Abstract
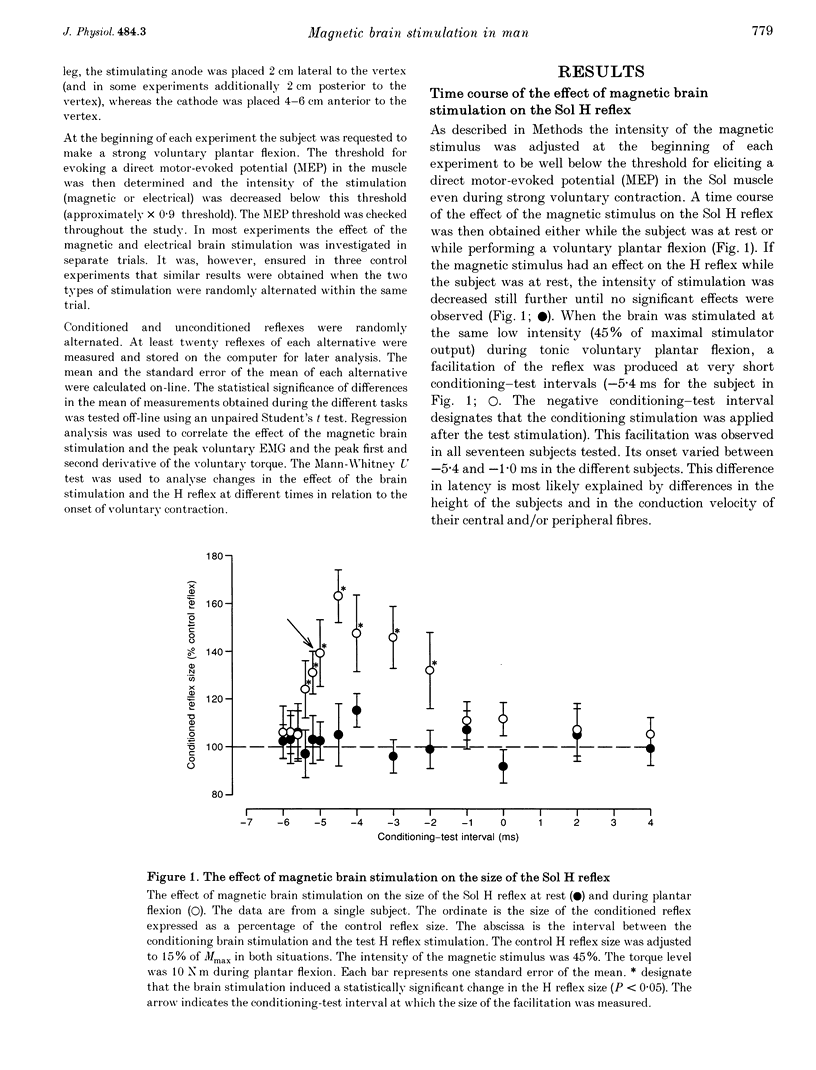
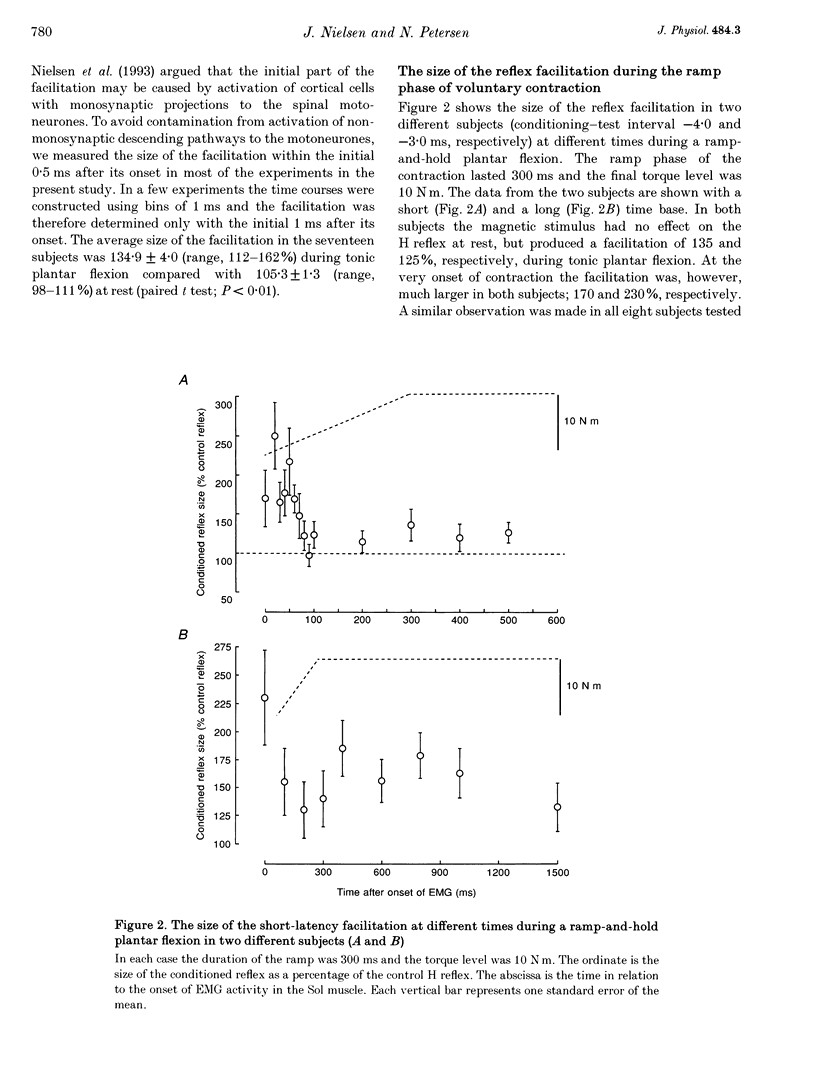
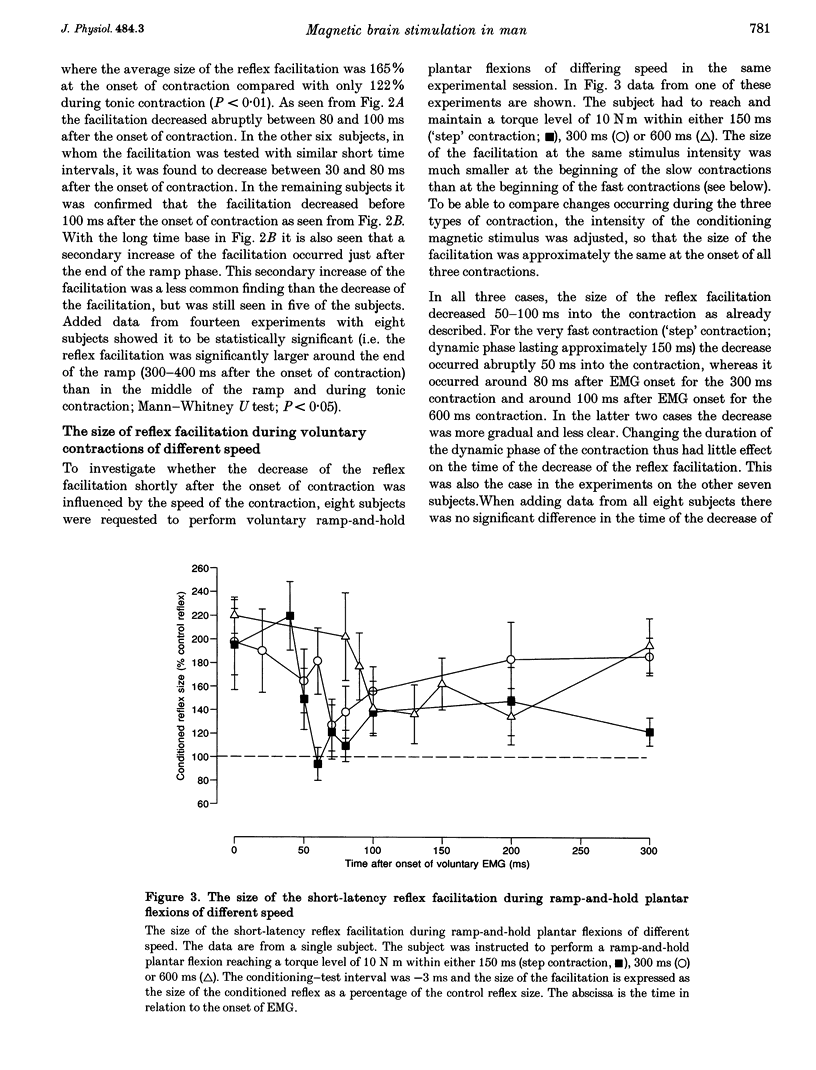
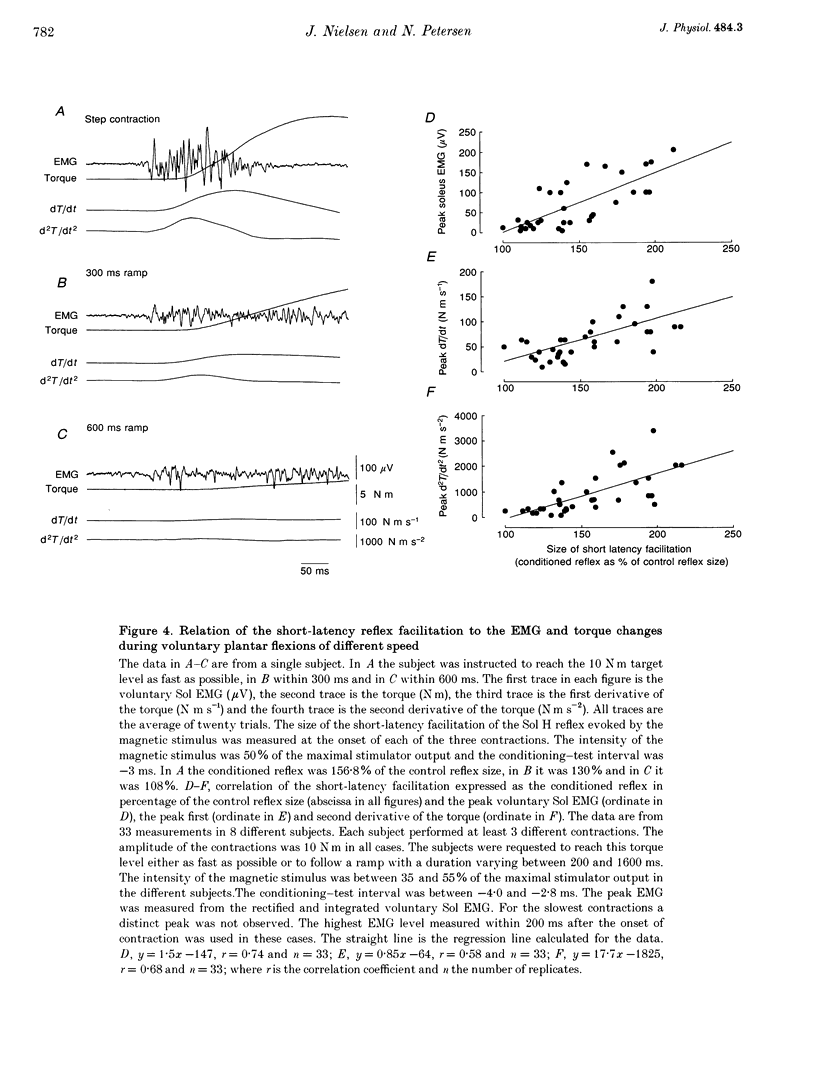
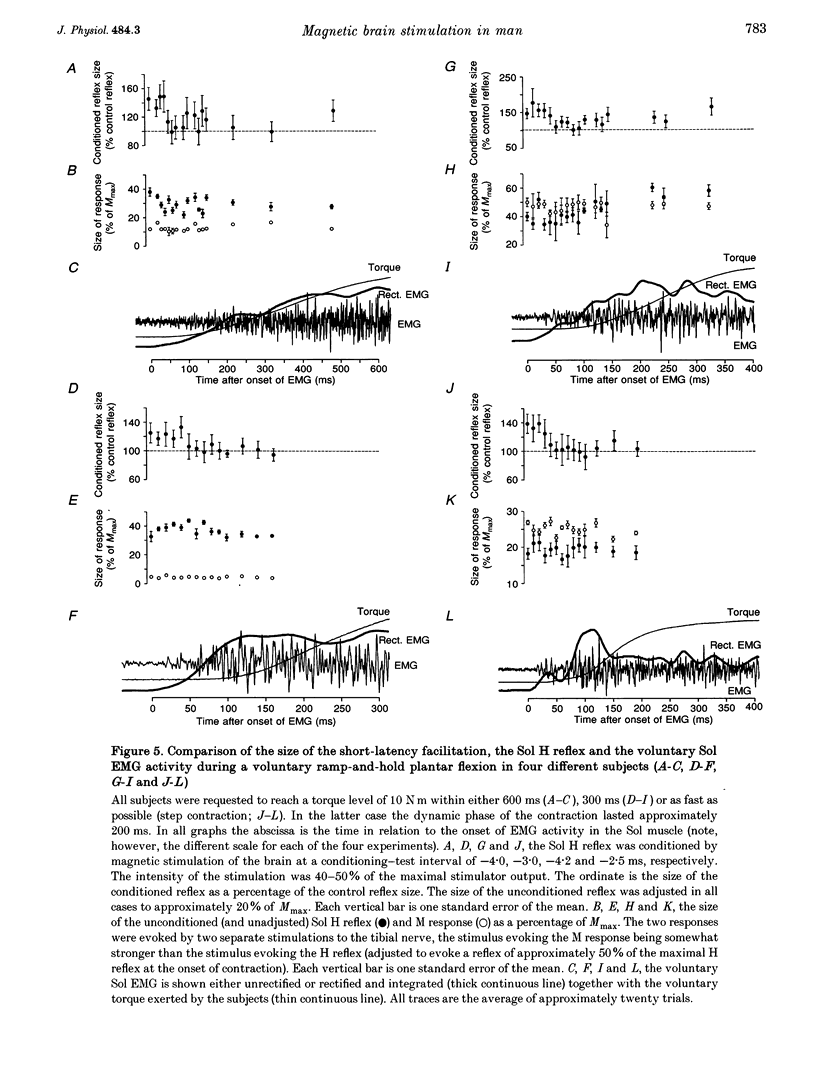
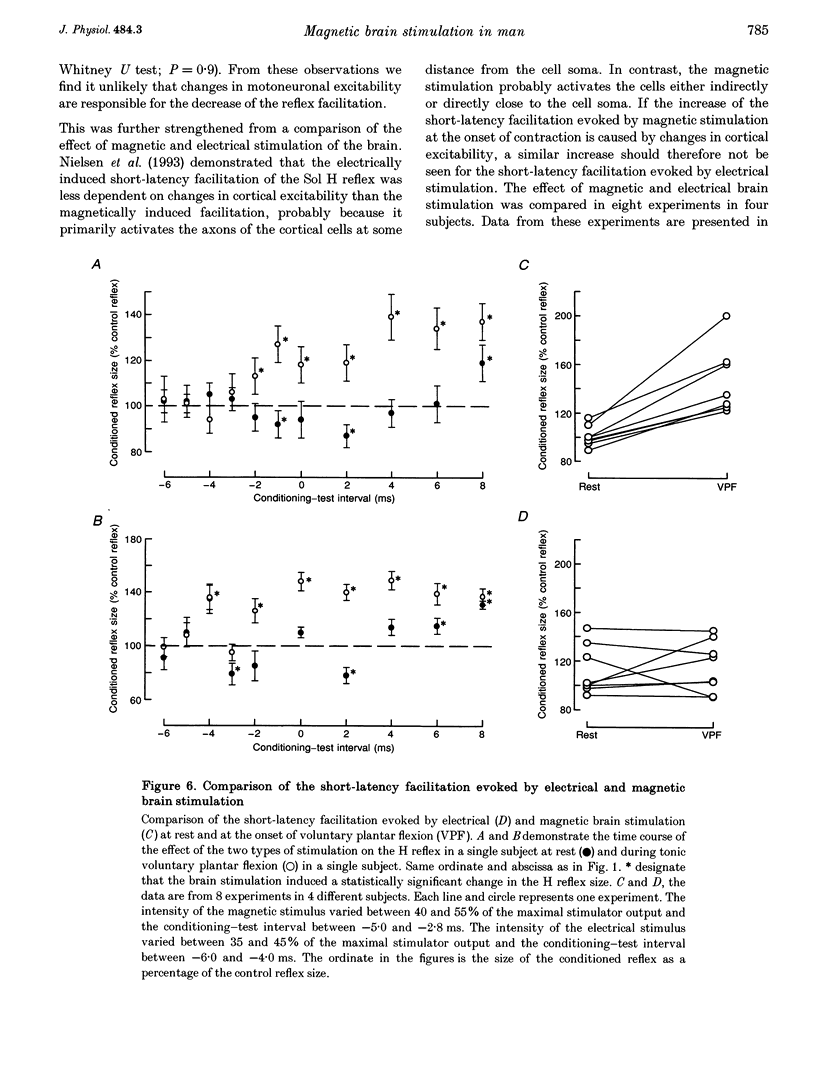
1. The soleus (Sol) H reflex was conditioned by magnetic stimulation of the contralateral motor cortex at rest and during voluntary contraction in healthy human subjects. The intensity of the magnetic stimulus was adjusted so as to have no effect on the H reflex at rest. During tonic voluntary contraction the same magnetic stimulus produced a facilitation with a short latency and a long duration, thus reflecting an increased excitation of Sol motoneurones by the magnetic stimulus during voluntary contraction. 2. The amount of reflex facilitation produced by brain stimulation within the initial 0.5-1 ms after its onset was investigated at different times during dynamic ramp-and-hold plantar flexion. The facilitation was largest at the onset of voluntary activity in the Sol muscle. It then decreased abruptly within 100 ms after the onset of the voluntary contraction. Neither the voluntary Sol activity nor the control H reflex decreased at this time. 3. Electrical stimulation of the brain with the anode placed lateral to the vertex produced a facilitation of the H reflex, which preceded the facilitation evoked by magnetic stimulation by 1-2 ms. The facilitation produced by the magnetic stimulus occurred or increased at the onset of contraction in relation to rest in all experiments. However, this was the case in only two out of eight experiments, when the brain was stimulated electrically. 