Abstract
1. Dendrites of rat neocortical layer V pyramidal neurons were loaded with the Ca2+ indicator dye Calcium Green-1 (CG-1) or fluo-3, and the mechanisms which govern action potential (AP)-evoked transient changes in dendritic cytosolic Ca2+ concentration ([Ca2+]i) were examined. APs were initiated either by synaptic stimulation or by depolarizing the soma or dendrite by current injection, and changes in fluorescence of the indicator dye were measured in the proximal 170 microns of the apical dendrite. 2. Simultaneous two-pipette recordings of APs from the soma and apical dendrite, and dendritic fluorescence imaging indicated that a single AP propagating from the soma into the apical dendrite evokes a rapid transient increase in fluorescence indicating a transient increase in [Ca2+]i. At 35-37 degrees C the decay time constant of the fluorescence transient following an AP was around 80 ms. 3. Voltage-activated Ca2+ channels (VACCs) of several subtypes mediated the AP-evoked fluorescence transient in the proximal (100-170 microns) apical dendrite. The AP-evoked fluorescence transient resulted from Ca2+ entry through L-type (nifedipine sensitive; 25%), N-type (omega-conotoxin GVIA sensitive; 28%) and P-type (omega-agatoxin IVA sensitive; 10%) Ca2+ channels and through Ca2+ channels (R-type) not sensitive to L-, N- and P-type Ca2+ channel blockers (cadmium ion sensitive; 37%). 4. The decay time course of the dendritic fluorescence transient was prolonged by the blockers of endoplasmic reticulum (ER) Ca(2+)-ATPase, cyclopiazonic acid and thapsigargin, suggesting that uptake of Ca2+ into the ER in dendrites governs clearance of dendritic Ca2+. 5. The decay time course of the fluorescence transient was slightly prolonged by benzamil, a blocker of plasma membrane Na(+)-Ca2+ exchange and by calmidazolium, a blocker of the calmodulin-dependent plasma membrane Ca(2+)-ATPase, suggesting that these pathways are less important for dendrite Ca2+ clearance following a single AP. Neither the mitochondrial uncoupler carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone (FCCP) nor the blocker of Ca2+ uptake into mitochondria, Ruthenium Red, had any measurable effect on the decay time course of the fluorescence transient. 6. Dendritic fluorescence transients measured during trains of dendritic APs began to summate at impulse frequencies of 5 APs s-1. At higher frequencies APs caused a concerted and maintained elevation of dendritic fluorescence during the train.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF











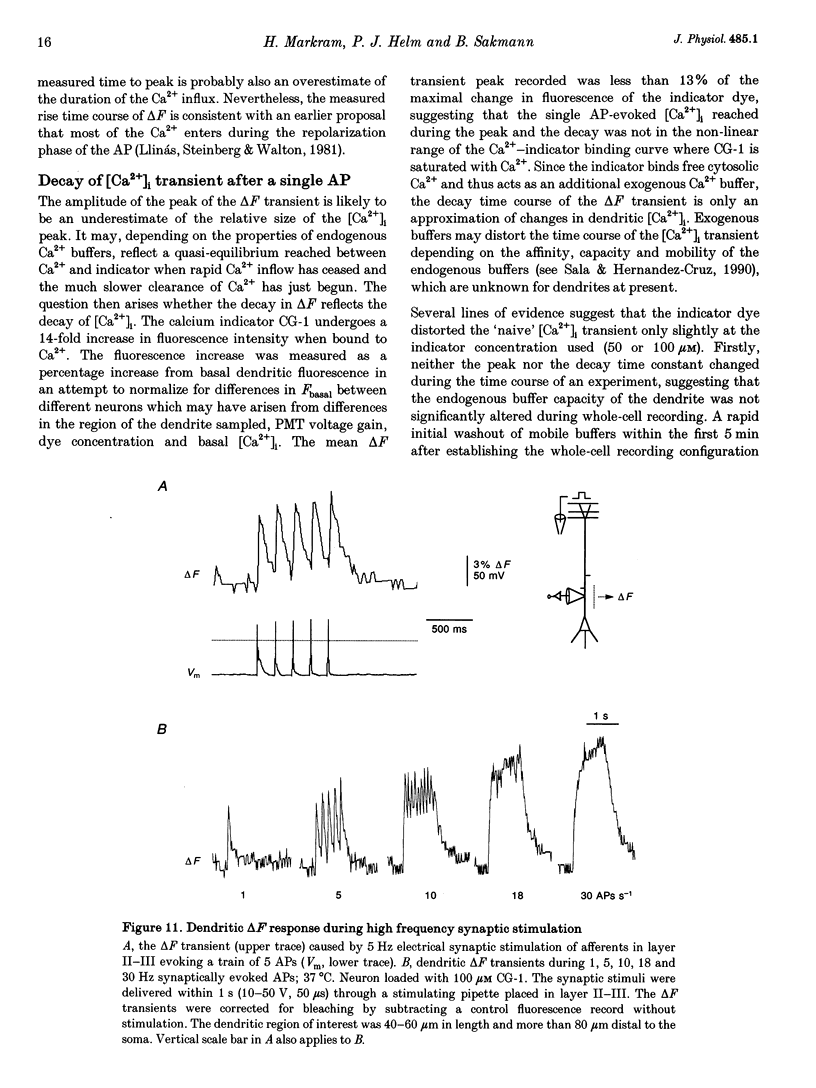




