Abstract
Background
Acute limb ischemia (ALI) is a medical emergency necessitating immediate action to avert irreversible tissue harm and limb loss. Rotarex mechanical thrombectomy (RMT) has become an efficient treatment alternative for ALI. However, there is a lack of data on RMT in Taiwan.
Methods
We retrospectively analyzed 61 ALI patients treated with RMT at our hospital between January 2016 and January 2022. We collected baseline characteristics, laboratory and angiographic data. We also examined the outcomes at 30 days, 6 months, and 1 year, including major amputations, minor amputations, all-cause mortality, and major adverse limb events (MALEs).
Results
Among the 61 RMT-treated patients, the average age was 70 ± 14 years. ALI affected the upper extremities in 9 cases and lower extremities in 52 cases. One-year outcomes revealed 2 major amputations (3.3%), 2 minor amputations (3.3%), 6 all-cause deaths (9.8%), and 10 MALEs (16.4%). After multiple logistic regression analysis, hemoglobin drop was significantly associated with 1-year all-cause mortality, and a history of peripheral artery disease (PAD) was significantly associated with MALEs.
Conclusions
Our research is the first investigation into the application of RMT for ALI in Taiwan. The short- and mid-term outcomes after RMT for ALI revealed reductions in amputation, mortality, and MALE rates. In addition, a decline in hemoglobin level was a significant predictor of increased mortality, and a history of PAD was a significant predictor of increased MALEs following RMT.
Keywords: Acute limb ischemia, All-cause mortality, Catheter-directed thrombolysis, Major adverse limb events, Rotarex mechanical thrombectomy
Abbreviations
ALI, Acute limb ischemia
CDT, Catheter-directed thrombolysis
EKOS, EkoSonic endovascular system
Hb, Hemoglobin
KMUH, Kaohsiung Medical University Hospital
MALE, Major adverse limb event
PAD, Peripheral artery disease
PMT, Pharmaco-mechanical thrombolysis
RMT, Rotarex mechanical thrombectomy
TIMI, Thrombolysis in Myocardial Infarction
INTRODUCTION
Acute limb ischemia (ALI) is a serious condition caused by the sudden disruption of blood flow to the limbs.1-6 ALI can be caused by various factors, including embolism, thrombosis, and injury. It is crucial to promptly diagnose and intervene to prevent limb amputation and improve patient outcomes. Thrombectomy is the primary treatment for ALI, with various methods available such as surgical thrombectomy, manual aspiration, rheolytic mechanical thrombectomy, rotational mechanical thrombectomy, and other novel techniques.3-8 Rotarex mechanical thrombectomy (RMT) is a useful and effective treatment option that can achieve a high success rate and reduce the risk of complications.9-15 However, the Rotarex device has only been introduced to Taiwan in recent years, with the first procedure at our hospital on January 11, 2016. Since then, our peripheral endovascular team has successfully completed over 70 cases using RMT for ALI. Of these cases, more than 60 have now reached one year of follow-up. In this study, we enrolled 61 of these patients and assessed their outcomes at 30 days, 6 months, and 1 year. Furthermore, we also evaluated the key predictors of 1-year mortality and major adverse limb events (MALEs).
METHODS
Study subjects
We conducted this retrospective study at Kaohsiung Medical University Hospital (KMUH) on patients with ALI who underwent RMT between January 11, 2016 and January 31, 2022. We excluded cases with Rutherford stage 3 and cases without 1-year follow-up data. Finally, 61 cases were enrolled for analysis. We collected and analyzed their demographic, baseline, laboratory, and angiographic data. Demographic and baseline data included age, gender, history of diabetes mellitus, hypertension, dyslipidemia, cerebrovascular disease, coronary artery disease, peripheral artery disease (PAD), heart failure, and atrial fibrillation. We also analyzed the duration of ALI, Rutherford stage, body mass index, and percentage of ALI involving lower extremities. Laboratory data included white blood count, platelet count, triglycerides, total cholesterol, low-density lipoprotein, HbA1c, uric acid, D-dimer, and hemoglobin (Hb) drop. Angiographic data included occlusive location of ALI, treatment strategy [RMT plus catheter-directed thrombolysis (CDT) or RMT only], additional treatment (angioplasty only or angioplasty plus stenting), and procedure success (complete success, partial success, or failure). The research protocol was approved and registered by the ethics committee (KMUH IRB) at our institution.
