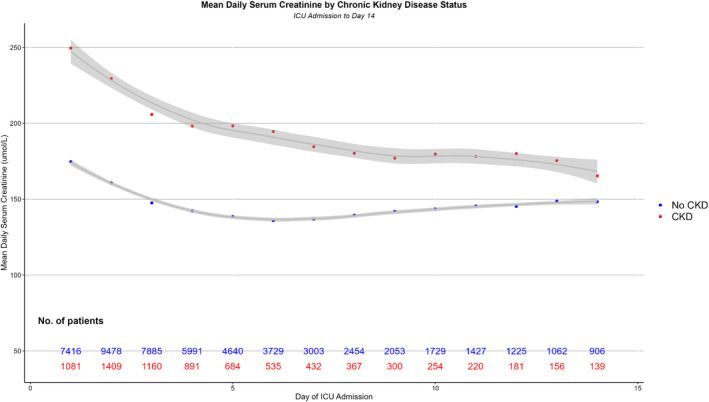
Mean daily serum creatinine by chronic kidney disease (CKD) status. ICU, intensive care unit.
The mean UO for patients with and without CKD is demonstrated in Figure 2. Patients with CKD had a steady improvement in mean hourly UO after ICU admission with a high of 1 mL/kg/h 120 hours after ICU admission. Similarly, patients without CKD had an improvement in UO to nearly 1.2 mL/kg/h at 96 hours after ICU admission.
FIGURE 2.
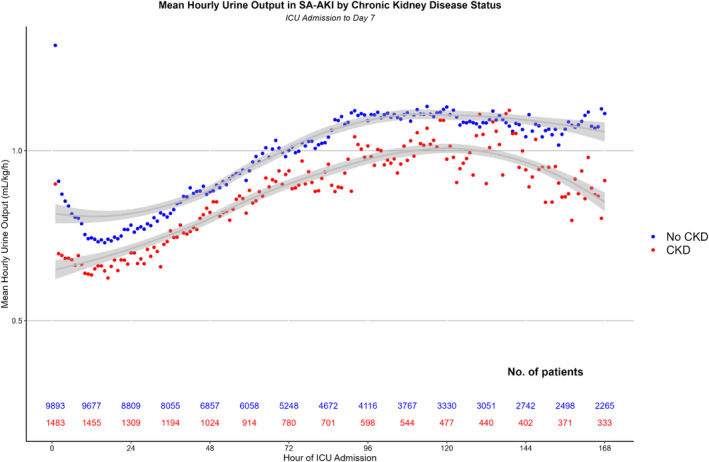
Mean hourly urine output by chronic kidney disease (CKD) status. ICU, intensive care unit; SA‐AKI, sepsis‐associated acute kidney injury.
The trajectory of renal function in patients with and without CKD over the first 7 days of SA‐AKI, as determined by the AKI stage, is shown in Figure 3. In CKD patients, nearly 60% of patients with CKD still had AKI by day seven of ICU. Further, the proportion of patients with Stage 3 AKI increased over time to greater than 30% on day seven. In contrast, the proportion of non‐CKD patients without AKI steadily increased to greater than 50% and patients with Stage 3 AKI remained less than 30% on day seven of ICU admission.
FIGURE 3.
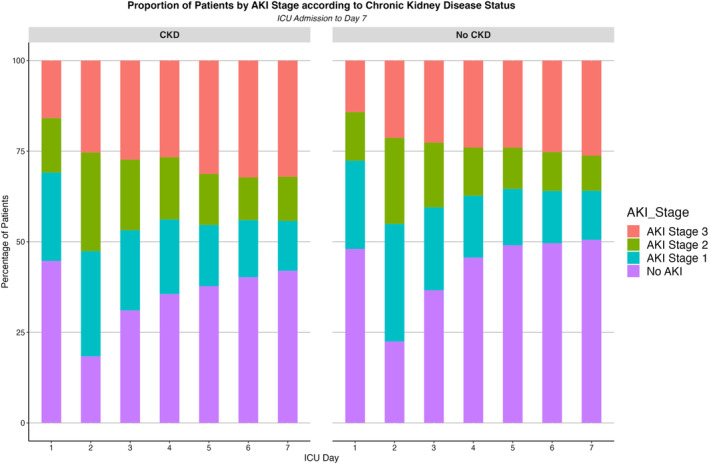
Proportion of patients by acute kidney injury (AKI) stage according to chronic kidney disease (CKD) status. ICU, intensive care unit.