4. The size of the reflex facilitation measured at the onset of contraction was larger the faster the contraction. Positive correlations were found between the size of the facilitation and the peak of the first and second derivative of the torque and the peak Sol EMG activity. 5. It is suggested that the observed changes in the size of the short-latency reflex facilitation produced by magnetic brain stimulation mainly reflects changes in the excitability of corticospinal cells, since similar changes were not observed in the size of the unconditioned Sol H reflex or in the short-latency reflex facilitation produced by electrical brain stimulation. The data support the hypothesis that fast conducting corticospinal fibres with monosynaptic projections to spinal motoneurones are involved in the initiation of voluntary movement in man.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker A. T., Jalinous R., Freeston I. L. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985 May 11;1(8437):1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- Berardelli A., Day B. L., Marsden C. D., Rothwell J. C. Evidence favouring presynaptic inhibition between antagonist muscle afferents in the human forearm. J Physiol. 1987 Oct;391:71–83. doi: 10.1113/jphysiol.1987.sp016726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney P. D., Fetz E. E. Functional classes of primate corticomotoneuronal cells and their relation to active force. J Neurophysiol. 1980 Oct;44(4):773–791. doi: 10.1152/jn.1980.44.4.773. [DOI] [PubMed] [Google Scholar]
- Cheney P. D., Mewes K., Fetz E. E. Encoding of motor parameters by corticomotoneuronal (CM) and rubromotoneuronal (RM) cells producing postspike facilitation of forelimb muscles in the behaving monkey. Behav Brain Res. 1988 Apr-May;28(1-2):181–191. doi: 10.1016/0166-4328(88)90095-2. [DOI] [PubMed] [Google Scholar]
- Crone C., Hultborn H., Mazières L., Morin C., Nielsen J., Pierrot-Deseilligny E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res. 1990;81(1):35–45. doi: 10.1007/BF00230098. [DOI] [PubMed] [Google Scholar]
- Datta A. K., Harrison L. M., Stephens J. A. Task-dependent changes in the size of response to magnetic brain stimulation in human first dorsal interosseous muscle. J Physiol. 1989 Nov;418:13–23. doi: 10.1113/jphysiol.1989.sp017826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B. L., Dressler D., Maertens de Noordhout A., Marsden C. D., Nakashima K., Rothwell J. C., Thompson P. D. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol. 1989 May;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day B. L., Riescher H., Struppler A., Rothwell J. C., Marsden C. D. Changes in the response to magnetic and electrical stimulation of the motor cortex following muscle stretch in man. J Physiol. 