Treatment strategy of endovascular therapy
After a patient had been diagnosed with ALI, we provided a clear explanation of the available treatment options and devices to both the patient and their family. Since our study specifically focused on patients using RMT devices, all of the enrolled patients signed informed consent forms for endovascular therapy and agreed to use the self-paid Rotarex device, providing their signature for device usage permits.
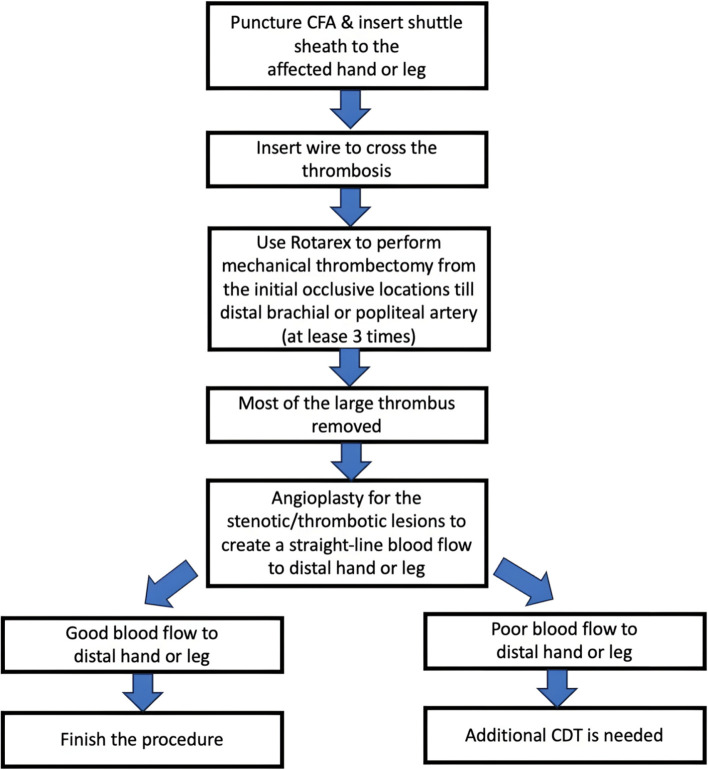
For ALI involving the lower extremities, we performed a contralateral common femoral artery puncture to insert the crossover shuttle sheath. After the wires had crossed the occlusive arteries, the Rotarex device was used for mechanical thrombectomy from the initial occlusive locations to the distal popliteal arteries. After at least three rounds of Rotarex mechanical thrombectomy, angiography was performed to evaluate its effect. If most of the thrombus burden had already been removed, we performed angioplasty in the affected leg to create a straight-line blood flow to the distal foot. If distal run-off blood flow was still poor after angioplasty, CDT was frequently performed in cases with below-the-knee thrombosis. Urokinase is used as the thrombolytic agent for CDT at our hospital, with a daily total dose of around 960 thousand units. The timing of the second endovascular therapy depended on the clinical symptoms/signs involved in the affected extremities. Further angioplasty may also have been indicated if the angiographic results were not satisfactory (Figure 1). For ALI involving the upper extremities, we often punctured the right common femoral artery and inserted a 90 cm shuttle sheath to the thrombotic hand. After the wires had crossed the occlusive arteries, the Rotarex device was used for mechanical thrombectomy from the occlusive location to the distal brachial arteries. After that, the strategy was similar to the procedure for the lower extremities. Cases needing CDT treatment were transferred to our cardiac care unit for further intensive care and close monitoring of fibrinogen and activated partial thromboplastin time levels under simultaneous heparinization.
Figure 1.
Treatment strategy of ALI using RMT in KMUH. ALI, acute limb ischemia; CDT, catheter-directed thrombolysis; CFA, common femoral artery; KMUH, Kaohsiung Medical University Hospital; RMT, Rotarex mechanical thrombectomy.
