Abstract
Background
Overweight is associated with dysrhythmia and sudden cardiac death, while sodium glucose co-transporter-2 inhibitors (SGLT2-is) have been shown to possess cardioprotective effects in patients with hyperglycemia.
Objectives
The aim of this study was to investigate the impact of overweight on cardiac remodeling and the potential effect of SGLT2-is.
Methods
Twenty-four rabbits were randomized into 4 groups: controls (Group 1), high-fat diet (HFD) (Group 2), controls treated with empagliflozin (Group 3), and HFD treated with empagliflozin (Group 4). All rabbits underwent electrophysiologic studies and ventricular tachycardia/ventricular fibrillation (VF) inducibility tests (maximal output with shortest 1:1 cycle length pacing). Atrial and ventricular myocardium were harvested for Western blot and Trichrome staining.
Results
Among all groups, Group 2 had the longest atrial effective refractory periods (ERPs) in both left and right atria, as well as the longest ventricular ERPs in both left and right ventricles. VF inducibility was highest in Group 2. The degree of fibrosis in both atria and ventricles was most severe in Group 2 and similar to that in Group 4. Enhanced calcium handling protein (CaV 1.2) expressions were noted in Group 2 compared to those in Group 1 and Group 3, respectively, and returned to baseline in Group 4.
Conclusions
Overweight causes atrial and ventricular remodeling with prolongation of effective refractoriness, increased vulnerability to VF induction, upregulation of calcium handling proteins, and advanced fibrosis. Empagliflozin attenuates these remodeling effects, leading to decreased cardiac arrhythmogenicity and a reduced risk of sudden cardiac death.
Keywords: Empagliflozin, Overweight, Remodeling, SGLT2 inhibitors, Ventricular fibrillation
Abbreviations
AF, Atrial fibrillation
DM, Diabetes mellitus
EMPA, Empagliflozin
ERP, Effective refractory period
HFD, High-fat diet
LA, Left atrium
LV, Left ventricle
RA, Right atrium
RV, Right ventricle
SGLT2-is, Sodium-glucose cotransporter-2 inhibitors
TBST, Tris-buffered saline with 0.1% Tween® 20 detergent
VF, Ventricular fibrillation
WOV, Window of vulnerability
INTRODUCTION
It is widely recognized that being overweight is associated with cardiac remodeling and an increased risk of sudden death.1 Ventricular arrhythmias, including ventricular tachycardia and ventricular fibrillation (VF), are among the most common causes of sudden death.2 In a randomized controlled trial, Pietrasik et al. found that patients who were overweight had a higher risk of appropriate implantable cardioverter-defibrillator therapy.3 Additionally, a study on obese rabbits reported that they developed ventricular remodeling and increased arrhythmogenesis.4 These findings suggest that being overweight is a risk factor for life-threatening ventricular arrhythmias. Furthermore, being overweight has been strongly associated with atrial arrhythmias. In an animal study, Abed et al. found that being overweight was linked to atrial electrical and structural remodeling.5 Our previous study also found that being overweight could induce cardiac remodeling through enhanced sympathetic activity.6
Recently, sodium-glucose cotransporter-2 inhibitors (SGLT2-is) have been shown to have cardioprotective effects. In the DECLARE-TIMI 58 clinical trial, dapagliflozin was shown to lower the incidence of cardiac death or hospitalization for heart failure in patients with type 2 diabetes mellitus (DM).7 This cardioprotective effect was also observed in non-diabetic patients. In the Dapagliflozin And Prevention of Adverse-outcomes in Heart Failure study, dapagliflozin was found to reduce the risk of worsening heart failure or cardiac death in patients without type 2 DM.8 Furthermore, SGLT2-is have been reported to be an emerging "magic bullet" for controlling overweight.9 However, the mechanisms underlying the cardioprotective effects of SGLT2-is are not yet fully understood. Therefore, in this study, we aimed to investigate the effects of being overweight on atrial and ventricular electrophysiology, and whether SGLT2-is can protect the heart from cardiac remodeling and ventricular arrhythmia in rabbits fed a high-fat diet (HFD).
METHODS
Animal preparation
This study was approved by the Institutional Animal Care and Use Committee of Taipei Veterans General Hospital (IACUC: 2021-034, 2021-116) and was conducted in strict accordance with the US National Institutes of Health or European Commission guidelines. All surgical procedures were performed under general anesthesia. All efforts were made to minimize suffering of the animals.
A total of 24 male New Zealand white rabbits, aged 12 weeks and with a baseline body weight of 2-3 kg, were obtained from the Shulin Breeding facility in New Taipei, Taiwan. The rabbits were individually housed in a temperature-regulated room with a 12:12 hours light-dark cycle.