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlijanian M. K., Westenbroek R. E., Catterall W. A. Subunit structure and localization of dihydropyridine-sensitive calcium channels in mammalian brain, spinal cord, and retina. Neuron. 1990 Jun;4(6):819–832. doi: 10.1016/0896-6273(90)90135-3. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H., Zablow L., Sabatini B. Evaluation of cellular mechanisms for modulation of calcium transients using a mathematical model of fura-2 Ca2+ imaging in Aplysia sensory neurons. Biophys J. 1992 Oct;63(4):1146–1164. doi: 10.1016/S0006-3495(92)81670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brorson J. R., Bleakman D., Gibbons S. J., Miller R. J. The properties of intracellular calcium stores in cultured rat cerebellar neurons. J Neurosci. 1991 Dec;11(12):4024–4043. doi: 10.1523/JNEUROSCI.11-12-04024.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne R. A., Parnavelas J. G., Lin C. S. Response properties of neurons in the visual cortex of the rat. Exp Brain Res. 1984;53(2):374–383. doi: 10.1007/BF00238168. [DOI] [PubMed] [Google Scholar]
- Deisz R. A., Fortin G., Zieglgänsberger W. Voltage dependence of excitatory postsynaptic potentials of rat neocortical neurons. J Neurophysiol. 1991 Feb;65(2):371–382. doi: 10.1152/jn.1991.65.2.371. [DOI] [PubMed] [Google Scholar]
- Duce I. R., Keen P. Can neuronal smooth endoplasmic reticulum function as a calcium reservoir? Neuroscience. 1978;3(9):837–848. doi: 10.1016/0306-4522(78)90036-2. [DOI] [PubMed] [Google Scholar]
- Eberhard M., Erne P. Calcium binding to fluorescent calcium indicators: calcium green, calcium orange and calcium crimson. Biochem Biophys Res Commun. 1991 Oct 15;180(1):209–215. doi: 10.1016/s0006-291x(05)81278-1. [DOI] [PubMed] [Google Scholar]
- Henzi V., MacDermott A. B. Characteristics and function of Ca(2+)- and inositol 1,4,5-trisphosphate-releasable stores of Ca2+ in neurons. Neuroscience. 1992;46(2):251–273. doi: 10.1016/0306-4522(92)90049-8. [DOI] [PubMed] [Google Scholar]
- Hillman D., Chen S., Aung T. T., Cherksey B., Sugimori M., Llinás R. R. Localization of P-type calcium channels in the central nervous system. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7076–7080. doi: 10.1073/pnas.88.16.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe D. B., Johnston D., Lasser-Ross N., Lisman J. E., Miyakawa H., Ross W. N. The spread of Na+ spikes determines the pattern of dendritic Ca2+ entry into hippocampal neurons. Nature. 1992 May 21;357(6375):244–246. doi: 10.1038/357244a0. [DOI] [PubMed] [Google Scholar]
- Jensen J. R., Rehder V. FCCP releases Ca2+ from a non-mitochondrial store in an identified Helisoma neuron. Brain Res. 1991 Jun 14;551(1-2):311–314. doi: 10.1016/0006-8993(91)90947-t. [DOI] [PubMed] [Google Scholar]
- Kaczorowski G. J., Barros F., Dethmers J. K., Trumble M. J., Cragoe E. J., Jr Inhibition of Na+/Ca2+ exchange in pituitary plasma membrane vesicles by analogues of amiloride. Biochemistry. 1985 Mar 12;24(6):1394–1403. doi: 10.1021/bi00327a017. [DOI] [PubMed] [Google Scholar]
- Lattanzio F. A., Jr, Bartschat D. K. The effect of pH on rate constants, ion selectivity and thermodynamic properties of fluorescent calcium and magnesium indicators. Biochem Biophys Res Commun. 1991 May 31;177(1):184–191. doi: 10.1016/0006-291x(91)91966-g. [DOI] [PubMed] [Google Scholar]
