Abstract
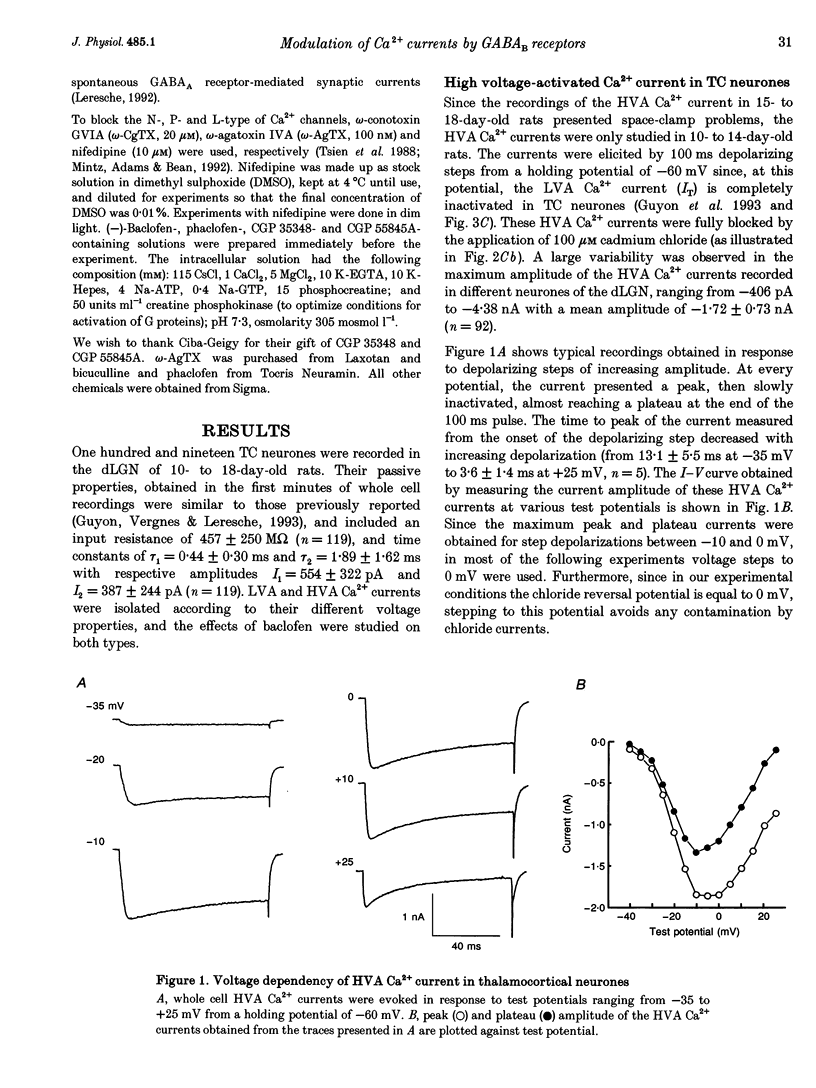
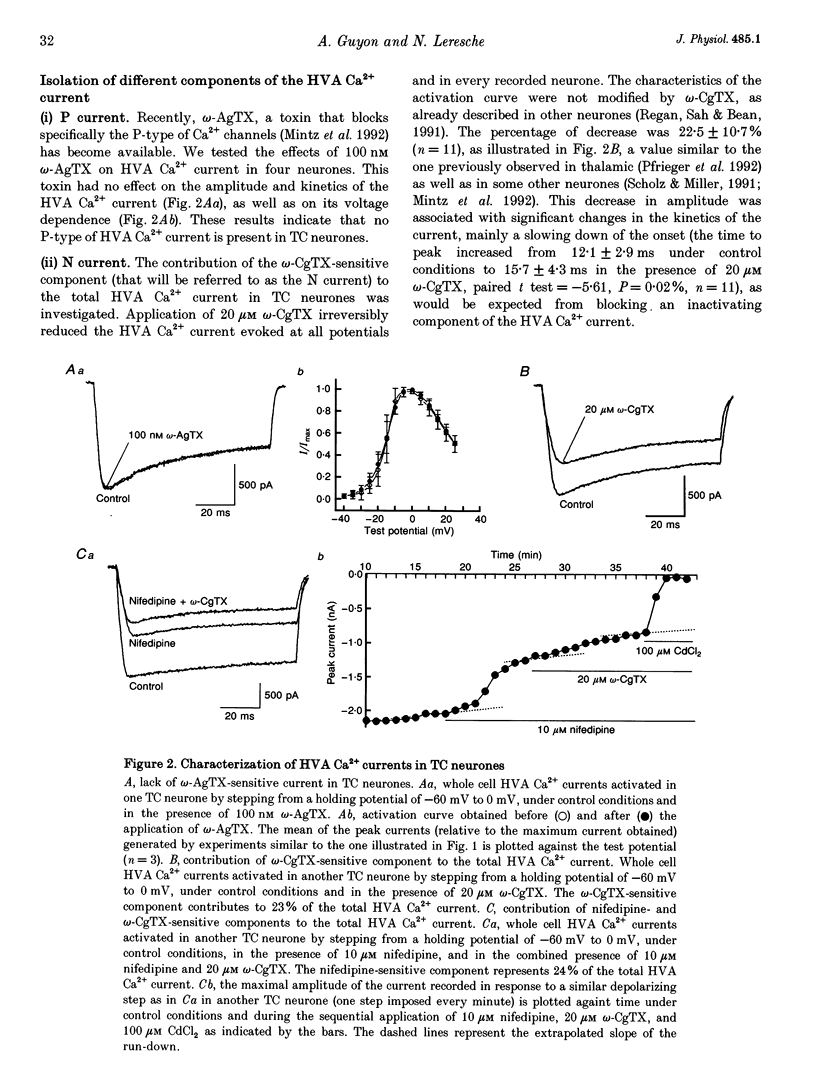
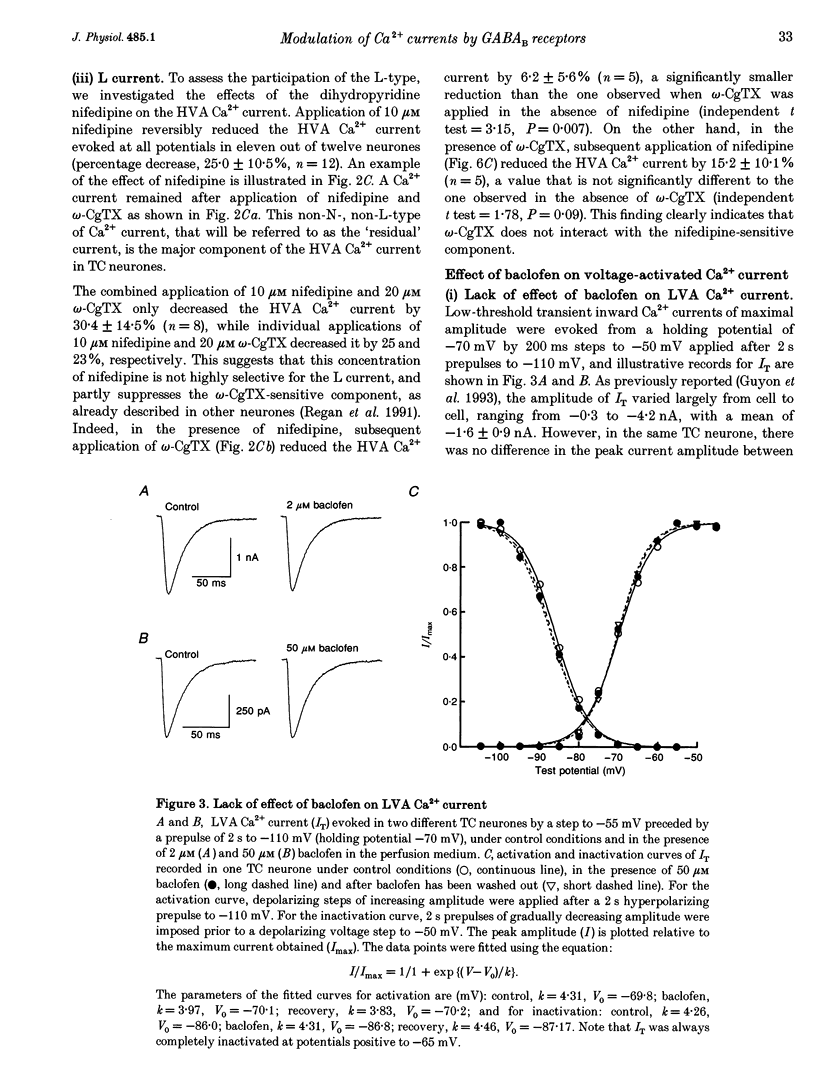
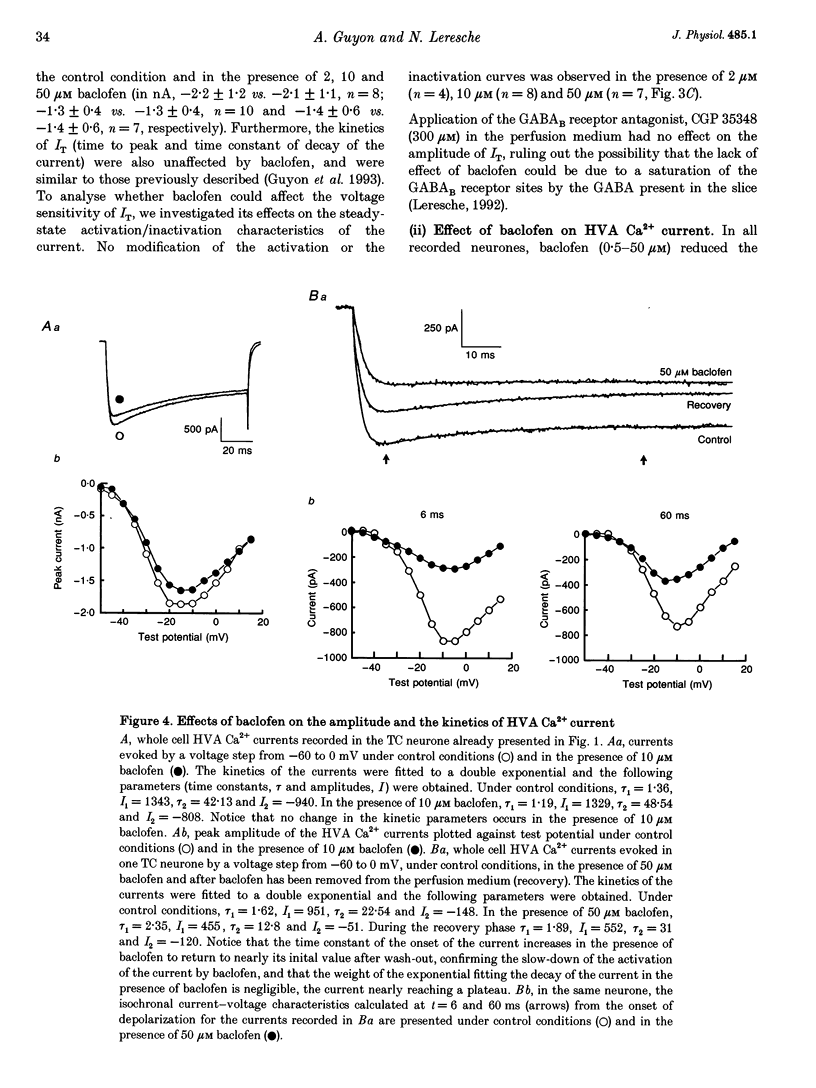
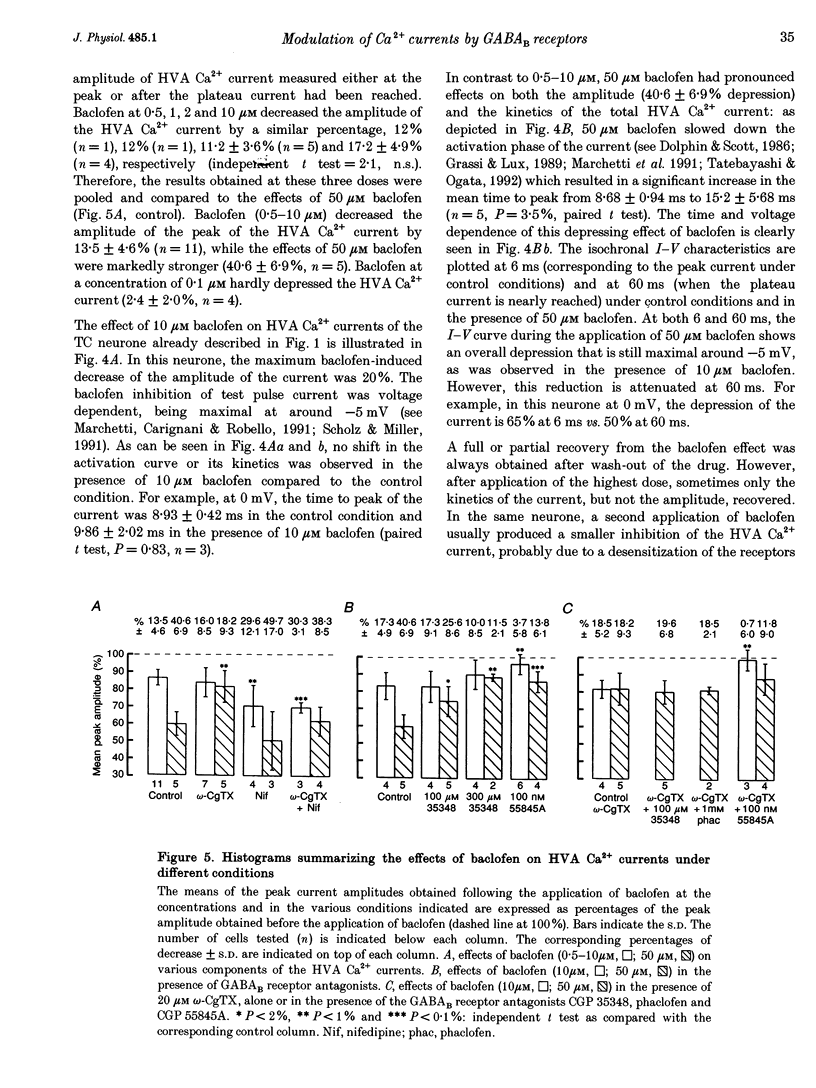
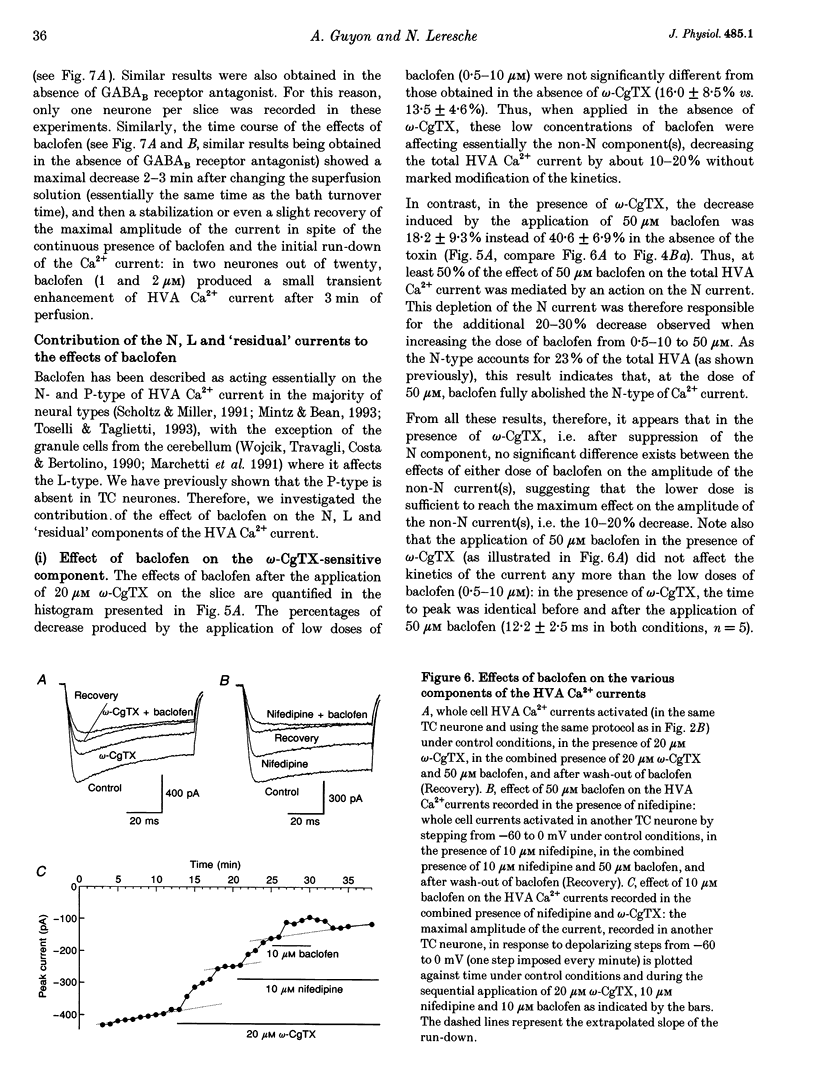
1. The effects of the GABAB receptor agonist baclofen on the voltage-dependent Ca2+ currents were studied in rat thalamocortical neurones with the use of whole cell voltage-clamp recordings in brain slices. 2. The contribution of N-, L- and P-types of Ca2+ channels to the total high voltage-activated Ca2+ (HVA Ca2+) current was assessed by the use of omega-conotoxin, nifedipine and omega-agatoxin IVA, respectively. No P-type current could be detected. Thus, the HVA Ca2+ current contained an N- and an L-type current (23 and 15% of the total current, respectively) and a residual current, which will be referred to as the 'R' component. 3. Baclofen (1-50 microM) had no effect on the low voltage-activated (LVA) Ca2+ current (IT). 