Twelve rabbits were assigned to the control group and were provided with unlimited high-fiber food (Laboratory Rabbit Diet 5326 HF, PMI, Richmond, IN, USA) containing 22.6% fiber and 6.6% fat, along with access to water. In order to induce progressive weight gain, the other 12 rabbits were fed a HFD consisting of normal food supplemented with an additional 15% fat (10% corn oil and 5% lard) for a period of 3 months, as described in a previous study.6
Experimental procedures
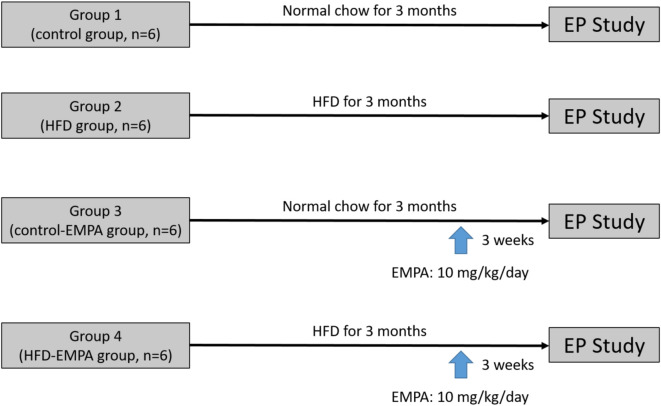
The rabbits were classified into 4 groups (Figure 1):
Figure 1.
Graphic illustration of the experiment protocol. EMPA, empagliflozin; EP, electrophysiological; HFD, high-fat diet.
Group 1 (control group, n = 6): this group did not receive a HFD or empagliflozin (EMPA).
Group 2 (HFD group, n = 6): this group received a HFD but did not receive EMPA. After administration of the HFD, the rabbits were observed for a period of 3 months before conducting the electrophysiology study.
Group 3 (control-EMPA group, n = 6): this group were fed with a control diet for 3 months and received EMPA (10 mg/kg/day, Boehringer Ingelheim pharmaceutical company, Ingelheim, Germany) orally for 3 weeks before the electrophysiology study.
Group 4 (HFD-EMPA group, n = 6): this group were fed with a HFD for 3 months and received EMPA (10 mg/kg/day, Boehringer Ingelheim pharmaceutical company, Ingelheim, Germany) orally for 3 weeks before the electrophysiology study.
General preparation
As described in our previous study,6 on the day of the electrophysiology study, a warming blanket was used to maintain the body temperature of the rabbits throughout the experiment. An intravenous line was set up to administer medication and fluids as needed. To induce anesthesia, all rabbits received an intraperitoneal injection of sodium pentobarbital at a dose of 40 mg/kg. They were then placed in a supine position and artificially ventilated using a cuffed endotracheal tube connected to a constant volume cycled respirator. The rabbits were ventilated with room air or oxygen as necessary to ensure adequate respiration. Next, a mid-thoracotomy was performed. The muscle tissue was carefully dissected, checking for any bleeding during the procedure, until the mediastinal space was exposed. Subsequently, the pericardium, the membrane surrounding the heart, was incised to completely expose the entire heart.
Conventional electrophysiology study and induction of ventricular fibrillation
As described in our previous study,6 programmed electrical stimulation was conducted in all rabbits using a custom-made stimulator (Model 5325, Medtronic, Ltd, Minneapolis, MN, USA). The stimulator delivered constant-current pulses of 1-ms duration. Initially, the effective refractory periods (ERPs) of the bi-atria and bi-ventricles were measured. Programmed electrical stimulation was delivered to the distal tips of the multielectrode catheter, which was sutured epicardially at the bi-atria and bi-ventricles. Stimulation was performed at 2 and 10 times the pacing threshold. The stimulation protocol involved delivering eight consecutive stimuli (S1-S1) with a cycle length of 300 ms, followed by a premature stimulus (S1-S2). The S1-S2 interval was initially decreased from 200 ms in decrements of 10 ms until the ERP was achieved. Afterward, the interval was decreased in decrements of 1 ms. The shortest S1-S2 interval that resulted in a propagated response in the atria and ventricles was determined as the atrial and ventricular ERPs, respectively. During ERP measurements, if atrial fibrillation (AF) was induced by decremental S1-S2 stimulation, the longest and shortest S1-S2 intervals at which AF was induced were determined. The difference between these two intervals was defined as the window of vulnerability (WOV), which was a quantitative measure of AF inducibility.10 Subsequently, burst S1S1 ventricular pacing was performed, with the cycle length decreasing from 250 ms until a failure of 1:1 ventricular capture occurred. VF inducibility was defined as the occurrence of sustained VF (> 30 s) induced by the shortest ventricular pacing at a 1:1 cycle length, typically between 100 and 150 ms. Burst S1S1 pacing was conducted for 10 s, with a maximum pacing output of 10 mA during 10 inductions, as previously described.11 A total of 10 VF induction tests were performed. The percentage of VF induction with induced VF was compared among the four groups. If VF was sustained for more than 30 s, external electrical defibrillation was performed to restore the heart to sinus rhythm. Therefore, all VF induced were longer than 30 s and external electrical defibrillation was applied.