- Lipscombe D., Madison D. V., Poenie M., Reuter H., Tsien R. W., Tsien R. Y. Imaging of cytosolic Ca2+ transients arising from Ca2+ stores and Ca2+ channels in sympathetic neurons. Neuron. 1988 Jul;1(5):355–365. doi: 10.1016/0896-6273(88)90185-7. [DOI] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents in squid giant synapse. Biophys J. 1981 Mar;33(3):289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Simon S. M. Transmission by presynaptic spike-like depolarization in the squid giant synapse. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2415–2419. doi: 10.1073/pnas.79.7.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H., Sakmann B. Calcium transients in dendrites of neocortical neurons evoked by single subthreshold excitatory postsynaptic potentials via low-voltage-activated calcium channels. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5207–5211. doi: 10.1073/pnas.91.11.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCobb D. P., Beam K. G. Action potential waveform voltage-clamp commands reveal striking differences in calcium entry via low and high voltage-activated calcium channels. Neuron. 1991 Jul;7(1):119–127. doi: 10.1016/0896-6273(91)90080-j. [DOI] [PubMed] [Google Scholar]
- McPherson P. S., Kim Y. K., Valdivia H., Knudson C. M., Takekura H., Franzini-Armstrong C., Coronado R., Campbell K. P. The brain ryanodine receptor: a caffeine-sensitive calcium release channel. Neuron. 1991 Jul;7(1):17–25. doi: 10.1016/0896-6273(91)90070-g. [DOI] [PubMed] [Google Scholar]
- Miller K. K., Verma A., Snyder S. H., Ross C. A. Localization of an endoplasmic reticulum calcium ATPase mRNA in rat brain by in situ hybridization. Neuroscience. 1991;43(1):1–9. doi: 10.1016/0306-4522(91)90410-p. [DOI] [PubMed] [Google Scholar]
- Miyakawa H., Lev-Ram V., Lasser-Ross N., Ross W. N. Calcium transients evoked by climbing fiber and parallel fiber synaptic inputs in guinea pig cerebellar Purkinje neurons. J Neurophysiol. 1992 Oct;68(4):1178–1189. doi: 10.1152/jn.1992.68.4.1178. [DOI] [PubMed] [Google Scholar]
- Moore C. L. Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochem Biophys Res Commun. 1971 Jan 22;42(2):298–305. doi: 10.1016/0006-291x(71)90102-1. [DOI] [PubMed] [Google Scholar]
- Muri R., Knöpfel T. Activity induced elevations of intracellular calcium concentration in neurons of the deep cerebellar nuclei. J Neurophysiol. 1994 Jan;71(1):420–428. doi: 10.1152/jn.1994.71.1.420. [DOI] [PubMed] [Google Scholar]
- Neering I. R., McBurney R. N. Role for microsomal Ca storage in mammalian neurones? Nature. 1984 May 10;309(5964):158–160. doi: 10.1038/309158a0. [DOI] [PubMed] [Google Scholar]
- Regehr W. G., Connor J. A., Tank D. W. Optical imaging of calcium accumulation in hippocampal pyramidal cells during synaptic activation. Nature. 1989 Oct 12;341(6242):533–536. doi: 10.1038/341533a0. [DOI] [PubMed] [Google Scholar]
