Abbreviations
ECG, Electrocardiogram
ICD, Implantable cardioverter-defibrillator
IKr, rapid K
OCTN2, Organic cation transporter novel family member 2
PCD, Primary carnitine deficiency
SLC22A5, Solute carrier family 22 member 5
VT, Ventricular tachycardia
INTRODUCTION
Carnitine, an essential quaternary amine facilitating the transport of long-chain fatty acids to the mitochondrial matrix, is predominantly derived from diet or biosynthesized from lysine and methionine.1 Carnitine can be transported into cells by organic cation transporter novel family member 2 (OCTN2)/solute carrier family 22 member 5 (SLC22A5), and defect of the transporters may lead to primary carnitine deficiency (PCD), an autosomal recessive disorder resulting from mutations in the SLC22A5 gene.2 PCD manifests across a spectrum of clinical presentations, from infancy decompensation to asymptomatic adults.3 Cardiomyopathy and arrhythmias, particularly short QT intervals and ventricular fibrillation, are prevalent cardiac manifestations of PCD.4 Here, we presented a case with mutations in SLC22A5 and manifestations of a short QT interval and idioventricular fibrillation.
CASE PRESENTATION
A 27-year-old previously healthy female has a disease history of idiopathic ventricular fibrillation, which led to the implantation of an external facility-placed implantable cardioverter-defibrillator (ICD) several years ago. She had a brother experiencing sudden cardiac arrest before. Subsequent syncope episodes related to defibrillation escalated in frequency. The initial electrocardiogram (ECG) displayed sinus rhythm with multiple interpolated ventricular premature complexes, an inverted T wave, and a short QT interval (Figure 1). A 24-hour ECG recorded sinus rhythm with 3062 multiform ventricular premature complexes and a short QT interval (QTc: 328 ms). The echocardiography revealed a structurally normal heart. Normal systolic function with left ventricular ejection fraction of 52% and normal diastolic function were found. In addition, no late gadolinium enhancement was detected by cardiac magnetic resonance image. Both examinations had no evidence of cardiomyopathy. An electrophysiological study confirmed idiopathic ventricular premature complexes with easily inducible polymorphic ventricular tachycardia (VT) originating from the right ventricle, accompanied by multiple episodes of fast VT. Therefore, the catheter ablation was performed targeting the moderate band of right ventricle. After the procedure, no ventricular premature complexes were detected anymore. The molecular genetic analysis showed a c.832C>T (NM_001308122.2) heterozygous variation and a c.51C>G (NM_001308122.2) heterozygous variation of the protein encoded by SLC22A5 gene (NP_001295051.1:p.Arg278Ter and p.Phe17Leu). The primary carnitine deficiency was identified by genetic testing with a low carnitine level (1.33 um, reference range: > 8.00 uM). We have checked the lipid profiles and there was no abnormal finding. Then, carnitine supplementation commenced at 6 g per day, divided into three doses. After two weeks, a follow-up ECG (Figure 2) revealed a normalized QT interval without inverted T waves and ventricular premature complexes.
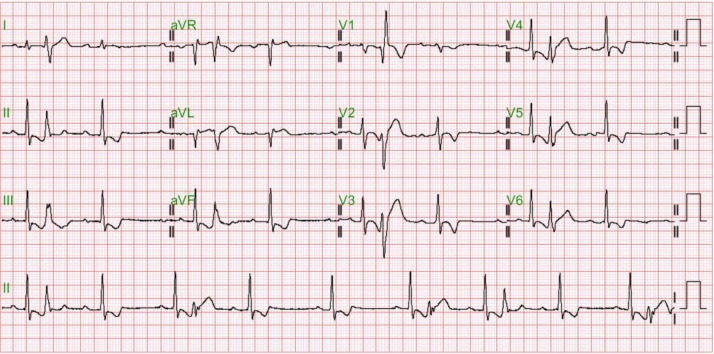
Figure 1.
Electrocardiogram was performed on March 17th, 2021. Sinus rhythm with multiple interpolated ventricular premature complexes and short QT interval is disclosed. Diffuse T-wave inversion is noticed. Positive T-wave in leads I and aVL. T-wave inversion in V3 is deeper than that in inferior leads.