Tissue collection
As described in our previous study,6 at the conclusion of the electrophysiology study, the rabbits were euthanized by exsanguination under anesthesia. The atrial and ventricular myocardia were then carefully collected. Tissue samples were obtained from the bi-atrial and bi-ventricular free walls of the heart. To ensure the samples were devoid of blood, they underwent thorough flushing. Subsequently, to preserve the tissue samples for further analysis, a fixation procedure was promptly initiated. The samples were immersed in two different solutions: 20% formalin and liquid nitrogen. Formalin fixation aids in preserving the cellular structure of the tissue, while liquid nitrogen freezing prevents sample degradation and preserves proteins and other biomolecules.
Western blot analysis
As described in our previous study,11 the collected myocardium samples underwent homogenization and were then loaded onto a 10% SDS-polyacrylamide gel for protein separation. The separated proteins were transferred to nitrocellulose filters. To prevent non-specific binding, the nitrocellulose filters were blocked with 5% albumin in tris-buffered saline with 0.1% Tween® 20 detergent (TBST) buffer for 30 minutes at room temperature. Next, the nitrocellulose membranes were exposed to primary antibodies overnight in a solution containing 2% albumin with TBST at 4 °C. Afterward, any excess primary antibodies were washed away from the nitrocellulose membranes with three 10-minute washes using TBS-T buffer. Subsequently, the membranes were incubated with the electrochemiluminescence anti-rabbit IgG fragment in TBS-T. Following another three rounds of 10-minute washes in TBS-T, the bound antibodies were detected using the Western Blotting Detection System. The expressions of the following ionic channel proteins were then analyzed: the α-subunit of cardiac sodium channels (Nav1.5, ASC-005, Alomone Labs, Jerusalem, Israel), cardiac calcium channels (CaV1.2, 21774-1-AP, Proteintech, IL, USA), inward-rectifier potassium ion channel (Kir2.1, 19965-1-AP, Proteintech, IL, USA), sodium/calcium-exchanger (NCX, N216, Merck, Darmstadt, Germany), cardiac ryanodine receptor-calcium release channel (P2808-RyR2, ab59225, Abcam, Cambridge, UK), and sarcoplasmic reticulum calcium ATPase (SERCA2, MA3-910, Thermo Fisher Scientific, Waltham, MA, USA). Twenty micrograms of protein was loaded per lane, and each was run on separate gels with a single glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Tissue fibrosis quantification
As described in our previous study,6 the collected bi-atrial and bi-ventricular tissues underwent dehydration through a series of ethanol washes, starting with 70% ethanol and progressing to 80%, 90%, and finally 100% ethanol. Subsequently, the tissues were embedded in paraffin. To analyze the tissue sections, slices were cut parallel to the plane of the atrioventricular annulus. These sections encompassed both the epicardial and endocardial aspects of the myocardium. The Masson’s trichrome staining technique was used, resulting in the visualization of fibrotic areas in blue, enriched with collagen, while cellular elements appeared red. Images of the Masson’s trichrome-stained myocardial sections were captured, and the collagen areas within the ventricles and atria were quantified as percentages of the total ventricular and atrial myocardial areas, respectively. This quantification was performed using an Image-Pro Plus 6.0 image analyzer (Media Cybernetics). By assessing the extent of collagen deposition, the results provided an estimation of the progression of fibrosis within the examined myocardial regions.
Statistical analysis
Continuous data were expressed as the mean value ± standard error, while categorical data were presented as absolute values and percentages. To compare variables among the different groups, the Kruskal-Wallis test was used. A p value of less than 0.05 was considered statistically significant. In addition, the Wilcoxon signed-rank test was used to assess whether there were any significant differences in body weight between baseline and 3-month time points. The statistical analysis was carried out using SPSS version 22.0 (IBM Inc., Armonk, NY).