4. At low concentrations (0.5-10 microM), baclofen decreased the HVA Ca2+ currents by about 10-20% without a marked modification on the kinetics, whereas 50 microM baclofen decreased the HVA Ca2+ currents by about 40% with a pronounced slowing down of the kinetics. 5. The 10-20% decrease of the total HVA Ca2+ currents produced by the low concentrations of baclofen occurred as the result of a 30% block of the 'R' component. The additional decrease observed with the dose of 50 microM was due to a full block of the N-type current. The L-type was unaffected by baclofen. 6. The effect of baclofen on the total HVA Ca2+ current was partially blocked by GABAB receptor antagonists indicating that it occurred through stimulation of GABAB receptors. 7. The effect of baclofen on the N-type current was abolished by CGP 35348 (100 microM) and CGP 55845A (100 nM). The effect on the 'R' component was also antagonized by CGP 55845A (100 nM) although with a lower potency, but was not blocked by CGP 35348 (100 microM). 8. We conclude that the effects of baclofen on the various components of the HVA Ca2+ currents occur through different types of GABAB receptors. One receptor has a high affinity for baclofen (i.e. saturated by concentrations as low as 0.5 microM), is insensitive to CGP 35348, is coupled to the 'R' component and is responsible for a maximum 20% decrease in the total HVA Ca2+ current. The other receptor has a lower affinity for baclofen (i.e. affected by a concentration of 50 microM), is sensitive to CGP 35348, is coupled to the N-type Ca2+ current and is responsible for the additional 20-30% decrease in the HVA Ca2+ current observed with 50 microM baclofen.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
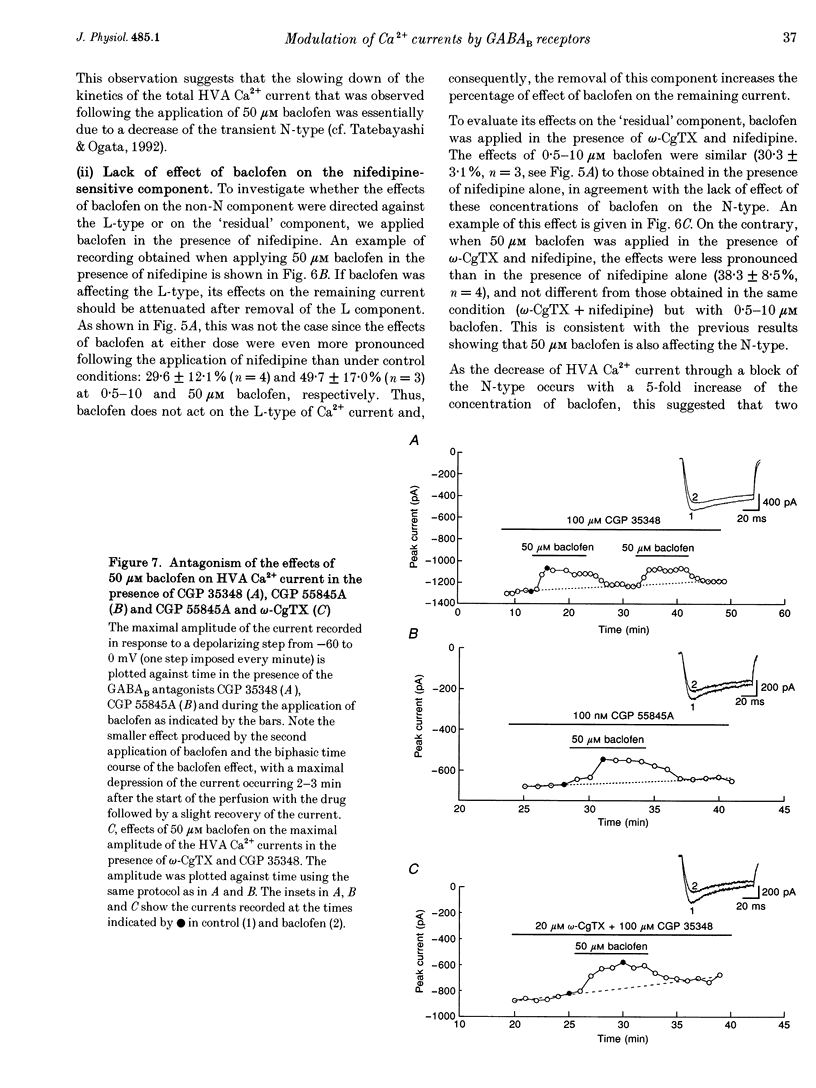
- Bonanno G., Raiteri M. Multiple GABAB receptors. Trends Pharmacol Sci. 1993 Jul;14(7):259–261. doi: 10.1016/0165-6147(93)90124-3. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Hudson A. L., Price G. W. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987 Feb;20(2):365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Calabresi P., Mercuri N. B., De Murtas M., Bernardi G. Involvement of GABA systems in feedback regulation of glutamate-and GABA-mediated synaptic potentials in rat neostriatum. J Physiol. 1991;440:581–599. doi: 10.1113/jphysiol.1991.sp018726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell V., Berrow N., Dolphin A. C. GABAB receptor modulation of Ca2+ currents in rat sensory neurones by the G protein G(0): antisense oligonucleotide studies. J Physiol. 1993 Oct;470:1–11. doi: 10.1113/jphysiol.1993.sp019842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter D. A., Huguenard J. R., Prince D. A. Calcium currents in rat thalamocortical relay neurones: kinetic properties of the transient, low-threshold current. J Physiol. 1989 Jul;414:587–604. doi: 10.1113/jphysiol.1989.sp017705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V., Haby M., Jassik-Gerschenfeld D., Leresche N., Pirchio M. Cl- - and K+-dependent inhibitory postsynaptic potentials evoked by interneurones of the rat lateral geniculate nucleus. J Physiol. 1988 May;399:153–176. doi: 10.1113/jphysiol.1988.sp017073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisz R. A., Billard J. M., Zieglgänsberger W. Pre- and postsynaptic GABAB receptors of rat neocortical neurons differ in their pharmacological properties. Neurosci Lett. 1993 May 14;154(1-2):209–212. doi: 10.1016/0304-3940(93)90209-4. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C., Scott R. H. Inhibition of calcium currents in cultured rat dorsal root ganglion neurones by (-)-baclofen. Br J Pharmacol. 1986 May;88(1):213–220. doi: 10.1111/j.1476-5381.1986.tb09489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. A physiological role for GABAB receptors in the central nervous system. Nature. 1988 Mar 10;332(6160):156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. Pre- and postsynaptic GABAB receptors in the hippocampus have different pharmacological properties. Neuron. 1988 Sep;1(7):585–591. doi: 10.1016/0896-6273(88)90108-0. [DOI] [PubMed] [Google Scholar]