3.5. Treatment during first 7 days of ICU admission
The treatment of patients with SA‐AKI by CKD status over the first 7 days of their ICU admission is shown in Table 2. Patients with CKD had similar ventilation and vasopressor treatment but were significantly more likely to require RRT. However, in patients receiving ventilation and vasopressors, the median duration of such support was comparable between groups.
TABLE 2.
Treatment during the first 7 days according to chronic kidney disease status.
| Chronic kidney disease | |||
|---|---|---|---|
| Variable | Yes, N = 1492 1 | No, N = 10027 1 | p value 2 |
| Any | |||
| Ventilation | 949 (64%) | 6635 (66%) | .053 |
| Invasive ventilation | 908 (61%) | 6373 (64%) | .044 |
| Non‐invasive ventilation | 88 (5.9%) | 498 (5.0%) | .13 |
| Vasopressors | 1118 (75%) | 7319 (73%) | .2 |
| Diuretics | 85 (5.7%) | 413 (4.1%) | .002 |
| RRT | 373 (25%) | 1726 (17%) | <.001 |
| Days of intervention | |||
| Ventilation | 2 (0, 5) | 2 (0, 5) | .032 |
| Invasive ventilation | 2 (0, 4) | 2 (0, 5) | .026 |
| Non‐invasive ventilation | 0 (0, 0) | 0 (0, 0) | .14 |
| Vasopressors | 2 (0, 5) | 2 (0, 5) | .032 |
| RRT | 2 (0, 4) | 2 (0, 3) | .014 |
| Mean daily | |||
| NEE score (ug/kg/min) | 0.05 (0.02, 0.10) | 0.05 (0.02, 0.10) | .3 |
Abbreviations: NEE, norepinephrine equivalent; RRT, renal replacement therapy.
Median (IQR) or frequency (%).
Pearson's chi‐squared test; Wilcoxon rank sum test.
3.6. Associated outcomes
The outcomes of patients with and without CKD are demonstrated in Table 3. The maximum AKI stage during ICU admission was greater in patients with CKD. The length of stay in ICU was comparable between the groups; however, those with CKD had a significantly longer hospital length of stay. Additionally, patients with CKD were less likely to have renal recovery at ICU discharge as well as at a significantly higher risk of MAKE‐30. When looking at mortality, CKD status was associated with an increased risk of ICU, hospital, 90‐day and 1‐year mortality.
TABLE 3.
Outcomes in all SA‐AKI patients according to chronic kidney disease status.
| Chronic kidney disease | |||
|---|---|---|---|
| Variable | Yes, N = 1492 1 | No, N = 10027 1 | p value 2 |
| Maximum AKI stage in ICU | <.001 | ||
| 1 | 505 (34%) | 3971 (40%) | |
| 2 | 394 (26%) | 2703 (27%) | |
| 3 | 593 (40%) | 3353 (33%) | |
| Any RRT during ICU admission | 392 (26%) | 1803 (18%) | <.001 |
| ICU LOS (days) | 4.0 (3.0, 8.0) | 4.0 (3.0, 8.0) | .4 |
| Hospital LOS (days) | 15 (8, 26) | 10 (5, 19) | <.001 |
| Renal recovery at ICU discharge | <.001 | ||
| Full | 709 (48%) | 6007 (60%) | |
| Partial | 90 (6.0%) | 518 (5.2%) | |
| None | 693 (46%) | 3502 (35%) | |
| MAKE‐30* | 568 (38%) | 2722 (27%) | <.001 |
| ICU mortality | 180 (12%) | 913 (9.1%) | <.001 |
| Hospital mortality | 311 (21%) | 1257 (13%) | <.001 |
| Day 30 mortality | 336 (23%) | 1352 (13%) | <.001 |
| Day 90 mortality | 410 (27%) | 1641 (16%) | <.001 |
| 1 year mortality | 535 (36%) | 2152 (21%) | <.001 |
Abbreviations: AKI, acute kidney injury; ICU, intensive care unit; LOS, length of stay; MAKE‐30, major adverse kidney events at day 30; RRT, renal replacement therapy.
