Abstract
We present a genome assembly from an individual leaf beetle, Galeruca laticollis (Arthropoda; Insecta; Coleoptera; Chrysomelidae). The genome sequence has a total length of 2,154.60 megabases. Most of the assembly (99.92%) is scaffolded into 12 chromosomal pseudomolecules, including the X and Y sex chromosomes. The mitochondrial genome has also been assembled and is 19.98 kilobases in length. Gene annotation of this assembly on Ensembl identified 32,229 protein-coding genes.
Keywords: Galeruca laticollis, leaf beetle, genome sequence, chromosomal, Coleoptera
Species taxonomy
Eukaryota; Opisthokonta; Metazoa; Eumetazoa; Bilateria; Protostomia; Ecdysozoa; Panarthropoda; Arthropoda; Mandibulata; Pancrustacea; Hexapoda; Insecta; Dicondylia; Pterygota; Neoptera; Endopterygota; Coleoptera; Polyphaga; Cucujiformia; Chrysomeloidea; Chrysomelidae; Galerucinae; Galerucini; Galerucites; Galeruca; Galeruca laticollis Sahlberg, C.R., 1838 (NCBI:txid2017130).
Background
Galeruca laticollis Sahlberg, C.R., 1838 is a leaf beetle in the family Chrysomelidae. It is ovate in shape with a strongly transverse pronotum, 6–9 mm in length, and can be recognised in the field by its black head, antennae, and legs on a brownish body rather than having the wholly black body of its congener, G. tanaceti (Linnaeus) ( Duff, 2016).
In recent years, adults have been found on a variety of plants, including thistles, growing in fenland. Larvae in the UK have been found on the leaves of Common Meadow-rue Thalictrum flavum ( Duff, 2016).
Galeruca laticollis was regarded formerly as very rare in the UK and listed as RDB1 – Endangered by Hyman and Parsons (1992) and possibly extinct, having not been seen since 1919 when it was found in Dorset. However, in the mid-1990s it was rediscovered at the same site from which the present material was collected in East Norfolk, where there is an apparently stable population. Outside the UK, it occurs in most of Europe and extends eastwards into temperate parts of Asia.
Here we present the first genome sequence for G. laticollis, based on an adult male specimen from Wheatfen Broad, England, United Kingdom, sequenced as part of the Darwin Tree of Life Project.
Genome sequence report