RESULTS
Baseline body weight
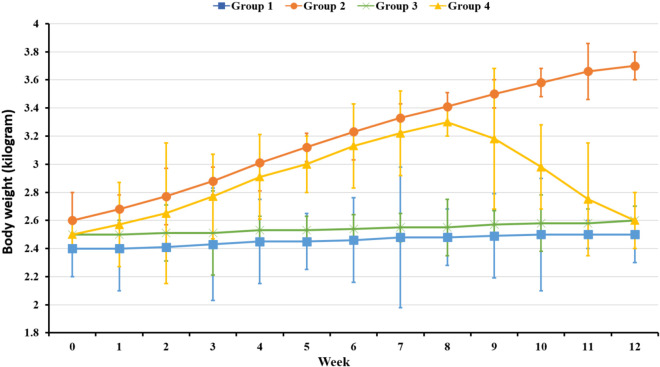
The baseline body weights were similar in all study groups. After 3 months of a HFD, the mean weight in Group 2 was higher than that in Group 1 (3.7 ± 0.2 kg vs. 2.5 ± 0.1 kg, p < 0.01). Prior to empagliflozin treatment, the mean weight in Group 4 was comparable to that in Group 2; however, after 3 weeks of empagliflozin treatment, the mean weight in Group 4 was significantly decreased compared to that in Group 2 (3.7 ± 0.2 kg vs. 2.6 ± 0.2 kg, p < 0.01), and was similar to that in Group 1 and Group 3 (Figure 2).
Figure 2.
Changes of body weights among 4 groups.
Electrophysiology study and AF/VF induction
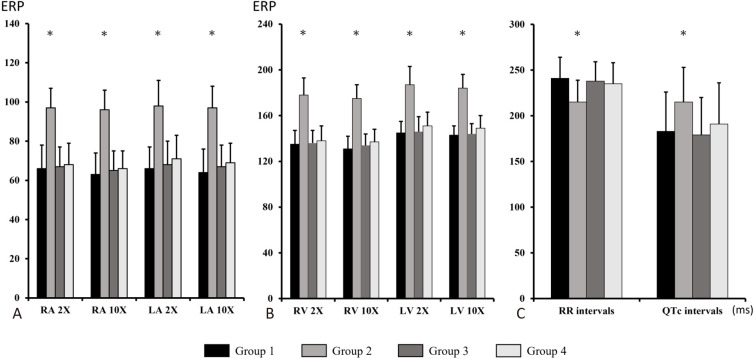
Figure 3 shows the atrial and ventricular ERPs and electrocardiographic data in all four groups. In Group 2, the ERPs were longer in both atria compared to those in Group 1 and Group 3. In Group 4, after treatment with empagliflozin, the ERPs in both atria were similar to those in Group 1 and Group 3 (Figure 3A). In Group 2, the ventricular ERPs were longer in the left ventricle (LV) compared to those in Group 1 and Group 3. In Group 4, after treatment with empagliflozin, the ERPs in both ventricles were similar to those in Group 1 and Group 3 (Figure 3B). Likewise, the RR intervals were shorter and the QTc intervals were longer in Group 2 compared to those in Group 1 and Group 3, and they were normal in Group 4 (Figure 3C).
Figure 3.
Electrophysiological studies of the study groups. (A) ERPs of RA and LA at 2 and 10 times the pacing threshold. (B) ERPs of RV and LV at 2 and 10 times the pacing threshold. (C) Electrocardiographic changes. ERP, effective refractory period; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle. * p < 0.05 compared to Group 1, 3, 4 respectively.
Among the 4 groups, AF inducibility was highest in Group 2. In Group 4, after treatment with empagliflozin, the mean WOV was markedly reduced compared to that in Group 2 (13 ± 6% vs. 53 ± 12, p < 0.05), and was similar to that in Group 1 and Group 3 (Figure 4A).
Figure 4.
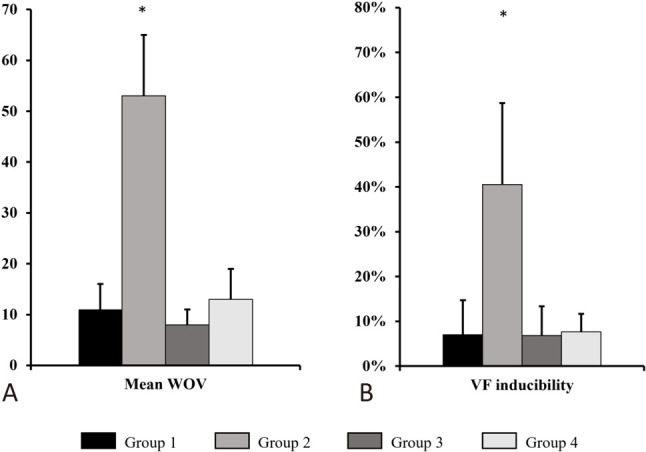
AF/VF inducibility of the study groups. (A) AF inducibility results (represented as WOV) in 4 groups. (B) VF inducibility results in 4 groups. AF, atrial fibrillation; VF, ventricular fibrillation; WOV, window of vulnerability. * p < 0.05 compared to Group 1, 3, 4 respectively.