Outcomes
The major outcomes evaluated in this study were major amputation, minor amputation, all-cause mortality, and MALEs. We assessed the 30-day, 6-month, and 1-year major outcomes during 1 year of follow-up. The outcome data were collected through a retrospective review of the patients’ medical charts. For endovascular therapy, technical success was defined as successfully wiring across thrombotic lesions and performing RMT smoothly. Procedural success was defined as successfully restoring continuous arterial patency of at least one calf artery down to the ankle with a Thrombolysis in Myocardial Infarction (TIMI) grade of 2 or 3. Partial success was defined as regaining only TIMI 1 flow after the intervention. Procedure failure was defined as TIMI flow 0 after the intervention, even after CDT treatment. We defined major bleeding as the safety outcome using the TIMI classification, as used in the VOYAGER study.16 In addition, major amputation was defined as above-knee or below-knee amputation. Minor amputation was defined as forefoot or toe amputation. A MALE was defined as severe limb ischemia leading to an intervention or major amputation. These definitions were the same as in the COMPASS study.17
Statistical analysis
We expressed all data as means ± standard deviation. We used the independent t-test to compare continuous variables between patients with and without 1-year all-cause mortality. Fisher’s exact test and chi-square test were used to compare categorical data. We further analyzed significantly correlated or relevant variables from the univariate analysis using binary logistic regression analysis to predict 1-year all-cause mortality and MALEs. We used a significance level of p < 0.05 for all two-sided p values. To conduct the statistical analysis, we used SPSS version 26.0 for Windows (IBM Inc., Armonk, NY).
RESULTS
Among the 61 patients, the mean age was 69.6 ± 14.1 years. All patients received RMT during endovascular therapy, and 48 cases (78.7%) were combined with CDT treatment. Technical success was achieved in all of the patients. The average urokinase dose during CDT treatment was 259 ± 181 × 103 U and the duration of urokinase infusion was 2.7 ± 1.9 days. After 1 year of follow-up, 56 patients were still alive, 6 had died, and 10 had MALEs. The major bleeding rate during hospitalization was 9.8%.
Comparisons of the clinical characteristics between the patients with and without MALEs at 1 year are shown in Table 1. More of the patients with MALEs had a history of PAD and a longer duration of ALI (6.9 ± 5.0 vs. 3.8 ± 3.9 days, p < 0.001) compared to the patients without MALEs. However, there were no significant differences in age, gender, Rutherford stage, percentages of diabetes mellitus, hypertension, dyslipidemia, cerebrovascular disease, coronary artery disease, chronic kidney disease, heart failure, atrial fibrillation, or percentage of lower extremity involvement.
Table 1. Baseline characteristics of patients with and without 1 year MALE.
| Baseline characteristics | MALE (–), n = 51 | MALE (+), n = 10 | p value |
| Age (yr) | 71 ± 14 | 63 ± 13 | 0.092 |
| Male gender (%) | 66.7 | 90.0 | 0.256 |
| ALI duration (day) | 3.8 ± 3.9 | 6.9 ± 5.0 | 0.039 |
| Rutherford stage (%) | 0.942 | ||
| Stage 1 | 15.7 | 20.0 | |
| Stage 2a | 33.3 | 30.0 | |
| Stage 2b | 51.0 | 50.0 | |
| Diabetes (%) | 45.1 | 20.0 | 0.176 |
| Hypertension (%) | 64.7 | 50.0 | 0.481 |
| Dyslipidemia (%) | 33.3 | 30.0 | 0.837 |
| CVD (%) | 21.6 | 20.0 | 0.912 |
| CAD (%) | 31.4 | 20.0 | 0.708 |
| PAD (%) | 25.5 | 60.0 | 0.041 |
| CKD (%) | 49.0 | 20.0 | 0.162 |
| Heart failure (%) | 25.5 | 10.0 | 0.429 |
| Atrial fibrillation (%) | 47.1 | 20.0 | 0.167 |
| Body mass index (kg/m2) | 25.0 ± 4.1 | 25.3 ± 4.5 | 0.847 |
| Lower extremities (%) | 88.2 | 70.0 | 0.157 |
ALI, acute limb ischemia; CAD, coronary artery disease; CKD, chronic kidney disease; CVD, cerebrovascular disease; MALE, major adverse limb events; PAD, peripheral artery disease.