Median (IQR) or frequency (%).
Pearson's chi‐squared test; Wilcoxon rank sum test.
MAKE‐30 defined as mortality at 30 days or serum creatinine is twice the estimated pre‐admission value at the time of ICU discharge, or the patient is still on renal replacement therapy at the time of ICU discharge.
In a multivariable logistic regression model for Day‐90 mortality (Table 4), after adjusting for patient characteristics and severity of illness, a history of CKD was not associated with an increased risk of mortality at 90‐day (OR 0.88; 95% CI 0.76–1.02; p = 0.08). A higher modified Charlson co‐morbidity index, admission post‐rapid response team review, a treatment limitation order on admission to ICU, and a higher APACHE 3 score were significantly associated with an increased risk of mortality. In addition, a multivariable logistic regression model for MAKE‐30 (Table S8), demonstrated no independent association with CKD status (OR 0.93; 95% CI 0.08–1.09; p = .40).
TABLE 4.
Multivariate regression analysis for Day 90 mortality.
| Variable | OR 1 | 95% CI 1 | p‐value |
|---|---|---|---|
| Chronic kidney disease | 0.88 | 0.76, 1.02 | .082 |
| Age | 0.97 | 0.91, 1.05 | .5 |
| Body mass index | 0.98 | 0.94, 1.03 | .4 |
| Female | 0.89 | 0.80, 1.00 | .042 |
| Charlson co‐morbidity index* | 1.50 | 1.40, 1.59 | <.001 |
| Elective admission | 0.95 | 0.47, 1.91 | .9 |
| Admitted post‐rapid response | 1.39 | 1.22, 1.58 | <.001 |
| Treatment limitation | 3.00 | 2.58, 3.49 | <.001 |
| APACHE 3 score | 2.76 | 2.59, 2.94 | <.001 |
| Any RRT at SA‐AKI diagnosis | 0.92 | 0.78, 1.10 | .4 |
| Any vasopressors at SA‐AKI diagnosis | 0.92 | 0.82, 1.04 | .2 |
| Any ventilation at SA‐AKI diagnosis | 1.06 | 0.95, 1.20 | .3 |
Abbreviations: APACHE, acute physiology and chronic health evaluation; RRT, renal replacement therapy; SA‐AKI, sepsis‐associated acute kidney injury; SOFA, sequential organ failure assessment.
OR = Odds Ratio, CI = Confidence interval.
Charlson co‐morbidity index calculation excluded chronic kidney disease component.
4. DISCUSSION
4.1. Key findings
In this retrospective multicentre study of approximately 85 000 critically ill admissions, we found that almost one in 10 patients had a history of CKD. Patients with CKD had a two‐fold higher prevalence of SA‐AKI than non‐CKD patients. They were older, had a higher burden of chronic disease, were more severely ill on admission to the ICU and, accordingly, had a higher rate of treatment limitation orders. Ventilation and vasopressor treatment did not differ between the two groups; however, patients with CKD had higher rates of RRT. Patients with CKD also had significantly worse unadjusted outcomes, with less renal recovery, higher MAKE‐30 rates, and increased risk of mortality at long‐term follow‐up. However, after adjusting for patient characteristics and severity of illness, CKD was not independently associated with a different risk of 90‐day mortality nor MAKE‐30 risk.
4.2. Relationship to literature
To our knowledge, no other study has examined the epidemiology of SA‐AKI in CKD patients admitted to ICU. Recent research examined the epidemiology of SA‐AKI in critically ill patients; however, it did not differentiate such epidemiology by CKD status. 3 A post‐hoc analysis of the REVIVAL study was conducted to investigate the efficacy of recombinant human alkaline phosphatase in 649 patients with SA‐AKI. 32 The study showed that the intervention had a different effect on the 49 patients with CKD compared with the majority of patients who did not have CKD.