Among the 4 groups, VF inducibility was highest in Group 2. In Group 4, after treatment with empagliflozin, VF inducibility was markedly decreased compared to that in Group 2 (8 ± 4% vs. 41 ± 18%, p < 0.05), and was similar to that in Group 1 and Group 3 (Figure 4B).
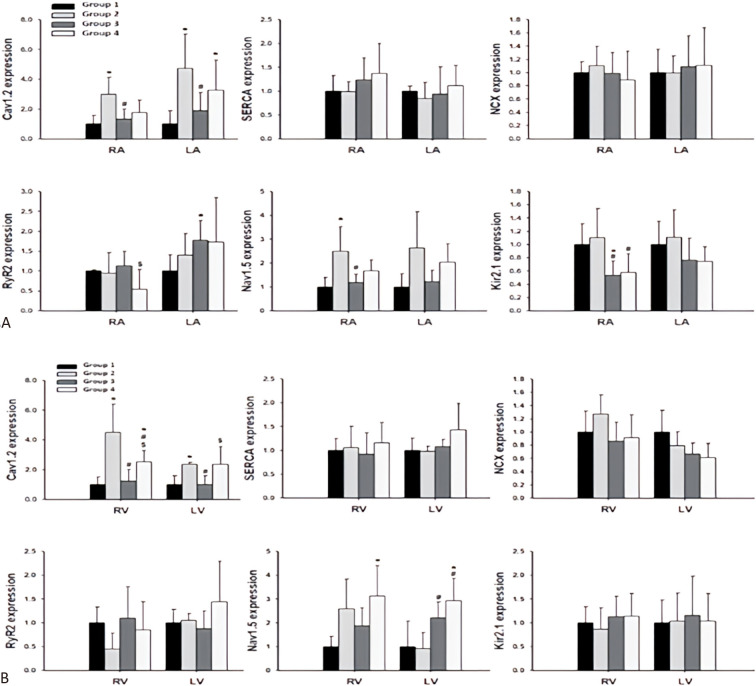
Expressions of cardiac ionic channels after empagliflozin treatment
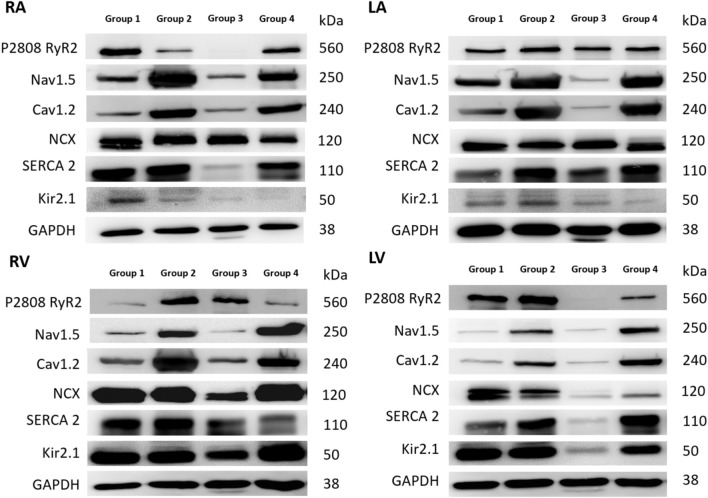
In Group 2, the expression of CaV 1.2 was upregulated in both atria compared to Group 1. In the RA, upregulation of NaV 1.5 was also observed in Group 2 compared to Group 1 (Figure 5 and 6A). Similarly, in both ventricles, upregulation of CaV 1.2 was observed in Group 2 compared to Group 1 (Figure 5 and 6B).
Figure 5.
The representative Western blots of the protein sample extracts in 4 groups. Cav1.2, subunit of L-type calcium current [ICaL]; Kir2.1, subunit of inward rectifier potassium current [IK1]; LA, left atrium; Nav1.5, α1-subunit of Na channel; NCX, Na+/Ca2+ exchanger; RA, right atrium; RyR, ryanodine receptor Ca2+ release channel; SERCA, sarcoplasmic reticulum Ca2+-ATPase.
Figure 6.
Immunoblots of ion channel proteins expression levels of both atria and ventricles in 4 groups. (A) Cardiac ionic channels expression among 4 groups in RA and LA. (B) Cardiac ionic channels expression among 4 groups in RV and LV. Each value represents the mean ± SD of all experiments. Cav1.2, subunit of L-type calcium current [ICaL]; Kir2.1, subunit of inward rectifier potassium current [IK1]; LA, left atrium; LV, left ventricle; Nav1.5, α1-subunit of Na channel; NCX, Na+/Ca2+ exchanger; RA, right atrium; RV, right ventricle; RyR, ryanodine receptor Ca2+ release channel; SD, standard deviation; SERCA, sarcoplasmic reticulum Ca2+-ATPase. * p < 0.05 compared to Group 1; # p < 0.05 compared to Group 2; $ p < 0.05 compared to Group 3.