- Ross W. N., Werman R. Mapping calcium transients in the dendrites of Purkinje cells from the guinea-pig cerebellum in vitro. J Physiol. 1987 Aug;389:319–336. doi: 10.1113/jphysiol.1987.sp016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala F., Hernández-Cruz A. Calcium diffusion modeling in a spherical neuron. Relevance of buffering properties. Biophys J. 1990 Feb;57(2):313–324. doi: 10.1016/S0006-3495(90)82533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayer R. J., Schwindt P. C., Crill W. E. High- and low-threshold calcium currents in neurons acutely isolated from rat sensorimotor cortex. Neurosci Lett. 1990 Dec 11;120(2):175–178. doi: 10.1016/0304-3940(90)90031-4. [DOI] [PubMed] [Google Scholar]
- Seidler N. W., Jona I., Vegh M., Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989 Oct 25;264(30):17816–17823. [PubMed] [Google Scholar]
- Simons D. J., Carvell G. E., Hershey A. E., Bryant D. P. Responses of barrel cortex neurons in awake rats and effects of urethane anesthesia. Exp Brain Res. 1992;91(2):259–272. doi: 10.1007/BF00231659. [DOI] [PubMed] [Google Scholar]
- Spruston N., Jaffe D. B., Johnston D. Dendritic attenuation of synaptic potentials and currents: the role of passive membrane properties. Trends Neurosci. 1994 Apr;17(4):161–166. doi: 10.1016/0166-2236(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Stuart G. J., Dodt H. U., Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflugers Arch. 1993 Jun;423(5-6):511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Stuart G. J., Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 1994 Jan 6;367(6458):69–72. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa A., Sharp A. H., Racchetti G., Podini P., Bole D. G., Dunn W. A., Pozzan T., Snyder S. H., Meldolesi J. The endoplasmic reticulum of Purkinje neuron body and dendrites: molecular identity and specializations for Ca2+ transport. Neuroscience. 1992 Jul;49(2):467–477. doi: 10.1016/0306-4522(92)90111-e. [DOI] [PubMed] [Google Scholar]
- Werth J. L., Thayer S. A. Mitochondria buffer physiological calcium loads in cultured rat dorsal root ganglion neurons. J Neurosci. 1994 Jan;14(1):348–356. doi: 10.1523/JNEUROSCI.14-01-00348.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek R. E., Hell J. W., Warner C., Dubel S. J., Snutch T. P., Catterall W. A. Biochemical properties and subcellular distribution of an N-type calcium channel alpha 1 subunit. Neuron. 1992 Dec;9(6):1099–1115. doi: 10.1016/0896-6273(92)90069-p. [DOI] [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., Supattapone S., Wilson V. S., Snyder S. H. Characterization of inositol trisphosphate receptor binding in brain. Regulation by pH and calcium. J Biol Chem. 1987 Sep 5;262(25):12132–12136. [PubMed] [Google Scholar]
- Zhang J. F., Randall A. D., Ellinor P. T., Horne W. A., Sather W. A., Tanabe T., Schwarz T. L., Tsien R. W. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993 Nov;32(11):1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]