Comparisons of the laboratory data between the patients with and without MALEs at 1 year are shown in Table 2. The results showed no significant differences in white blood count, platelet count, triglyceride levels, total cholesterol levels, low-density lipoprotein levels, HbA1c levels, uric acid levels, D-dimer levels, lowest fibrinogen levels, and hemoglobin drop.
Table 2. Laboratory data between patients with and without 1 year MALE.
| Baseline characteristics | MALE (–), n = 51 | MALE (+), n = 10 | p value |
| White blood count (×103/uL) | 10.7 ± 4.7 | 10.2 ± 4.3 | 0.761 |
| Platelet (×103/uL) | 228.5 ± 96.7 | 239.5 ± 120.2 | 0.755 |
| Triglyceride (mg/dL) | 105.9 ± 58.0 | 79.7 ± 27.0 | 0.193 |
| Total cholesterol (mg/dL) | 171.0 ± 43.2 | 146.5 ± 28.1 | 0.131 |
| Low-density lipoprotein (mg/dL) | 104.8 ± 37.4 | 93.2 ± 27.6 | 0.381 |
| HbA1c (%) | 6.8 ± 2.4 | 5.8 ± 0.8 | 0.310 |
| Uric acid (mg/dL) | 5.7 ± 2.2 | 6.4 ± 2.8 | 0.538 |
| D-dimer (mg/L FEU) | 4.4 ± 4.1 | 4.7 ± 3.9 | 0.909 |
| Lowest fibrinogen (mg/dL) | 269.3 ± 90.9 | 296.7 ± 96.3 | 0.503 |
| Hemoglobin drop (g/dL) | 2.0 ± 1.9 | 3.0 ± 1.9 | 0.139 |
HbA1c, glycated hemoglobin; MALE, major adverse limb events.
Comparisons of the angiographic data between the patients with and without 1-year MALEs are shown in Table 3. There were no significant differences in the location of the occlusive artery, treatment strategy (Rotarex alone or combined with CDT), additional treatment (angioplasty alone or combined with stenting), and procedure success.
Table 3. Angiographic data between patients with and without 1 year MALE.
| Baseline characteristics | MALE (–), n = 51 | MALE (+), n = 10 | p value |
| Occlusive location from | 0.424 | ||
| Iliac artery (LE) | 37.3% | 20.0% | |
| Femoral artery (LE) | 47.1% | 40.0% | |
| Popliteal artery (LE) | 3.9% | 10.0% | |
| Subclavian artery (UE) | 5.9% | 20.0% | |
| Axillary artery (UE) | 3.9% | 0% | |
| Brachial artery (UE) | 2.0% | 10.0% | |
| Treatment strategy | 0.397 | ||
| Rotarex (RMT) + CDT | 82.4% | 70.0% | |
| Rotarex (RMT) only | 17.6% | 30.0% | |
| Additional treatment | 0.143 | ||
| Angioplasty only | 39.2% | 10.0% | |
| Angioplasty + stenting | 60.8% | 90.0% | |
| Procedure success | 0.156 | ||
| Complete success | 90.2% | 80.0% | |
| Partial success | 9.8% | 10.0% | |
| Failure | 0% | 10.0% |
CDT, catheter directed thrombolysis; LE, lower extremities; MALE, major adverse limb events; RMT, Rotarex mechanical thrombectomy; UE, upper extremities.
The outcomes of major amputation, minor amputation, all-cause mortality, and MALEs at 30 days, 6 months, and 1 year are presented in Table 4. The rates of major amputation, minor amputation, all-cause mortality, and MALEs at 30 days were 3.3%, 1.6%, 8.2%, and 4.9% respectively. At 6 months, the rates were 3.3% for major amputation, 3.3% for minor amputation, 9.8% for all-cause mortality, and 9.8% for MALEs. Finally, at 1 year, the rates were 3.3% for major amputation, 3.3% for minor amputation, 9.8% for all-cause mortality, and 16.4% for MALEs.
Table 4. 30 days, 6 months, and 1 year outcome of major amputation, minor amputation, all-cause mortality, and MALE.
| Outcomes | 30 days | 6 months | 1 year |
| Major amputation | 3.3% | 3.3% | 3.3% |
| Minor amputation | 1.6% | 3.3% | 3.3% |
| All-cause mortality | 8.2% | 9.8% | 9.8% |
| MALE | 4.9% | 9.8% | 16.4% |
MALE, major adverse limb events.