The genome of an adult male Galeruca laticollis ( Figure 1) was sequenced using Pacific Biosciences single-molecule HiFi long reads, generating a total of 74.49 Gb (gigabases) from 7.04 million reads, providing approximately 35-fold coverage. Primary assembly contigs were scaffolded with chromosome conformation Hi-C data, which produced 135.01 Gb from 894.11 million reads, yielding an approximate coverage of 63-fold. Specimen and sequencing information is summarised in Table 1.
Figure 1. Photographs of the Galeruca laticollis (icGalLati1) specimen used for genome sequencing.
a) Dorsal view, b) Ventral view.
Table 1. Specimen and sequencing data for Galeruca laticollis.
| Project information | |||
|---|---|---|---|
| Study title | Galeruca laticollis | ||
| Umbrella BioProject | PRJEB65735 | ||
| BioSample | SAMEA112964282 | ||
| NCBI taxonomy ID | 2017130 | ||
| Specimen information | |||
| Technology | ToLID | BioSample accession | Organism part |
| PacBio long read sequencing | icGalLati1 | SAMEA112975451 | Whole organism |
| Hi-C sequencing | icGalLati1 | SAMEA112975451 | Whole organism |
| Sequencing information | |||
| Platform | Run accession | Read count | Base count (Gb) |
| Hi-C Illumina NovaSeq 6000 | ERR12035317 | 8.94e+08 | 135.01 |
| PacBio Revio | ERR12015776 | 7.04e+06 | 74.49 |
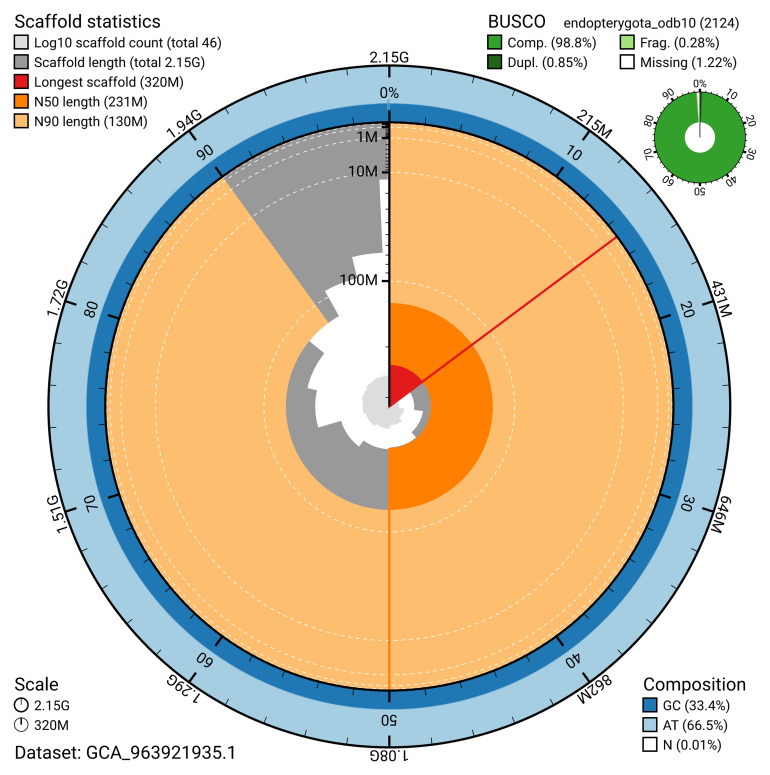
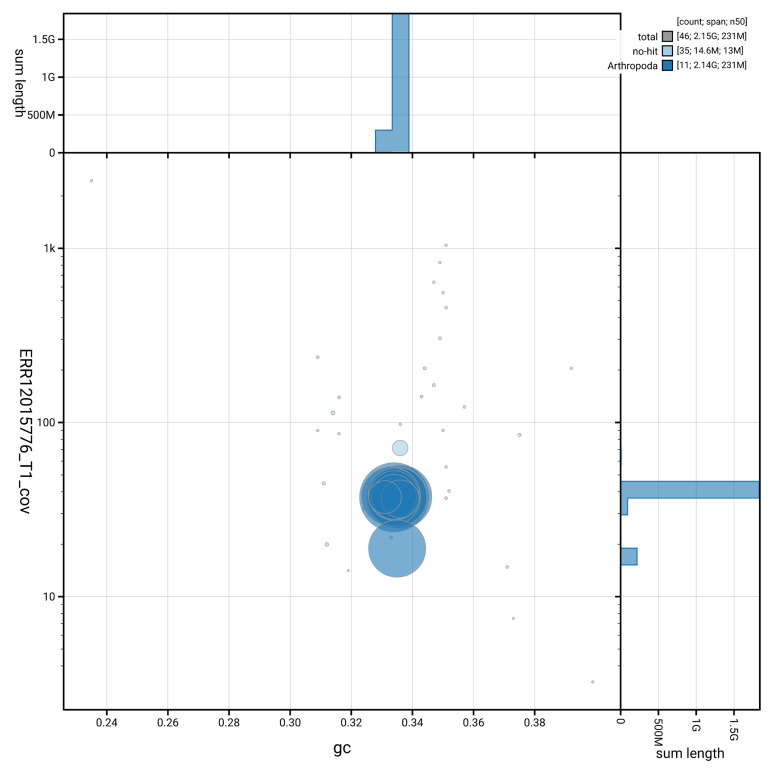
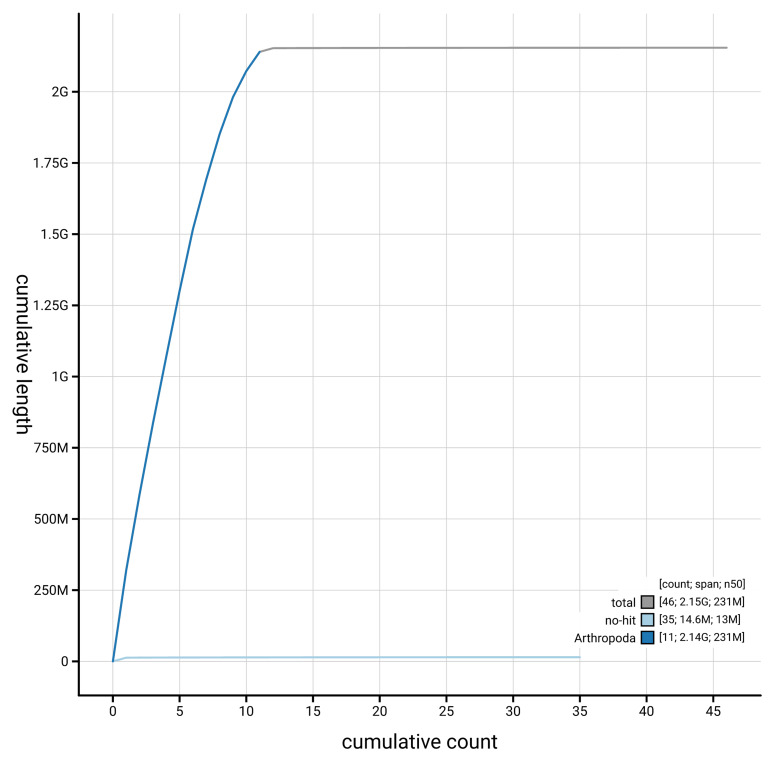
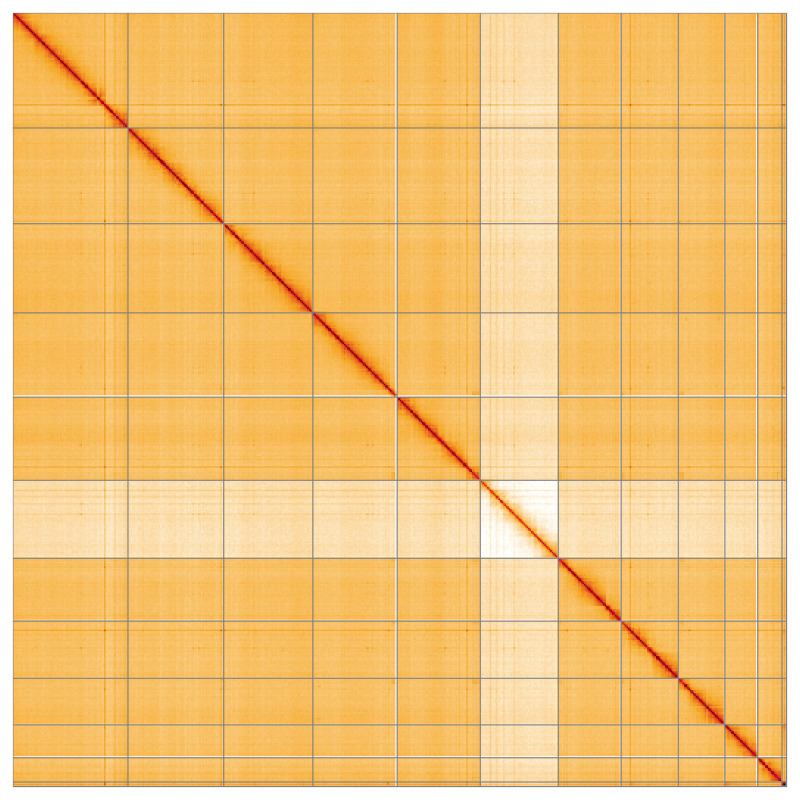
Manual assembly curation corrected 35 missing joins or mis-joins, reducing the scaffold number by 30.3%. The final assembly has a total length of 2,154.60 Mb in 45 sequence scaffolds, with 597 gaps, and a scaffold N50 of 231.3 Mb ( Table 2). The snail plot in Figure 2 provides a summary of the assembly statistics, while the distribution of assembly scaffolds on GC proportion and coverage is shown in Figure 3. The cumulative assembly plot in Figure 4 shows curves for subsets of scaffolds assigned to different phyla. Most (99.92%) of the assembly sequence was assigned to 12 chromosomal-level scaffolds, representing 10 autosomes and the X and Y sex chromosomes. Chromosome-scale scaffolds confirmed by the Hi-C data are named in order of size ( Figure 5; Table 3). Chromosome X was identified by homology with the Lochmaea crataegi assembly (GCA_947563755.1) ( Crowley et al., 2024), and chromosome Y was identified based on coverage. While not fully phased, the assembly deposited is of one haplotype. Contigs corresponding to the second haplotype have also been deposited. The mitochondrial genome was also assembled and can be found as a contig within the multifasta file of the genome submission.