In Group 3, the upregulated expressions of CaV 1.2, NaV 1.5 and Kir 2.1 in the RA decreased after treatment with empagliflozin (Figure 5 and 6A), and the upregulated expression of CaV 1.2 in both the LV and RV decreased after treatment with empagliflozin (Figure 5 and 6B). Compared with Group 1, RyR2 expression was increased in the LA (Figure 6A).
In Group 4, the upregulated expression CaV 1.2 in the RV decreased after treatment with empagliflozin (Figure 5 and 6B). Compared with Group 3, RyR2 expression was decreased in the RA (Figure 6A).
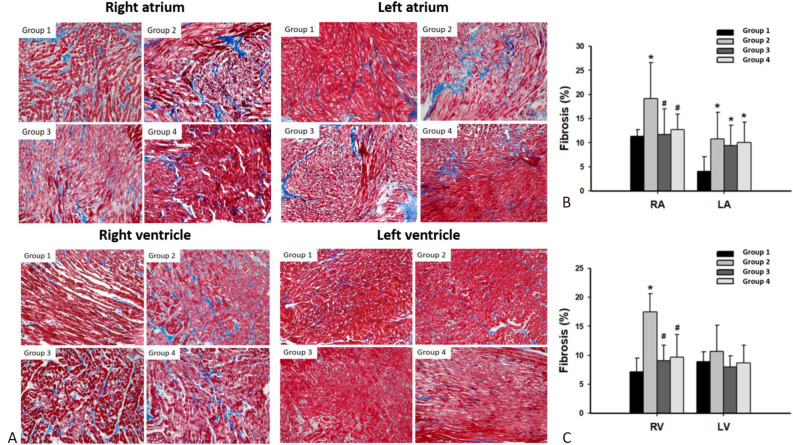
Tissue fibrosis in atrium and ventricle after empagliflozin treatment
In both the RA and LA, Group 2 showed the highest degree of fibrosis. However, after empagliflozin treatment, fibrosis was less abundant in both Group 3 and Group 4 relative to Group 2 in the RA (Figures 7A and 7B). In the RV, Group 2 also had the highest degree of fibrosis; however, after empagliflozin treatment, fibrosis was less abundant in both Group 3 and Group 4 relative to Group 2 in the RV (Figures 7A and 7C).
Figure 7.
Evaluation of fibrosis analyzed by Masson’s trichrome stain. (A) Representation of Masson’s trichrome stain of both atria and ventricles in 4 groups. (B) Quantification of fibrosis among 4 groups in RA and LA. (C) Quantification of fibrosis among 4 groups in RV and LV. Each value represents the mean ± SD of all experiments. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; SD, standard deviation;. * p < 0.05 compared to Group 1; # p < 0.05 compared to Group 2.
DISCUSSION
Main findings
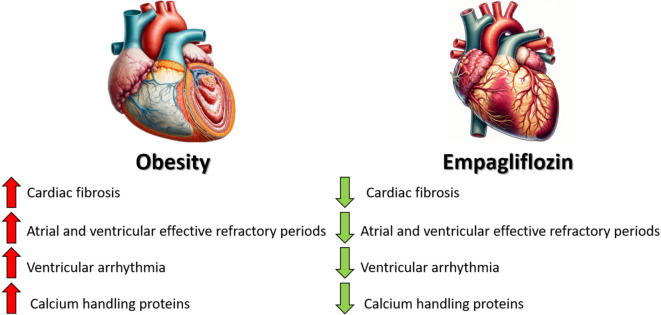
The results of this study showed that overweight rabbits had atrial and ventricular remodeling, as evidenced by prolonged ERPs, upregulation of calcium handling proteins, and myocardial fibrosis. Moreover, the overweight rabbits had higher VF inducibility. However, treatment with empagliflozin led to a reduction in atrial and ventricular refractoriness, VF inducibility, calcium handling protein expressions, and fibrosis. Therefore, empagliflozin may reduce cardiac remodeling, resulting in decreased risks of dysrhythmia and sudden cardiac death.
Cardiac structural and electrical remodeling associated with overweight
Overweight has been identified as a risk factor for cardiac remodeling, which can lead to malignant tachyarrhythmia and sudden cardiac death.3 Consistent with our previous study,6 the present study demonstrated electrical remodeling including prolonged ventricular ERPs and higher VF inducibility in overweight rabbits. These findings suggest that overweight leads to structural and electrical remodeling caused by upregulation of calcium handling proteins and advanced fibrosis.