1991 Feb;433:41–57. doi: 10.1113/jphysiol.1991.sp018413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschl G., Michels R., Berardelli A., Schenck E., Inghilleri M., Lücking C. H. Effects of electric and magnetic transcranial stimulation on long latency reflexes. Exp Brain Res. 1991;83(2):403–410. doi: 10.1007/BF00231165. [DOI] [PubMed] [Google Scholar]
- Evarts E. V. Relation of pyramidal tract activity to force exerted during voluntary movement. J Neurophysiol. 1968 Jan;31(1):14–27. doi: 10.1152/jn.1968.31.1.14. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Cheney P. D. Functional relations between primate motor cortex cells and muscles: fixed and flexible. Ciba Found Symp. 1987;132:98–117. doi: 10.1002/9780470513545.ch7. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Cheney P. D., Mewes K., Palmer S. Control of forelimb muscle activity by populations of corticomotoneuronal and rubromotoneuronal cells. Prog Brain Res. 1989;80:437–430. doi: 10.1016/s0079-6123(08)62241-4. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Cheney P. D. Postspike facilitation of forelimb muscle activity by primate corticomotoneuronal cells. J Neurophysiol. 1980 Oct;44(4):751–772. doi: 10.1152/jn.1980.44.4.751. [DOI] [PubMed] [Google Scholar]