Figure 2. Genome assembly of Galeruca laticollis, icGalLati1.1: metrics.
The BlobToolKit snail plot shows N50 metrics and BUSCO gene completeness. The main plot is divided into 1,000 size-ordered bins around the circumference with each bin representing 0.1% of the 2,154,579,676 bp assembly. The distribution of scaffold lengths is shown in dark grey with the plot radius scaled to the longest scaffold present in the assembly (320,044,732 bp, shown in red). Orange and pale-orange arcs show the N50 and N90 scaffold lengths (231,279,273 and 129,513,413 bp), respectively. The pale grey spiral shows the cumulative scaffold count on a log scale with white scale lines showing successive orders of magnitude. The blue and pale-blue area around the outside of the plot shows the distribution of GC, AT and N percentages in the same bins as the inner plot. A summary of complete, fragmented, duplicated and missing BUSCO genes in the endopterygota_odb10 set is shown in the top right. An interactive version of this figure is available at https://blobtoolkit.genomehubs.org/view/GCA_963921935.1/dataset/GCA_963921935.1/snail.
Figure 3. Genome assembly of Galeruca laticollis: Blot plot of base coverage in the raw data against GC proportion for sequences in icGalLati1.1.
Sequences are coloured by phylum. Circles are sized in proportion to sequence length. Histograms show the distribution of sequence length sum along each axis. An interactive version of this figure is available at https://blobtoolkit.genomehubs.org/view/GCA_963921935.1/dataset/GCA_963921935.1/blob.
Figure 4. Genome assembly of Galeruca laticollis icGalLati1.1: BlobToolKit cumulative sequence plot.
The grey line shows cumulative length for all scaffolds. Coloured lines show cumulative lengths of scaffolds assigned to each phylum using the buscogenes taxrule. An interactive version of this figure is available at https://blobtoolkit.genomehubs.org/view/GCA_963921935.1/dataset/GCA_963921935.1/cumulative.
Figure 5. Genome assembly of Galeruca laticollis icGalLati1.1: Hi-C contact map of the icGalLati1.1 assembly, visualised using HiGlass.
Chromosomes are shown in order of size from left to right and top to bottom. An interactive version of this figure may be viewed at https://genome-note-higlass.tol.sanger.ac.uk/l/?d=WQfcah4sQICI-kvqtLjt0A.