Several studies have reported that overweight is highly associated with chronic inflammation, as shown by increased fibrosis.12-14 Activation of the innate immune system can induce the subsequent enrollment of immune cells, such as macrophages and T cells, leading to extensive fibrosis.15,16 Our previous study also suggested that overweight-related adipocytes could directly cause cardiac remodeling by modifying electrophysiological properties and ion channels.17 Inflammation and fibrosis induced by these adipocytes could further enhance arrhythmogenesis in cardiomyocytes.18 In the present study, we found that the overweight rabbits had more ventricular and atrial fibrosis than controls, which suggests that overweight leads to advanced structural remodeling and is consistent with previous findings.
Effect of empagliflozin on cardiac remodeling associated with overweight
The cardioprotective effects of empagliflozin have been extensively investigated. Previous literature19,20 has shown that SGLT2-is reduce cytoplasmic sodium concentrations in cardiomyocytes. The reduced intracellular sodium concentration diminishes the driving force for the sodium-calcium exchanger, thereby reducing intracellular calcium overload. Xu et al. demonstrated that empagliflozin can reverse the progression of overweight in mice fed with a HFD by enhancing energy expenditure, reducing oxidative stress, inflammation, and fibrosis.21 Similarly, Sun et al. found that empagliflozin could reduce myocardial hypertrophy and improve cardiac function through regulating cardiac Sesn2-mediated AMPK-mTOR signaling and preserving cardiac redox homeostasis.22 These studies suggest that empagliflozin exerts cardioprotective effects by reducing inflammation, fibrosis, and structural cardiac remodeling, which could be the possible mechanisms by which SGLT2-is reduce fibrosis in the atria and ventricles.
In our study, we observed that the overweight rabbits had upregulated expressions of calcium handling proteins including CaV 1.2 and NaV 1.5, and that these changes could be reversed by treatment with empagliflozin. Of note, the degree of decrease in the expression of RyR2 after empagliflozin treatment was more prominent in the overweight rabbits. This is in accordance with a previous study in which empagliflozin was shown to inhibit activation of Ca2+/calmodulin-dependent protein kinase type-II (CaMKII) and further decrease RyR2, especially in diabetic fatty animal models.23 Moreover, we demonstrated that empagliflozin not only decreased cardiac fibrosis but also reduced the upregulation of calcium handling proteins, altered cardiac refractoriness, and decreased VF inducibility. In other words, our study further supports the notion that empagliflozin can significantly decrease both cardiac structural and electrical remodeling. Although we did not directly verify the correlation with inflammation, we showed that empagliflozin can prevent cardiac structural and electrical remodeling through body weight reduction and anti-fibrosis mechanisms.
Limitations
There were several limitations to this study. First, we did not measure action potential duration since it might require techniques such as optical mapping. In addition, we only checked the protein expressions of ion channels that could affect ERPs. The lack of a functional study at the cellular level (such as patch clamp) or optical mapping is the main limitation of this study. We plan to conduct future investigations on this issue. Future works on RNA sequencing, RNA and/or protein lysate would be beneficial to evaluate the expressions of these genes and their causal relationships with changes in electrophysiology or inducibility of VF.
New knowledge gained
Overweight contributes to heart remodeling, increasing the risk of arrhythmias and sudden cardiac death through prolonged electrical recovery periods, altered calcium protein levels, and heart fibrosis. Empagliflozin can counteract these changes, thereby reducing the incidence of dysrhythmias and the risk of sudden death (Central Illustration).
Central Illustration.
Overweight-induced cardiac remodeling increases arrhythmia risk, but empagliflozin treatment can mitigate these effects and lower sudden cardiac death risk.
CONCLUSIONS
Overweight can lead to remodeling of both the atria and ventricles, resulting in prolonged ERPs, increased susceptibility to ventricular fibrillation induction, upregulation of calcium handling proteins, and myocardial fibrosis. Empagliflozin can reverse this remodeling, leading to a decrease in dysrhythmias and sudden cardiac death risk. This action of empagliflozin highlights its potential in managing overweight-related cardiac issues.
DECLARATION OF CONFLICT OF INTEREST
All the authors declare no conflict of interest.
Acknowledgments
The present work was supported by the Taipei Veterans General Hospital (V108C-073, V108-031, V109C-001, V109C-005, V110C-024, V110-014) Ministry of Science and Technology (MOST 107-2314-B-010-057, MOST 108-2314-B-010-051-MY3, MOST 108-2811-B-010-542, MOST 109-2811-B-010-529, MOST 109-2314-B-075A-011-MY3).