- Flament D., Goldsmith P., Buckley C. J., Lemon R. N. Task dependence of responses in first dorsal interosseous muscle to magnetic brain stimulation in man. J Physiol. 1993 May;464:361–378. doi: 10.1113/jphysiol.1993.sp019639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez C., Gordon J. Trajectory control in targeted force impulses. I. Role of opposing muscles. Exp Brain Res. 1987;67(2):225–240. doi: 10.1007/BF00248545. [DOI] [PubMed] [Google Scholar]
- Gordon J., Ghez C. Trajectory control in targeted force impulses. II. Pulse height control. Exp Brain Res. 1987;67(2):241–252. doi: 10.1007/BF00248546. [DOI] [PubMed] [Google Scholar]
- Gordon J., Ghez C. Trajectory control in targeted force impulses. III. Compensatory adjustments for initial errors. Exp Brain Res. 1987;67(2):253–269. doi: 10.1007/BF00248547. [DOI] [PubMed] [Google Scholar]
- Hepp-Reymond M. C., Wiesendanger M. Unilateral pyramidotomy in monkeys: effect on force and speed of a conditioned precision grip. Brain Res. 1972 Jan 14;36(1):117–131. doi: 10.1016/0006-8993(72)90770-6. [DOI] [PubMed] [Google Scholar]
- Hepp-Reymond, Trouche E., Wiesendanger M. Effects of unilateral and bilateral pyramidotomy on a conditioned rapid precision grip in monkeys (Macaca fascicularis). Exp Brain Res. 1974;21(5):519–527. doi: 10.1007/BF00237170. [DOI] [PubMed] [Google Scholar]
- Kernell D., Hultborn H. Synaptic effects on recruitment gain: a mechanism of importance for the input-output relations of motoneurone pools? Brain Res. 1990 Jan 15;507(1):176–179. doi: 10.1016/0006-8993(90)90542-j. [DOI] [PubMed] [Google Scholar]