Previous literature has examined the outcomes of critically ill CKD patients with AKI. 6 , 7 Using the Swedish intensive care registry, investigators compared 998 patients with AKI and a history of CKD to 92 509 patients with no renal disease and no AKI, demonstrating an increased mortality risk at 5 years with CKD. Other investigators examined 524 mechanically ventilated patients with CKD and reported that higher stages of CKD were associated with a greater risk of one‐year mortality. Unlike our study, neither study mentioned critically ill patients with SA‐AKI based on the presence or absence of CKD or adjusted for detailed baseline characteristics.
4.3. Implications of the study findings
Our study findings indicate that CKD is prevalent among critically ill patients with sepsis. Furthermore, they suggest that critically ill patients with CKD face an increased risk of developing SA‐AKI. Additionally, the findings demonstrate that CKD patients who develop SA‐AKI differ significantly from those with CKD who do not develop SA‐AKI. Specifically, they have a higher burden of chronic disease, require different ICU therapies, follow different trajectories and experience worse unadjusted outcomes. Moreover, our findings imply that patients with CKD who develop SA‐AKI represent a distinct pathophysiological group. Finally, they imply that future interventional trials examining SA‐AKI should stratify randomization based on CKD status.
4.4. Strengths and limitations
Our study has several strengths. First, to diagnose SA‐AKI, we relied on previously published methods that utilised the ADQI consensus definition. By utilising established techniques, we were able to ensure that our classification was accurate and reliable. Second, our research involved patients who were in critical condition and receiving treatment in 12 distinct intensive care units, thus highly likely to be representative of the broader Australian population and probably of similar populations in resource rich countries. Third, our study made use of routinely collected detailed data, which allowed us to accurately detect cases of AKI based on urine output criteria. Additionally, this data enabled us to conduct a detailed analysis of the trajectory of SA‐AKI in patients with CKD, which had not been previously observed.
We acknowledge some limitations. First, the determination of SA‐AKI and CKD was done electronically and though these methods have been previously used, misclassification could have occurred. Second, the study cohort's baseline serum creatinine was not available and needed to be estimated. This is a frequent problem as the availability to pre‐admission is uncommon in Australia as shown by the RENAL trial 33 and such estimation is often necessary. As estimates of baseline serum creatinine are imperfect, to ensure greater accuracy and reproducibility, we conducted a sensitivity analysis using four different methods to estimate baseline serum creatinine, which did not demonstrate a difference in our study's conclusion. Furthermore, the sensitivity analysis is robust as it included the Larsen equations that were validated from known pre‐admission serum creatinine values in Australian patients with and without CKD who were admitted to the hospital. 29 , 30 The results indicated minimal variation in SA‐AKI incidence and outcomes, indicating that the approach to baseline creatine estimation would likely not affect the study's conclusions. Third, the censoring of RRT data, and dependent outcomes such as MAKE‐30 at ICU discharge, may introduce an ascertainment bias. Fourth, we lacked data on the stage of CKD; thus, the outcomes demonstrated may vary for patients with different stages of CKD. Fifth, we did not have microbiological data, therefore, we could not control for micro‐organisms in our analysis. As CKD patients have a higher risk of multiresistent and opportunistic infections there may be unaccounted for bias. Finally, CKD was detected by electronic coding, and though previously validated, this approach may have unintentionally misclassified some patients.
5. CONCLUSION
Our study shows that patients with a history of CKD have a higher risk of SA‐AKI and, when diagnosed with SA‐AKI, had worse unadjusted outcomes compared with those without CKD. Additionally, CKD patients with SA‐AKI required RRT often and had lower rates renal recovery.
However, our study also found that, once adjustment for baseline features was applied, CKD was not an independent risk factor for mortality or MAKE‐30. This suggests that the identification and stratification for CKD status is crucial for future interventional trials in patients with SA‐AKI.
AUTHOR CONTRIBUTIONS
The study conception and design (KW, KL, RB); data acquisition (KW, RH, PC); analysis (KW, AS); interpretation of data (all authors); article drafting (KW, KL, RB), article revision for important intellectual content (all authors); final approval of the version submitted for publication (all authors); agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved (KW, KL, RB).
FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
ETHICS STATEMENT
This study was approved by the Metro South Hospital and Health Service Human Research Ethics Committee (HREC/2022/QMS/82024) with an individual waiver of consent granted.
Supporting information
Table S1. Supporting Information.
ACKNOWLEDGEMENTS
The authors acknowledge the Statistical Analysis and Linkage Unit of the Statistical Services Branch (SSB), Queensland Health for linking the data sets used in this project. The authors thank the ANZICS CORE management committee and the clinicians, data collectors and researchers at the following contributing sites: Caboolture Hospital, Cairns Hospital, Gold Coast University Hospital, Logan Hospital, Mackay Base Hospital, Princess Alexandra Hospital, Redcliffe Hospital, Rockhampton Hospital, Royal Brisbane and Women's Hospital, Sunshine Coast University Hospital, The Prince Charles Hospital, and The Townsville Hospital.
Collaborators—Queensland Critical Care Research Network Group: Mahesh Ramanan, Prashanti Marella, Patrick Young, Phillipa McIlroy, Ben Nash, James McCullough, Kerina J Denny, Mandy Tallott, Andrea Marshall, David Moore, Hayden White, Sunil Sane, Aashish Kumar, Lynette Morrison, Pam Dipplesman, Jennifer Taylor, Stephen Luke, Anni Paasilahti, Ray Asimus, Jennifer Taylor, Kyle White, Jason Meyer, Rod Hurford, Meg Harward, James Walsham, Neeraj Bhadange, Wayne Stevens, Kevin Plumpton, Sainath Raman, Andrew Barlow, Alexis Tabah, Hamish Pollock, Stuart Baker, Kylie Jacobs, Antony G. Attokaran, David Austin, Jacobus Poggenpoel, Josephine Reoch, Kevin B. Laupland, Felicity Edwards, Tess Evans, Jayesh Dhanani, Marianne Kirrane, Pierre Clement, Nermin Karamujic, Paula Lister, Vikram Masurkar, Lauren Murray, Jane Brailsford, Todd Erbacher, Kiran Shekar, Jayshree Lavana, George Cornmell, Siva Senthuran, Stephen Whebell, Michelle Gatton, Sam Keogh. Open access publishing facilitated by Queensland University of Technology, as part of the Wiley ‐ Queensland University of Technology agreement via the Council of Australian University Librarians.
White KC, Bellomo R, Tabah A, et al. Sepsis‐associated acute kidney injury in patients with chronic kidney disease: Patient characteristics, prevalence, timing, trajectory, treatment and associated outcomes. Nephrology. 2024;29(12):838‐848. doi: 10.1111/nep.14392
QCCRN group details are provided in Acknowledgments section.
Contributor Information
Kyle C. White, Email: kyle.white@health.qld.gov.au.
the Queensland Critical Care Research Network (QCCRN):
Mahesh Ramanan, Prashanti Marella, Patrick Young, Phillipa McIlroy, Ben Nash, James McCullough, Kerina J Denny, Mandy Tallott, Andrea Marshall, David Moore, Hayden White, Sunil Sane, Aashish Kumar, Lynette Morrison, Pam Dipplesman, Jennifer Taylor, Stephen Luke, Anni Paasilahti, Ray Asimus, Jennifer Taylor, Kyle White, Jason Meyer, Rod Hurford, Meg Harward, James Walsham, Neeraj Bhadange, Wayne Stevens, Kevin Plumpton, Sainath Raman, Andrew Barlow, Alexis Tabah, Hamish Pollock, Stuart Baker, Kylie Jacobs, Antony G. Attokaran, David Austin, Jacobus Poggenpoel, Josephine Reoch, Kevin B. Laupland, Felicity Edwards, Tess Evans, Jayesh Dhanani, Marianne Kirrane, Pierre Clement, Nermin Karamujic, Paula Lister, Vikram Masurkar, Lauren Murray, Jane Brailsford, Todd Erbacher, Kiran Shekar, Jayshree Lavana, George Cornmell, Siva Senthuran, Stephen Whebell, Michelle Gatton, and Sam Keogh
DATA AVAILABILITY STATEMENT
Data cannot be shared publicly due to institutional ethics, privacy and confidentiality regulations. Data released for research under Sect. 280 of the Public Health Act 2005 requires an application to the Director‐General of Queensland Health (pha@health.qld.gov.au).