Multivariate logistic regression analysis was conducted to identify the predictors of 1-year all-cause mortality and MALEs (Table 5). In predicting 1-year all-cause mortality, significant variables of age, Hb drop, and procedure success were used. Among these variables, only Hb drop remained a significant predictor of 1-year all-cause mortality (p = 0.022). On the other hand, in predicting 1-year MALEs, significant variables of ALI duration and history of PAD were included. Among these variables, only a history of PAD was a significant predictor of 1-year MALEs (p = 0.040).
Table 5. Predictors of 1 year all-cause mortality & MALE using multiple logistic regression analysis.
| Significant parameters | Multivariate analysis (all-cause mortality) | p value |
| In univariate analysis | Hazard ratio (95% confidence interval) | |
| Age | 1.163 (0.967-1.399) | 0.110 |
| Hemoglobin drop | 4.337 (1.231-15.274) | 0.022 |
| Procedure success | 0.338 (0.045-2.556) | 0.293 |
| Significant parameters | Multivariate analysis (major adverse limb events) | p value |
| In univariate analysis | Hazard ratio (95% confidence interval) | |
| ALI duration | 1.138 (0.973-1.332) | 0.106 |
| History of PAD | 4.385 (1.067-18.017) | 0.040 |
ALI, acute limb ischemia; MALE, major adverse limb events; PAD, peripheral artery disease.
DISCUSSION
In this study, we aimed to evaluate short- and mid-term clinical outcomes after using RMT in Taiwanese patients with ALI. There were several major findings. First, RMT achieved good clinical outcomes regarding both major and minor amputations and also short- and mid-term all-cause mortality and MALEs. Second, Hb drop during hospitalization was associated with increased 1-year all-cause mortality after multivariate analysis. Third, a history of PAD was associated with an increased 1-year MALE rate after multivariate analysis. Fourth, 14.8% of our cases had ALI in the upper extremities, and few studies have discussed the outcomes of ALI treated by RMT in the upper extremities.
RMT involves using a rotating and aspirating catheter that can remove the thrombus from the affected artery. The catheter’s rotational speed creates a low-pressure zone that facilitates the fragmentation and aspiration of the thrombus.9-15 The technique can be performed under local anesthesia, and the procedure time can be significantly reduced after mechanical thrombectomy. RMT also has several advantages, such as lower risk of distal embolization and higher success rates.
According to the literature, complications are common among patients with ALI. Even with early revascularization, the 30-day amputation rates and mortality rates remain high, typically ranging from 10% to 15%, or even higher.18-20 Higashitani et al. reported the results of the Edo registry on 1-year limb outcomes and mortality in ALI patients undergoing revascularization. In their study, the 1-year rates of major amputation, all-cause mortality, and MALEs were 5.7%, 28.6%, and 40.0%, respectively.21 In contrast, clinical studies have reported relatively favorable outcomes with RMT for ALI,9-15 which is similar to our study. Another Taiwanese study also reported the use of RMT for ALI. Wang et al. compared the use of pharmaco-mechanical thrombolysis (PMT) and CDT for ALI,15 and used RMT as the mechanical thrombectomy device in their PMT group. However, 62.1% of patients in this group also used the EkoSonic endovascular system (EKOS). EKOS is a useful tool for local thrombolysis, as it releases ultrasonic waves to thin and separate fibrin strands and accelerate lytic dispersion deeper into the clot. Therefore, the outcomes in their study reflect the combined effect of Rotarex and EKOS devices, which is different from our study.