- Lawrence D. G., Kuypers H. G. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain. 1968 Mar;91(1):1–14. doi: 10.1093/brain/91.1.1. [DOI] [PubMed] [Google Scholar]
- Lemon R. N., Mantel G. W. The influence of changes in discharge frequency of corticospinal neurones on hand muscles in the monkey. J Physiol. 1989 Jun;413:351–378. doi: 10.1113/jphysiol.1989.sp017658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertens de Noordhout A., Rothwell J. C., Day B. L., Dressler D., Nakashima K., Thompson P. D., Marsden C. D. Effect of digital nerve stimuli on responses to electrical or magnetic stimulation of the human brain. J Physiol. 1992 Feb;447:535–548. doi: 10.1113/jphysiol.1992.sp019016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S., Pierrot-Deseilligny E. Gating of the afferent volley of the monosynaptic stretch reflex during movement in man. J Physiol. 1989 Dec;419:753–763. doi: 10.1113/jphysiol.1989.sp017896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J., Petersen N., Ballegaard M. Latency of effects evoked by electrical and magnetic brain stimulation in lower limb motoneurones in man. J Physiol. 1995 May 1;484(Pt 3):791–802. doi: 10.1113/jphysiol.1995.sp020704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J., Petersen N. Changes in motor cortex excitability preceding voluntary ramp-and-hold plantarflexion in man. Acta Physiol Scand. 1992 Nov;146(3):399–400. doi: 10.1111/j.1748-1716.1992.tb09435.x. [DOI] [PubMed] [Google Scholar]