REFERENCES
- 1.Bharati S, Lev M. Cardiac conduction system involvement in sudden death of obese young people. Am Heart J. 1995;129:273–281. doi: 10.1016/0002-8703(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 2.Weaver WD, Hill D, Fahrenbruch CE, et al. Use of the automatic external defibrillator in the management of out-of-hospital cardiac arrest. N Engl J Med. 1988;319:661–666. doi: 10.1056/NEJM198809153191101. [DOI] [PubMed] [Google Scholar]
- 3.Pietrasik G, Goldenberg I, McNitt S, et al. Obesity as a risk factor for sustained ventricular tachyarrhythmias in MADIT II patients. J Cardiovasc Electrophysiol. 2007;18:181–184. doi: 10.1111/j.1540-8167.2006.00680.x. [DOI] [PubMed] [Google Scholar]
- 4.Zarzoso M, Mironov S, Guerrero-Serna G, et al. Ventricular remodelling in rabbits with sustained high-fat diet. Acta Physiol. 2014;211:36–47. doi: 10.1111/apha.12185. [DOI] [PubMed] [Google Scholar]
- 5.Abed HS, Samuel CS, Lau DH, et al. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm. 2013;10:90–100. doi: 10.1016/j.hrthm.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 6.Yamada S, Lo LW, Chou YH, et al. Renal denervation ameliorates the risk of ventricular fibrillation in overweight and heart failure. Europace. 2020;22:657–666. doi: 10.1093/europace/euz335. [DOI] [PubMed] [Google Scholar]
- 7.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 8.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 9.Pereira MJ, Eriksson JW. Emerging role of SGLT-2 inhibitors for the treatment of obesity. Drugs. 2019;79:219–230. doi: 10.1007/s40265-019-1057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu Z, Nie L, He B, et al. Increase in vulnerability of atrial fibrillation in an acute intermittent hypoxia model: importance of autonomic imbalance. Auton Neurosci. 2013;177:148–153. doi: 10.1016/j.autneu.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Yamada S, Lo LW, Chou YH, et al. Beneficial effect of renal denervation on ventricular premature complex induced cardiomyopathy. J Am Heart Assoc. 2017;6:e004479. doi: 10.1161/JAHA.116.004479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 13.Kitade H, Sawamoto K, Nagashimada M, et al. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes. 2012;61:1680–1690. doi: 10.2337/db11-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei Y, Kim TJ, Peng DH, et al. Fibroblast-specific inhibition of TGF-β1 signaling attenuates lung and tumor fibrosis. J Clin Invest. 2017;127:3675–3688. doi: 10.1172/JCI94624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu H, Ghosh S, Perrard XD, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 17.Lin YK, Chen YC, Chen JH, et al. Adipocytes modulate the electrophysiology of atrial myocytes: implications in obesity-induced atrial fibrillation. Basic Res Cardiol. 2012;107:293. doi: 10.1007/s00395-012-0293-1. [DOI] [PubMed] [Google Scholar]
- 18.Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12:230–243. doi: 10.1038/nrcardio.2015.2. [DOI] [PubMed] [Google Scholar]
- 19.Philippaert K, Kalyaanamoorthy S, Fatehi M, et al. Cardiac late sodium channel current is a molecular target for the sodium/glucose cotransporter 2 inhibitor empagliflozin. Circulation. 2021;143:2188–2204. doi: 10.1161/CIRCULATIONAHA.121.053350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arow M, Waldman M, Yadin D, et al. Sodium-glucose cotransporter 2 inhibitor dapagliflozin attenuates diabetic cardiomyopathy. Cardiovasc Diabetol. 2020;19:7. doi: 10.1186/s12933-019-0980-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu L, Nagata N, Chen G, et al. Empagliflozin reverses obesity and insulin resistance through fat browning and alternative macrophage activation in mice fed a high-fat diet. BMJ Open Diabetes Res Care. 2019;7:e000783. doi: 10.1136/bmjdrc-2019-000783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun X, Han F, Lu Q, et al. Empagliflozin ameliorates obesity-related cardiac dysfunction by regulating sestrin2-mediated AMPK-mTOR signaling and redox homeostasis in high-fat diet-induced obese mice. Diabetes. 2020;69:1292–1305. doi: 10.2337/db19-0991. [DOI] [PubMed] [Google Scholar]
- 23.Gao J, Xue G, Zhan G, et al. Benefits of SGLT2 inhibitors in arrhythmias. Front Cardiovasc Med. 2022;9:1011429. doi: 10.3389/fcvm.2022.1011429. [DOI] [PMC free article] [PubMed] [Google Scholar]