Table 2. Genome assembly data for Galeruca laticollis, icGalLati1.1.
| Genome assembly | ||
|---|---|---|
| Assembly name | icGalLati1.1 | |
| Assembly accession | GCA_963921935.1 | |
| Accession of alternate haplotype | GCA_963921905.1 | |
| Span (Mb) | 2,154.60 | |
| Number of contigs | 643 | |
| Contig N50 length (Mb) | 6.4 | |
| Number of scaffolds | 45 | |
| Scaffold N50 length (Mb) | 231.3 | |
| Longest scaffold (Mb) | 320.04 | |
| Assembly metrics * | Benchmark | |
| Consensus quality (QV) | 58.3 | ≥ 50 |
| k-mer completeness | 99.99% | ≥ 95% |
| BUSCO ** | C:98.7%[S:97.9%,D:0.8%], F:0.3%,M:1.0%,n:2,124 | C ≥ 95% |
| Percentage of assembly mapped to chromosomes | 99.92% | ≥ 95% |
| Sex chromosomes | XY | localised homologous pairs |
| Organelles | Mitochondrial genome: 19.98 kb | complete single alleles |
| Genome annotation of assembly GCA_963921935.1 at Ensembl | ||
| Number of protein-coding genes | 32,229 | |
| Number of non-coding genes | 830 | |
| Number of gene transcripts | 47,793 | |
Assembly metric benchmarks are adapted from column VGP-2020 of “Table 1: Proposed standards and metrics for defining genome assembly quality” from Rhie et al. (2021).
BUSCO scores based on the endopterygota_odb10 BUSCO set using version 5.4.3. C = complete [S = single copy, D = duplicated], F = fragmented, M = missing, n = number of orthologues in comparison. A full set of BUSCO scores is available at https://blobtoolkit.genomehubs.org/view/Galeruca_laticollis/dataset/GCA_963921935.1/busco.
Table 3. Chromosomal pseudomolecules in the genome assembly of Galeruca laticollis, icGalLati1.
| INSDC accession | Name | Length (Mb) | GC% |
|---|---|---|---|
| OY998175.1 | 1 | 320.04 | 33.5 |
| OY998176.1 | 2 | 266.52 | 33.5 |
| OY998177.1 | 3 | 248.29 | 33.5 |
| OY998178.1 | 4 | 234.71 | 33.5 |
| OY998179.1 | 5 | 231.28 | 33.5 |
| OY998181.1 | 6 | 174.9 | 33.5 |
| OY998182.1 | 7 | 159.02 | 33.5 |
| OY998183.1 | 8 | 129.51 | 33.5 |
| OY998184.1 | 9 | 91.58 | 33.5 |
| OY998185.1 | 10 | 67.33 | 33.0 |
| OY998180.1 | X | 216.78 | 33.5 |
| OY998186.1 | Y | 12.97 | 33.5 |
| OY998187.1 | MT | 0.02 | 23.5 |
The estimated Quality Value (QV) of the final assembly is 58.3 with k-mer completeness of 99.99%, and the assembly has a BUSCO v5.4.3 completeness of 98.7% (single = 97.9%, duplicated = 0.8%), using the endopterygota_odb10 reference set ( n = 2,124).
Metadata for specimens, BOLD barcode results, spectra estimates, sequencing runs, contaminants and pre-curation assembly statistics are given at https://links.tol.sanger.ac.uk/species/2017130
Genome annotation report
The Galeruca laticollis genome assembly (GCA_963921935.1) was annotated at the European Bioinformatics Institute (EBI) on Ensembl Rapid Release. The resulting annotation includes 47,793 transcribed mRNAs from 32,229 protein-coding and 830 non-coding genes ( Table 2; https://rapid.ensembl.org/Galeruca_laticollis_GCA_963921935.1/Info/Index). The average transcript length is 31,279.23. There are 1.45 coding transcripts per gene and 4.60 exons per transcript.
Methods
Sample acquisition and DNA barcoding
An adult male Galeruca laticollis (specimen ID NHMUK015059395, ToLID icGalLati1) was collected from Wheatfen Broad, England, United Kingdom (latitude 52.6, longitude 1.44) on 2022-07-03. The specimen was collected and identified by Roger Booth (Natural History Museum) and preserved by dry freezing at –80 °C.
The initial identification was verified by an additional DNA barcoding process according to the framework developed by Twyford et al. (2024). A small sample was dissected from the specimens and stored in ethanol, while the remaining parts were shipped on dry ice to the Wellcome Sanger Institute (WSI). The tissue was lysed, the COI marker region was amplified by PCR, and amplicons were sequenced and compared to the BOLD database, confirming the species identification ( Crowley et al., 2023). Following whole genome sequence generation, the relevant DNA barcode region was also used alongside the initial barcoding data for sample tracking at the WSI ( Twyford et al., 2024). The standard operating procedures for Darwin Tree of Life barcoding have been deposited on protocols.io ( Beasley et al., 2023).
Nucleic acid extraction
The workflow for high molecular weight (HMW) DNA extraction at the Wellcome Sanger Institute (WSI) Tree of Life Core Laboratory includes a sequence of core procedures: sample preparation and homogenisation, DNA extraction, fragmentation and purification. Detailed protocols are available on protocols.io ( Denton et al., 2023b). The icGalLati1 sample was prepared for DNA extraction by weighing and dissecting it on dry ice ( Jay et al., 2023), and tissue from the whole organism was homogenised using a PowerMasher II tissue disruptor ( Denton et al., 2023a).