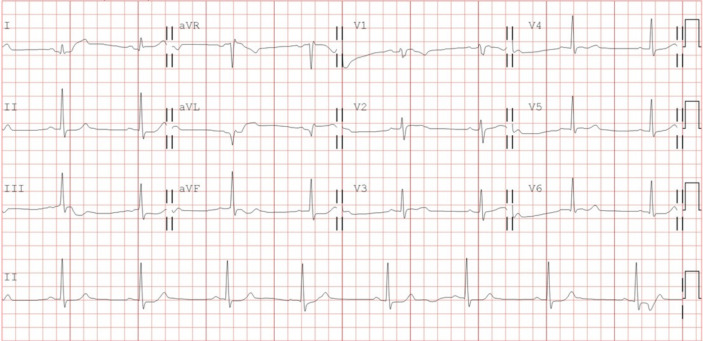
Figure 2.
Electrocardiogram was recorded after 2 weeks of carnitine supplement on April 26th, 2021. A normalized QT interval is displayed and there is no inverted T waves and ventricular premature complexes.
DISCUSSION
Long-chain fatty acids can penetrate the mitochondrial membrane by assembling the carnitine in the carnitine shutter and providing energy by β-oxidation in mitochondria.5 PCD is one of the genetic fatty acid disorders induced by reduced or absent OCTN2/SLC22A5 activity. Patients with impaired transporters have low serum carnitine levels and decreased carnitine accumulation inside cells, which results in a shortage of energy supplements. Because of the metabolic compensation, systemic PCD patients will have low ketone body hypoglycemia or even cranial neuropathy in early life. In childhood, skeletal or cardiac myopathy will be presented. Our patient didn’t have these clinical manifestations and clinically looks the same as a healthy person.
Most arrhythmogenic effects of fatty acid disorders are attributed to the accumulation of long-chain acylcarnitines, one of the products in the carnitine shutter.6 The made acylcarnitine can easily diffuse through the mitochondrial membrane and affect the cellular ion channels. In contrast, our case was detected as having a low level of carnitine due to the defects of OCTN2/SLC22A5. The cellular concentration of long-chain acetylcarnitine is theoretically very low or absent. In this situation, increased rapid K current (IKr) has ever been reported in the absence of long-chain acylcarnitine,4 which can explain the observed QT shortening in our patients.
Short QT syndrome is typically characterized by accelerated repolarization, which results in short QT intervals and the formation of tall peaked T waves on the ECG.7 The T-wave morphology in our case, who manifested a diffuse T-wave inversion, differs from the typical pattern. One of the causes of diffuse T-wave inversion is memory T-waves.8 Memory T-waves are T-wave inversions with normal sinus rhythm when abnormal ventricular activation transiently happens.9 The diagnostic criteria for differentiating memory T-waves from ischemic T-wave inversions are positive T in lead aVL, positive/isoelectric T in lead I, and precordial T-wave inversion larger than inferior T-wave inversion.8 In isolated myocytes with memory T-waves, endocardial IKr current increased, while epicardial IKr current decreased. The heterogeneous change resulted in the transmural gradient10 and probably supported the influence of an absence of long-chain acylcarnitine on IKr current in PCD patients.
Besides, studies utilizing a mouse model of carnitine deficiency induced by MET88(3-(2,2,2-trimethylhydrazinium) propionate), an OCTN2 competitor inhibitor, have demonstrated electrophysiological remodeling characterized by shortened ventricular repolarization time and increased arrhythmogenic potential, with frequent ventricular premature complex, sustained VT, and ventricular fibrillation.4 It is also compatible with the clinical manifestations of our patients.
Gradual normalization of ECG ensued following carnitine supplementation. Despite the return of the ECG to normal, the patient retained a heightened risk of sudden cardiac death, justifying the ongoing use of the ICD. The presence of short QT syndrome with inherited carnitine deficiency heightens the susceptibility to cardiac arrhythmias and unexpected sudden death.