REFERENCES
- 1. Zarbock A, Nadim MK, Pickkers P, et al. Sepsis‐associated acute kidney injury: consensus report of the 28th acute disease quality initiative workgroup. Nat Rev Nephrol. 2023;19:401‐417. [DOI] [PubMed] [Google Scholar]
- 2. Granado RCD, James MT, Legrand M. Tackling sepsis‐associated acute kidney injury using routinely collected data. Intensive Care Med. 2023;49(9):1100‐1102. [DOI] [PubMed] [Google Scholar]
- 3. White KC, Serpa‐Neto A, Hurford R, et al. Sepsis‐associated acute kidney injury in the intensive care unit: incidence, patient characteristics, timing, trajectory, treatment, and associated outcomes. A multicenter, observational study. Intensive Care Med. 2023;49(9):1079‐1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. White KC, Laupland KB, Tabah A, Ramanan M, Bellomo R. Details and the devil within: the case of sepsis associated AKI. Intensive Care Med. 2023;49(11):1426‐1427. [DOI] [PubMed] [Google Scholar]
- 5. Gómez H, Zarbock A. Details and the devil within—the case of sepsis associated acute kidney injury. Intensivē Care Med. 2023;49(11):1424‐1425. [DOI] [PubMed] [Google Scholar]
- 6. Rimes‐Stigare C, Frumento P, Bottai M, Mårtensson J, Martling CR, Bell M. Long‐term mortality and risk factors for development of end‐stage renal disease in critically ill patients with and without chronic kidney disease. Crit Care. 2015;19(1):383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lebiedz P, Knickel L, Engelbertz C, et al. Impact of preexisting chronic kidney disease on acute and long‐term outcome of critically ill patients on a medical intensive care unit. J Nephrol. 2014;27(1):73‐80. [DOI] [PubMed] [Google Scholar]
- 8. Rimes‐Stigare C, Frumento P, Bottai M, et al. Evolution of chronic renal impairment and long‐term mortality after de novo acute kidney injury in the critically ill; a Swedish multi‐centre cohort study. Crit Care. 2015;19(1):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abdalrahim MS, Khalil AA, Alramly M, Alshlool KN, Abed MA, Moser DK. Pre‐existing chronic kidney disease and acute kidney injury among critically ill patients. Heart Lung. 2020;49(5):626‐629. [DOI] [PubMed] [Google Scholar]
- 10. Bagshaw SM, Neto AS, Smith O, et al. Impact of renal‐replacement therapy strategies on outcomes for patients with chronic kidney disease: a secondary analysis of the STARRT‐AKI trial. Intensive Care Med. 2022;48(12):1736‐1750. [DOI] [PubMed] [Google Scholar]
- 11. White K, Tabah A, Ramanan M, Shekar K, Edwards F, Laupland KB. 90‐day case‐fatality in critically ill patients with chronic liver disease influenced by presence of portal hypertension, results from a multicentre retrospective cohort study. J Crit Care. 2023;1(38):5‐10. [DOI] [PubMed] [Google Scholar]
- 12. Marella P, Ramanan M, Shekar K, Tabah A, Laupland KB. Determinants of 90‐day case fatality among older patients admitted to intensive care units: a retrospective cohort study. Aust Crit Care. 2023;37(1):18‐24. [DOI] [PubMed] [Google Scholar]
- 13. Laupland KB, Ramanan M, Shekar K, Edwards F, Clement P, Tabah A. Long‐term outcome of prolonged critical illness: a multicentered study in North Brisbane, Australia. PLoS One. 2021;16(4):e0249840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sieben NA, Dash S. A retrospective evaluation of multiple definitions for ventilator associated pneumonia (VAP) diagnosis in an Australian regional intensive care unit. Infect Dis Health. 2022;27(4):191‐197. [DOI] [PubMed] [Google Scholar]
- 15. Bagshaw SM, George C, Bellomo R, Committe ADM. A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23(5):1569‐1574. [DOI] [PubMed] [Google Scholar]
- 16. Raith EP, Udy AA, Bailey M, et al. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in‐hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA. 2017;317(3):290‐300. [DOI] [PubMed] [Google Scholar]