HMW DNA was extracted in the WSI Scientific Operations core using the Automated MagAttract v2 protocol ( Oatley et al., 2023). The DNA was sheared into an average fragment size of 12–20 kb in a Megaruptor 3 system ( Bates et al., 2023). Sheared DNA was purified by solid-phase reversible immobilisation, using AMPure PB beads to eliminate shorter fragments and concentrate the DNA ( Strickland et al., 2023). The concentration of the sheared and purified DNA was assessed using a Nanodrop spectrophotometer and Qubit Fluorometer using the Qubit dsDNA High Sensitivity Assay kit. Fragment size distribution was evaluated by running the sample on the FemtoPulse system.
Hi-C preparation
The icGalLati1 sample was processed at the WSI Scientific Operations core, using the Arima-HiC v2 kit. In brief, frozen tissue (stored at –80°C) was fixed, and the DNA crosslinked using a TC buffer with 22% formaldehyde. After crosslinking, the tissue was homogenised using the Diagnocine Power Masher-II and BioMasher-II tubes and pestles. Following the kit manufacturer's instructions, crosslinked DNA was digested using a restriction enzyme master mix. The 5’-overhangs were then filled in and labelled with biotinylated nucleotides and proximally ligated. An overnight incubation was carried out for enzymes to digest remaining proteins and for crosslinks to reverse. A clean up was performed with SPRIselect beads prior to library preparation.
Library preparation and sequencing
Library preparation and DNA sequencing were performed at the WSI Scientific Operations core. Pacific Biosciences HiFi circular consensus DNA sequencing libraries were prepared using the PacBio Express Template Preparation Kit v2.0 (Pacific Biosciences, California, USA) as per the manufacturer’s instructions. The kit includes the reagents required for removal of single-strand overhangs, DNA damage repair, end repair/A-tailing, adapter ligation, and nuclease treatment. Library preparation also included a library purification step using 0.8X AMPure PB beads (Pacific Biosciences, California, USA) and size selection step to remove templates < 5 kb using AMPure PB modified SPRI. Samples were sequenced on the Revio system (Pacific Biosciences, California, USA).
For Hi-C library preparation, DNA was fragmented to a size of 400 to 600 bp using a Covaris E220 sonicator. The DNA was then enriched, barcoded, and amplified using the NEBNext Ultra II DNA Library Prep Kit following manufacturers’ instructions. The Hi-C sequencing was performed using paired-end sequencing with a read length of 150 bp on an Illumina NovaSeq 6000 instrument.
Genome assembly, curation and evaluation
Assembly
The HiFi reads were first assembled using Hifiasm ( Cheng et al., 2021) with the --primary option. Haplotypic duplications were identified and removed using purge_dups ( Guan et al., 2020). The Hi-C reads were mapped to the primary contigs using bwa-mem2 ( Vasimuddin et al., 2019). The contigs were further scaffolded using the provided Hi-C data ( Rao et al., 2014) in YaHS ( Zhou et al., 2023) using the --break option. The scaffolded assemblies were evaluated using Gfastats ( Formenti et al., 2022), BUSCO ( Manni et al., 2021) and MERQURY.FK ( Rhie et al., 2020).
The mitochondrial genome was assembled using MitoHiFi ( Uliano-Silva et al., 2023), which runs MitoFinder ( Allio et al., 2020) and uses these annotations to select the final mitochondrial contig and to ensure the general quality of the sequence.
Assembly curation
The assembly was decontaminated using the Assembly Screen for Cobionts and Contaminants (ASCC) pipeline (article in preparation). Flat files and maps used in curation were generated in TreeVal ( Pointon et al., 2023). Manual curation was primarily conducted using PretextView ( Harry, 2022), with additional insights provided by JBrowse2 ( Diesh et al., 2023) and HiGlass ( Kerpedjiev et al., 2018). Scaffolds were visually inspected and corrected as described by Howe et al. (2021). Any identified contamination, missed joins, and mis-joins were corrected, and duplicate sequences were tagged and removed. Sex chromosomes were identified using both read coverage and synteny analysis. The curation process is documented at https://gitlab.com/wtsi-grit/rapid-curation (article in preparation).
Evaluation of the final assembly
The final assembly was post-processed and evaluated using the three Nextflow ( Di Tommaso et al., 2017) DSL2 pipelines: sanger-tol/readmapping ( Surana et al., 2023a), sanger-tol/genomenote ( Surana et al., 2023b), and sanger-tol/blobtoolkit ( Muffato et al., 2024). The readmapping pipeline aligns the Hi-C reads using bwa-mem2 ( Vasimuddin et al., 2019) and combines the alignment files with SAMtools ( Danecek et al., 2021). The genomenote pipeline converts the Hi-C alignments into a contact map using BEDTools ( Quinlan & Hall, 2010) and the Cooler tool suite ( Abdennur & Mirny, 2020). The contact map is visualised in HiGlass ( Kerpedjiev et al., 2018). This pipeline also generates assembly statistics using the NCBI datasets report ( Sayers et al., 2024), computes k-mer completeness and QV consensus quality values with FastK and MERQURY.FK, and runs BUSCO ( Manni et al., 2021) to assess completeness.