ECG can diagnose short QT syndrome efficiently. Because the QT is defectively adapted to heart rate changes in patients with short QT syndrome, experts advise measuring the QT interval on ECG with a heart rate of 50-70 beats per minute. Patients with a corrected QT interval (QTc) ≤ 300 ms may augment the risk of sudden cardiac death, and quinidine should be considered to lengthen the QTc. Patients who have a history of cardiac arrest or documented VT are at increased risk of recurrent arrhythmic events, and the estimated risk of recurrent sudden cardiac arrest is 10% each year. Consequently, an ICD might be necessary for secondary prevention. If patients have recurrent ICD shocks, quinidine has been demonstrated to prevent further ICD discharges. Isoprenaline infusion, a treatment option that can increase heart rate, can be useful for controlling electrical storms or refractory ventricular fibrillation. This intervention is typically used in situations where other treatments have been ineffective or when there is a need to restore and maintain sinus rhythm. For patients with a QTc of 300-360 ms, the main predictor for recurrent arrhythmic events is a history of cardiac arrest or documented VT. Asymptomatic patients with a QTc of 300-360 ms can have no prophylactic medication, but regular follow-ups are crucial for them, ensuring their ongoing care and health monitoring. Genetic testing, a significant step in the diagnostic process, is strongly recommended for patients with suspected SQTS, including KCNH2, KCNQ1, KCNJ2, CACNA1C, CACNB2, CACNA2D1, and SLC4A3. For certain situations, such as suspecting PCD, SLC22A5 can also be considered.7
LEARNING POINTS
• Pathologic/likely pathologic mutations in SLC22A5 gene are probably associated with PCD.
• Patient with cardiac PCD can present a short QT interval.
• Carnitine supplementation can reverse the cardiac electric manifestations.
DECLARATION OF CONFLICT OF INTEREST
All the authors declare no conflict of interest.
Acknowledgments
No funding to declare.
REFERENCES
- 1.Longo N, Frigeni M, Pasquali M. Carnitine transport and fatty acid oxidation. Biochim Biophys Acta. 2016;1863:2422–2435. doi: 10.1016/j.bbamcr.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Hattab AW, Scaglia F. Disorders of carnitine biosynthesis and transport. Mol Genet Metab. 2015;116:107–112. doi: 10.1016/j.ymgme.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Magoulas PL, El-Hattab AW. Systemic primary carnitine deficiency: an overview of clinical manifestations, diagnosis, and management. Orphanet J Rare Dis. 2012;7:68. doi: 10.1186/1750-1172-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roussel J, Labarthe F, Thireau J, et al. Carnitine deficiency induces a short QT syndrome. Heart Rhythm. 2016;13:165–174. doi: 10.1016/j.hrthm.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 5.Juraszek B, Nałęcz KA. SLC22A5 (OCTN2) carnitine transporter-indispensable for cell metabolism, a jekyll and hyde of human cancer. Molecules. 2019;25:14. doi: 10.3390/molecules25010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnet D, Martin D, De Lonlay P, et al. Arrhythmias and conduction defects as presenting symptoms of fatty acid oxidation disorders in children. Circulation. 1999;100:2248–2253. doi: 10.1161/01.cir.100.22.2248. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz PJ, Ackerman MJ, Antzelevitch C, et al. Inherited cardiac arrhythmias. Nat Rev Dis Primers. 2020;6:58. doi: 10.1038/s41572-020-0188-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunaseelan R, Sasikumar M, Aswin K, et al. Memory T-waves, a rare cause of T-wave inversion in the emergency department. J Emerg Trauma Shock. 2020;13:312–316. doi: 10.4103/JETS.JETS_70_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obreztchikova MN, Patberg KW, Plotnikov AN, et al. I(Kr) contributes to the altered ventricular repolarization that determines long-term cardiac memory. Cardiovasc Res. 2006;71:88–96. doi: 10.1016/j.cardiores.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 10.Shvilkin A, Huang HD, Josephson ME. Cardiac memory: diagnostic tool in the making. Circ Arrhythm Electrophysiol. 2015;8:475–482. doi: 10.1161/CIRCEP.115.002778. [DOI] [PubMed] [Google Scholar]