- Nielsen J., Petersen N., Deuschl G., Ballegaard M. Task-related changes in the effect of magnetic brain stimulation on spinal neurones in man. J Physiol. 1993 Nov;471:223–243. doi: 10.1113/jphysiol.1993.sp019899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J., Petersen N. Is presynaptic inhibition distributed to corticospinal fibres in man? J Physiol. 1994 May 15;477(Pt 1):47–58. doi: 10.1113/jphysiol.1994.sp020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer E., Ashby P. Evidence that a long latency stretch reflex in humans is transcortical. J Physiol. 1992 Apr;449:429–440. doi: 10.1113/jphysiol.1992.sp019094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. Early facilitation at corticomotoneuronal synapses. J Physiol. 1970 May;207(3):733–745. doi: 10.1113/jphysiol.1970.sp009091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudomín P., Núez R., Madrid J. Modulation of synaptic effectiveness of Ia and descending fibers in cat spinal cord. J Neurophysiol. 1975 Sep;38(5):1181–1195. doi: 10.1152/jn.1975.38.5.1181. [DOI] [PubMed] [Google Scholar]
- Vallbo A. B. Muscle spindle response at the onset of isometric voluntary contractions in man. Time difference between fusimotor and skeletomotor effects. J Physiol. 1971 Oct;218(2):405–431. doi: 10.1113/jphysiol.1971.sp009625. [DOI] [PMC free article] [PubMed] [Google Scholar]