The blobtoolkit pipeline is a Nextflow port of the previous Snakemake Blobtoolkit pipeline ( Challis et al., 2020). It aligns the PacBio reads in SAMtools and minimap2 ( Li, 2018) and generates coverage tracks for regions of fixed size. In parallel, it queries the GoaT database ( Challis et al., 2023) to identify all matching BUSCO lineages to run BUSCO ( Manni et al., 2021). For the three domain-level BUSCO lineages, the pipeline aligns the BUSCO genes to the UniProt Reference Proteomes database ( Bateman et al., 2023) with DIAMOND ( Buchfink et al., 2021) blastp. The genome is also split into chunks according to the density of the BUSCO genes from the closest taxonomic lineage, and each chunk is aligned to the UniProt Reference Proteomes database with DIAMOND blastx. Genome sequences without a hit are chunked with seqtk and aligned to the NT database with blastn ( Altschul et al., 1990). The blobtools suite combines all these outputs into a blobdir for visualisation.
The genome assembly and evaluation pipelines were developed using nf-core tooling ( Ewels et al., 2020) and MultiQC ( Ewels et al., 2016), relying on the Conda package manager, the Bioconda initiative ( Grüning et al., 2018), the Biocontainers infrastructure ( da Veiga Leprevost et al., 2017), as well as the Docker ( Merkel, 2014) and Singularity ( Kurtzer et al., 2017) containerisation solutions.
Table 4 contains a list of relevant software tool versions and sources.
Table 4. Software tools: versions and sources.
Genome annotation
The Ensembl Genebuild annotation system ( Aken et al., 2016) was used to generate annotation for the Galeruca laticollis assembly (GCA_963921935.1) in Ensembl Rapid Release at the EBI. Annotation was created primarily through alignment of transcriptomic data to the genome, with gap filling via protein-to-genome alignments of a select set of proteins from UniProt ( UniProt Consortium, 2019).
Wellcome Sanger Institute – Legal and Governance
The materials that have contributed to this genome note have been supplied by a Darwin Tree of Life Partner. The submission of materials by a Darwin Tree of Life Partner is subject to the ‘Darwin Tree of Life Project Sampling Code of Practice’, which can be found in full on the Darwin Tree of Life website here. By agreeing with and signing up to the Sampling Code of Practice, the Darwin Tree of Life Partner agrees they will meet the legal and ethical requirements and standards set out within this document in respect of all samples acquired for, and supplied to, the Darwin Tree of Life Project.
Further, the Wellcome Sanger Institute employs a process whereby due diligence is carried out proportionate to the nature of the materials themselves, and the circumstances under which they have been/are to be collected and provided for use. The purpose of this is to address and mitigate any potential legal and/or ethical implications of receipt and use of the materials as part of the research project, and to ensure that in doing so we align with best practice wherever possible. The overarching areas of consideration are:
Ethical review of provenance and sourcing of the material
Legality of collection, transfer and use (national and international)
Each transfer of samples is further undertaken according to a Research Collaboration Agreement or Material Transfer Agreement entered into by the Darwin Tree of Life Partner, Genome Research Limited (operating as the Wellcome Sanger Institute), and in some circumstances other Darwin Tree of Life collaborators.
Funding Statement
This work was supported by Wellcome through core funding to the Wellcome Sanger Institute [206194, <a href=https://doi.org/10.35802/206194>https://doi.org/10.35802/206194</a>] and the Darwin Tree of Life Discretionary Award [218328, <a href=https://doi.org/10.35802/218328>https://doi.org/10.35802/218328 </a>].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 2 approved]
Data availability
European Nucleotide Archive: Galeruca laticollis. Accession number PRJEB65735; https://identifiers.org/ena.embl/PRJEB65735 ( Wellcome Sanger Institute, 2024). The genome sequence is released openly for reuse. The Galeruca laticollis genome sequencing initiative is part of the Darwin Tree of Life (DToL) project. All raw sequence data and the assembly have been deposited in INSDC databases. Raw data and assembly accession identifiers are reported in Table 1 and Table 2.
Author information
Members of the Natural History Museum Genome Acquisition Lab are listed here: https://doi.org/10.5281/zenodo.12159242.
Members of the Wellcome Sanger Institute Tree of Life Management, Samples and Laboratory team are listed here: https://doi.org/10.5281/zenodo.12162482.
Members of Wellcome Sanger Institute Scientific Operations: Sequencing Operations are listed here: https://doi.org/10.5281/zenodo.12165051.
Members of the Wellcome Sanger Institute Tree of Life Core Informatics team are listed here: https://doi.org/10.5281/zenodo.12160324.
Members of the Tree of Life Core Informatics collective are listed here: https://doi.org/10.5281/zenodo.12205391.
Members of the Darwin Tree of Life Consortium are listed here: https://doi.org/10.5281/zenodo.4783558.