In addition, despite being developed many years ago, RMT is a relatively new technique in Taiwan. KMUH started using RMT in Taiwan in January 2016, and it has gradually gained popularity as a safe and effective treatment option for ALI. In our study of patients with ALI, relatively good outcomes were achieved in all cases at 30 days, 6 months, and 1 year, not only in terms of major and minor amputation, but also in terms of all-cause mortality and MALEs. However, despite its advantages, RMT is not without its limitations and potential complications, including the risk of vessel injury and Hb drop related to thrombectomy. Our experience at KMUH suggests that RMT should be avoided in relatively small arteries, such as those below the popliteal artery in the lower extremities and below the brachial artery in the upper extremities to avoid the possibility of vessel injury. Moreover, Hb drop is also an important issue after RMT, especially for patients combined with CDT treatment. The average major bleeding rate in our study during hospitalization was 9.8%. The patients who died also had a much higher major bleeding rate (66.7% vs. 3.6%) than the patients who survived. However, after analyzing the causes of 1-year mortality in our patients, we found that three patients died due to sepsis progression, two patients died due to reperfusion injury, and 1 patient died due to in-hospital cardiac arrest. Consequently, none of these deaths were due to major bleeding. Therefore, although Hb drop was an important predictor of 1-year mortality, it was not directly associated with mortality. In addition, a history of PAD was also found to be a significant predictor of MALEs. Because MALEs included amputation and re-intervention events in this study, it is reasonable that the patients with PAD may have had a higher risk of major adverse cardiac events and MALEs.22-25 Careful patient selection and operator experience are essential to minimize the risks and maximize the benefits of RMT.
Study limitations
There were some limitations to this study. First, the sample size was not very large. However, this is the first study to focus on RMT for ALI in Taiwan, and we evaluated both short- and mid-term clinical outcomes. Second, we did not compare RMT treatment with other novel thrombectomy strategies, such as Angiojet, Indigo System, or CDT treatment in this study.
New knowledge gained
This study is the first to focus on RMT for patients with ALI in Taiwan. The results showed good short- and mid-term outcomes for minor amputation, major amputation, all-cause mortality, and MALEs. In addition, we found that a drop in Hb levels was an important predictor of all-cause mortality, and that a history of PAD was an important predictor of MALEs (Central Illustration).
Central Illustration.
Short- and mid-term outcome of Rotarex mechanical thrombectomy for acute limb ischemia. ALI, acute limb ischemia; MALE, major adverse limb ischemia; PAD, peripheral artery disease; RMT, Rotarex mechanical thrombectomy.
CONCLUSIONS
RMT is a safe and effective treatment option for ALI, with high success rates and low risk of complications. Our results showed good short- and mid-term outcomes of RMT. In addition, we found that Hb drop may predict 1-year all-cause mortality, and that a history of PAD may predict 1-year MALEs in patients with ALI.
DECLARATION OF CONFLICT OF INTEREST
All the authors declare no conflict of interest.
Acknowledgments
Nil.
REFERENCES
- 1.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45 Suppl S:S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 2.Walker TG. Acute limb ischemia. Tech Vasc Interv Radiol. 2009;12:117–129. doi: 10.1053/j.tvir.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Kashyap VS, Gilani R, Bena JF, et al. Endovascular therapy for acute limb ischemia. J Vasc Surg. 2011;53:340–346. doi: 10.1016/j.jvs.2010.08.064. [DOI] [PubMed] [Google Scholar]
- 4.Hynes BG, Margey RJ, Ruggiero N, 2nd, et al. Endovascular management of acute limb ischemia. Ann Vasc Surg. 2012;26:110–124. doi: 10.1016/j.avsg.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Obara H, Matsubara K, Kitagawa Y. Acute limb ischemia. Ann Vasc Dis. 2018;11:443–448. doi: 10.3400/avd.ra.18-00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veenstra EB, van der Laan MJ, Zeebregts CJ, et al. A systematic review and meta-analysis of endovascular and surgical revascularization techniques in acute limb ischemia. J Vasc Surg. 2020;71:654–668 e3. doi: 10.1016/j.jvs.2019.05.031. [DOI] [PubMed] [Google Scholar]