- 17. Corrigan C, Duke G, Millar J, et al. Admissions of children and adolescents with deliberate self‐harm to intensive care during the SARS‐CoV‐2 outbreak in Australia. JAMA Netw Open. 2022;5(5):e2211692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kirsi‐Maija K, Michael B, David P, Jamie CD, Rinaldo B. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 2015;372(17):1629‐1638. [DOI] [PubMed] [Google Scholar]
- 19. Vallmuur K, Cameron CM, Watson A, Warren J. Comparing the accuracy of ICD‐based severity estimates to trauma registry‐based injury severity estimates for predicting mortality outcomes. Injury. 2021;52(7):1732‐1739. [DOI] [PubMed] [Google Scholar]
- 20. Watson A, Watson B, Vallmuur K. Estimating under‐reporting of road crash injuries to police using multiple linked data collections. Accid Anal Prev. 2015;83:18‐25. [DOI] [PubMed] [Google Scholar]
- 21. Nghiem S, Afoakwah C, Byrnes J, Scuffham P. Lifetime costs of hospitalised cardiovascular disease in Australia: an incidence‐based estimate. Heart Lung Circ. 2021;30(8):1207‐1212. [DOI] [PubMed] [Google Scholar]
- 22. Win KTH, Thomas B, Emeto TI, et al. A comparison of clinical characteristics and outcomes between indigenous and non‐indigenous patients presenting to Townsville hospital emergency department with chest pain. Heart Lung Circ. 2022;31(2):183‐193. [DOI] [PubMed] [Google Scholar]
- 23. O'Beirne J, Skoien R, Leggett BA, et al. Diabetes mellitus and the progression of non‐alcoholic fatty liver disease to decompensated cirrhosis: a retrospective cohort study. Med J Aust. 2023;219:358‐365. [DOI] [PubMed] [Google Scholar]
- 24. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD‐10 version of the Charlson comorbidity index predicted in‐hospital mortality. J Clin Epidemiol. 2004;57(12):1288‐1294. [DOI] [PubMed] [Google Scholar]
- 25. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43(11):1130‐1139. [DOI] [PubMed] [Google Scholar]
- 26. Khanna A, English SW, Wang XS, et al. Angiotensin II for the treatment of vasodilatory shock. N Engl J Medicine. 2017;377(5):419‐430. [DOI] [PubMed] [Google Scholar]
- 27. Zhang J, Healy HG, Baboolal K, et al. Frequency and consequences of acute kidney injury in patients with CKD: a registry study in Queensland Australia. Kidney Med. 2019;1(4):180‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179‐c184. [DOI] [PubMed] [Google Scholar]
- 29. Larsen T, See EJ, Holmes NE, Bellomo R. Estimating baseline creatinine to detect acute kidney injury in patients with chronic kidney disease. Nephrol Ther. 2023;28(8):434‐445. [DOI] [PubMed] [Google Scholar]
- 30. Larsen T, See EJ, Holmes N, Bellomo R. Estimating baseline kidney function in hospitalized adults with acute kidney injury. Nephrol Ther. 2022;27(7):588‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Feise R. Do multiple outcome measures require p‐value adjustment? BMC Med Res Methodol. 2002;2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pickkers P, Angus DC, Bass K, et al. Phase‐3 trial of recombinant human alkaline phosphatase for patients with sepsis‐associated acute kidney injury (REVIVAL). Intensive Care Med. 2024;50(1):68‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bellomo R, Cass A, Cole L, et al. Intensity of continuous renal‐replacement therapy in critically ill patients. N Engl J Med. 2009;361(17):1627‐1638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Supporting Information.
Data Availability Statement
Data cannot be shared publicly due to institutional ethics, privacy and confidentiality regulations. Data released for research under Sect. 280 of the Public Health Act 2005 requires an application to the Director‐General of Queensland Health (pha@health.qld.gov.au).