References
- Abdennur N, Mirny LA: Cooler: scalable storage for Hi-C data and other genomically labeled arrays. Bioinformatics. 2020;36(1):311–316. 10.1093/bioinformatics/btz540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aken BL, Ayling S, Barrell D, et al. : The ensembl gene annotation system. Database (Oxford). 2016;2016: baw093. 10.1093/database/baw093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allio R, Schomaker-Bastos A, Romiguier J, et al. : MitoFinder: efficient automated large-scale extraction of mitogenomic data in target enrichment phylogenomics. Mol Ecol Resour. 2020;20(4):892–905. 10.1111/1755-0998.13160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, et al. : Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- Bateman A, Martin MJ, Orchard S, et al. : UniProt: the universal protein knowledgebase in 2023. Nucleic Acids Res. 2023;51(D1):D523–D531. 10.1093/nar/gkac1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates A, Clayton-Lucey I, Howard C: Sanger Tree of Life HMW DNA fragmentation: diagenode Megaruptor ®3 for LI PacBio. protocols.io. 2023. 10.17504/protocols.io.81wgbxzq3lpk/v1 [DOI] [Google Scholar]
- Beasley J, Uhl R, Forrest LL, et al. : DNA barcoding SOPs for the Darwin Tree of Life project. protocols.io. 2023. [Accessed 25 June 2024]. 10.17504/protocols.io.261ged91jv47/v1 [DOI] [Google Scholar]
- Buchfink B, Reuter K, Drost HG: Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat Methods 2021;18(4):366–368. 10.1038/s41592-021-01101-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis R, Kumar S, Sotero-Caio C, et al. : Genomes on a Tree (GoaT): a versatile, scalable search engine for genomic and sequencing project metadata across the eukaryotic tree of life [version 1; peer review: 2 approved]. Wellcome Open Res. 2023;8:24. 10.12688/wellcomeopenres.18658.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challis R, Richards E, Rajan J, et al. : BlobToolKit – interactive quality assessment of genome assemblies. G3 (Bethesda). 2020;10(4):1361–1374. 10.1534/g3.119.400908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Concepcion GT, Feng X, et al. : Haplotype-resolved de novo assembly using phased assembly graphs with hifiasm. Nat Methods. 2021;18(2):170–175. 10.1038/s41592-020-01056-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley L, Allen H, Barnes I, et al. : A sampling strategy for genome sequencing the British terrestrial arthropod fauna [version 1; peer review: 2 approved]. Wellcome Open Res. 2023;8:123. 10.12688/wellcomeopenres.18925.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley LM, Telfer MG, Escalona HE, et al. : The genome sequence of the hawthorn leaf beetle, Lochmaea crataegi (Forster, 1771) [version 1; peer review: 2 approved]. Wellcome Open Res. 2024;9:80. 10.12688/wellcomeopenres.20911.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Veiga Leprevost F, Grüning BA, Alves Aflitos S, et al. : BioContainers: an open-source and community-driven framework for software standardization. Bioinformatics. 2017;33(16):2580–2582. 10.1093/bioinformatics/btx192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danecek P, Bonfield JK, Liddle J, et al. : Twelve years of SAMtools and BCFtools. GigaScience. 2021;10(2): giab008. 10.1093/gigascience/giab008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton A, Oatley G, Cornwell C, et al. : Sanger Tree of Life sample homogenisation: PowerMash. protocols.io. 2023a. 10.17504/protocols.io.5qpvo3r19v4o/v1 [DOI] [Google Scholar]
- Denton A, Yatsenko H, Jay J, et al. : Sanger Tree of Life wet laboratory protocol collection V.1. protocols.io. 2023b. 10.17504/protocols.io.8epv5xxy6g1b/v1 [DOI] [Google Scholar]
- Di Tommaso P, Chatzou M, Floden EW, et al. : Nextflow enables reproducible computational workflows. Nat Biotechnol. 2017;35(4):316–319. 10.1038/nbt.3820 [DOI] [PubMed] [Google Scholar]
- Diesh C, Stevens GJ, Xie P, et al. : JBrowse 2: a modular genome browser with views of synteny and structural variation. Genome Biol. 2023;24(1): 74. 10.1186/s13059-023-02914-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff AG: Beetles of Britain and Ireland. Cerambycidae to curculionidae. West Runton: A.G. Duff publishing,2016;4. Reference Source [Google Scholar]
- Ewels P, Magnusson M, Lundin S, et al. : MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 2016;32(19):3047–3048. 10.1093/bioinformatics/btw354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewels PA, Peltzer A, Fillinger S, et al. : The nf-core framework for community-curated bioinformatics pipelines. Nat Biotechnol. 2020;38(3):276–278. 10.1038/s41587-020-0439-x [DOI] [PubMed] [Google Scholar]
- Formenti G, Abueg L, Brajuka A, et al. : Gfastats: conversion, evaluation and manipulation of genome sequences using assembly graphs. Bioinformatics. 2022;38(17):4214–4216. 10.1093/bioinformatics/btac460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüning B, Dale R, Sjödin A, et al. : Bioconda: sustainable and comprehensive software distribution for the life sciences. Nat Methods. 2018;15(7):475–476. 10.1038/s41592-018-0046-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan D, McCarthy SA, Wood J, et al. : Identifying and removing haplotypic duplication in primary genome assemblies. Bioinformatics. 2020;36(9):2896–2898. 10.1093/bioinformatics/btaa025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry E: PretextView (Paired Read Texture Viewer): a desktop application for viewing pretext contact maps.2022. Reference Source