- 7.Khan S, Hawkins BM. Acute limb ischemia interventions. Interv Cardiol Clin. 2020;9:221–228. doi: 10.1016/j.iccl.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 8.King EG, Farber A. What is the best treatment for acute limb ischemia? Adv Surg. 2022;56:287–304. doi: 10.1016/j.yasu.2022.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Stanek F, Ouhrabkova R, Prochazka D. Mechanical thrombectomy using the Rotarex catheter--safe and effective method in the treatment of peripheral arterial thromboembolic occlusions. Vasa. 2010;39:334–340. doi: 10.1024/0301-1526/a000058. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenberg M, Stahlhoff FW, Boese D. Endovascular treatment of acute limb ischemia and proximal deep vein thrombosis using rotational thrombectomy: a review of published literature. Cardiovasc Revasc Med. 2013;14:343–348. doi: 10.1016/j.carrev.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Freitas B, Steiner S, Bausback Y, et al. Rotarex mechanical debulking in acute and subacute arterial lesions. Angiology. 2017;68:233–241. doi: 10.1177/0003319716646682. [DOI] [PubMed] [Google Scholar]
- 12.Heller S, Lubanda JC, Varejka P, et al. Percutaneous mechanical thrombectomy using Rotarex(R) S device in acute limb ischemia in infrainguinal occlusions. Biomed Res Int. 2017;2017:2362769. doi: 10.1155/2017/2362769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bulvas M, Sommerova Z, Vanek I, Weiss J. Prospective single-arm trial of endovascular mechanical debulking as initial therapy in patients with acute and subacute lower limb ischemia: one-year outcomes. J Endovasc Ther. 2019;26:291–301. doi: 10.1177/1526602819840697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loffroy R, Falvo N, Galland C, et al. Percutaneous rotational mechanical atherectomy plus thrombectomy using Rotarex S device in patients with acute and subacute lower limb ischemia: a review of safety, efficacy, and outcomes. Front Cardiovasc Med. 2020;7:557420. doi: 10.3389/fcvm.2020.557420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang CC, Lu CR, Hsieh LC, et al. Comparison of pharmaco-mechanical thrombolysis and catheter-directed thrombolysis for treating thrombotic or embolic arterial occlusion of the lower limb. Int Angiol. 2022;41:292–302. doi: 10.23736/S0392-9590.22.04809-X. [DOI] [PubMed] [Google Scholar]
- 16.Bonaca MP, Bauersachs RM, Anand SS, et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med. 2020;382:1994–2004. doi: 10.1056/NEJMoa2000052. [DOI] [PubMed] [Google Scholar]
- 17.Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. doi: 10.1056/NEJMoa1709118. [DOI] [PubMed] [Google Scholar]
- 18.Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. The STILE trial. Ann Surg. 1994;220:251–266; discussion 266-8. doi: 10.1097/00000658-199409000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eliason JL, Wainess RM, Proctor MC, et al. A national and single institutional experience in the contemporary treatment of acute lower extremity ischemia. Ann Surg. 2003;238:382–389; discussion 389-90. doi: 10.1097/01.sla.0000086663.49670.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Earnshaw JJ, Whitman B, Foy C. National Audit of Thrombolysis for Acute Leg Ischemia (NATALI): clinical factors associated with early outcome. J Vasc Surg. 2004;39:1018–1025. doi: 10.1016/j.jvs.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Higashitani M, Anzai H, Mizuno A, et al. One-year limb outcome and mortality in patients undergoing revascularization therapy for acute limb ischemia: short-term results of the Edo registry. Cardiovasc Interv Ther. 2021;36:226–236. doi: 10.1007/s12928-020-00662-6. [DOI] [PubMed] [Google Scholar]
- 22.Hess CN, Norgren L, Ansel GM, et al. A structured review of antithrombotic therapy in peripheral artery disease with a focus on revascularization: a TASC (InterSociety Consensus for the Management of Peripheral Artery Disease) initiative. Circulation. 2017;135:2534–2555. doi: 10.1161/CIRCULATIONAHA.117.024469. [DOI] [PubMed] [Google Scholar]
- 23.Kaplovitch E, Eikelboom JW, Dyal L, et al. Rivaroxaban and aspirin in patients with symptomatic lower extremity peripheral artery disease: a subanalysis of the COMPASS randomized clinical trial. JAMA Cardiol. 2021;6:21–29. doi: 10.1001/jamacardio.2020.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JK, Hsieh IC, Su CH, et al. Referral, diagnosis, and pharmacological management of peripheral artery disease: perspectives from Taiwan. Acta Cardiol Sin. 2023;39:97–108. doi: 10.6515/ACS.202301_39(1).20220815A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu YW, Wang CY, Cheng NC, et al. 2024 TSOC/TSPS Joint Consensus: strategies for advanced vascular wound management in arterial and venous diseases. Acta Cardiol Sin. 2024;40:1–44. doi: 10.6515/ACS.202401_40(1).20231220A. [DOI] [PMC free article] [PubMed] [Google Scholar]