- Howe K, Chow W, Collins J, et al. : Significantly improving the quality of genome assemblies through curation. GigaScience. 2021;10(1): giaa153. 10.1093/gigascience/giaa153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman PS, Parsons MS: A review of the scarce and threatened Coleoptera of great Britain: Part 1. Peterborough: Joint Nature Conservation Committee,1992. Reference Source [Google Scholar]
- Jay J, Yatsenko H, Narváez-Gómez JP, et al. : Sanger Tree of Life sample preparation: triage and dissection. protocols.io. 2023. 10.17504/protocols.io.x54v9prmqg3e/v1 [DOI] [Google Scholar]
- Kerpedjiev P, Abdennur N, Lekschas F, et al. : HiGlass: web-based visual exploration and analysis of genome interaction maps. Genome Biol. 2018;19(1): 125. 10.1186/s13059-018-1486-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzer GM, Sochat V, Bauer MW: Singularity: scientific containers for mobility of compute. PLoS One. 2017;12(5): e0177459. 10.1371/journal.pone.0177459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H: Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34(18):3094–3100. 10.1093/bioinformatics/bty191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manni M, Berkeley MR, Seppey M, et al. : BUSCO update: novel and streamlined workflows along with broader and deeper phylogenetic coverage for scoring of eukaryotic, prokaryotic, and viral genomes. Mol Biol Evol. 2021;38(10):4647–4654. 10.1093/molbev/msab199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel D: Docker: lightweight Linux containers for consistent development and deployment. Linux J. 2014;2014(239): 2. [Accessed 2 April 2024]. Reference Source [Google Scholar]
- Muffato M, Butt Z, Challis R, et al. : sanger-tol/blobtoolkit: v0.3.0 – poliwag.2024. 10.5281/zenodo.10649272 [DOI] [Google Scholar]
- Oatley G, Denton A, Howard C: Sanger Tree of Life HMW DNA extraction: automated MagAttract v.2. protocols.io. 2023. 10.17504/protocols.io.kxygx3y4dg8j/v1 [DOI] [Google Scholar]
- Pointon DL, Eagles W, Sims Y, et al. : sanger-tol/treeval v1.0.0 – ancient Atlantis.2023. 10.5281/zenodo.10047654 [DOI]
- Quinlan AR, Hall IM: BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26(6):841–842. 10.1093/bioinformatics/btq033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SSP, Huntley MH, Durand NC, et al. : A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159(7):1665–1680. 10.1016/j.cell.2014.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhie A, McCarthy SA, Fedrigo O, et al. : Towards complete and error-free genome assemblies of all vertebrate species. Nature. 2021;592(7856):737–746. 10.1038/s41586-021-03451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhie A, Walenz BP, Koren S, et al. : Merqury: reference-free quality, completeness, and phasing assessment for genome assemblies. Genome Biol. 2020;21(1): 245. 10.1186/s13059-020-02134-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers EW, Cavanaugh M, Clark K, et al. : GenBank 2024 update. Nucleic Acids Res. 2024;52(D1):D134–D137. 10.1093/nar/gkad903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland M, Cornwell C, Howard C: Sanger Tree of Life fragmented DNA clean up: manual SPRI. protocols.io. 2023. 10.17504/protocols.io.kxygx3y1dg8j/v1 [DOI] [Google Scholar]
- Surana P, Muffato M, Qi G: sanger-tol/readmapping: sanger-tol/readmapping v1.1.0 - Hebridean Black (1.1.0). Zenodo. 2023a. 10.5281/zenodo.7755669 [DOI] [Google Scholar]
- Surana P, Muffato M, Sadasivan Baby C: sanger-tol/genomenote (v1.0.dev). Zenodo. 2023b. 10.5281/zenodo.6785935 [DOI] [Google Scholar]
- Twyford AD, Beasley J, Barnes I, et al. : A DNA barcoding framework for taxonomic verification in the Darwin Tree of Life project [version 1; peer review: awaiting peer review]. Wellcome Open Res. 2024;9:339. 10.12688/wellcomeopenres.21143.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uliano-Silva M, Ferreira JGRN, Krasheninnikova K, et al. : MitoHiFi: a python pipeline for mitochondrial genome assembly from PacBio high fidelity reads. BMC Bioinformatics. 2023;24(1): 288. 10.1186/s12859-023-05385-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- UniProt Consortium: UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47(D1):D506–D515. 10.1093/nar/gky1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasimuddin Md, Misra S, Li H, et al. : Efficient architecture-aware acceleration of BWA-MEM for multicore systems. In: 2019 IEEE International Parallel and Distributed Processing Symposium (IPDPS). IEEE,2019;314–324. 10.1109/IPDPS.2019.00041 [DOI] [Google Scholar]
- Wellcome Sanger Institute: The genome sequence of a leaf beetle, Galeruca laticollis Sahlberg, C.R., 1838. European Nucleotide Archive. [dataset], accession number PRJEB65735,2023.
- Zhou C, McCarthy SA, Durbin R: YaHS: yet another Hi-C scaffolding tool. Bioinformatics. 2023;39(1): btac808. 10.1093/bioinformatics/btac808 [DOI] [PMC free article] [PubMed] [Google Scholar]