- Fraser D. D., MacVicar B. A. Low-threshold transient calcium current in rat hippocampal lacunosum-moleculare interneurons: kinetics and modulation by neurotransmitters. J Neurosci. 1991 Sep;11(9):2812–2820. doi: 10.1523/JNEUROSCI.11-09-02812.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi F., Lux H. D. Voltage-dependent GABA-induced modulation of calcium currents in chick sensory neurons. Neurosci Lett. 1989 Oct 23;105(1-2):113–119. doi: 10.1016/0304-3940(89)90021-9. [DOI] [PubMed] [Google Scholar]
- Green K. A., Cottrell G. A. Actions of baclofen on components of the Ca-current in rat and mouse DRG neurones in culture. Br J Pharmacol. 1988 May;94(1):235–245. doi: 10.1111/j.1476-5381.1988.tb11520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyon A., Vergnes M., Leresche N. Thalamic low threshold calcium current in a genetic model of absence epilepsy. Neuroreport. 1993 Sep 10;4(11):1231–1234. doi: 10.1097/00001756-199309000-00005. [DOI] [PubMed] [Google Scholar]
- Hernández-Cruz A., Pape H. C. Identification of two calcium currents in acutely dissociated neurons from the rat lateral geniculate nucleus. J Neurophysiol. 1989 Jun;61(6):1270–1283. doi: 10.1152/jn.1989.61.6.1270. [DOI] [PubMed] [Google Scholar]
- Hillman D., Chen S., Aung T. T., Cherksey B., Sugimori M., Llinás R. R. Localization of P-type calcium channels in the central nervous system. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7076–7080. doi: 10.1073/pnas.88.16.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhász G., Emri Z., Kékesi K. A., Salfay O., Crunelli V. Blockade of thalamic GABAB receptors decreases EEG synchronization. Neurosci Lett. 1994 May 19;172(1-2):155–158. doi: 10.1016/0304-3940(94)90685-8. [DOI] [PubMed] [Google Scholar]
- Leresche N. Synaptic Currents in Thalamo-cortical Neurons of the Rat Lateral Geniculate Nucleus. Eur J Neurosci. 1992;4(7):595–602. doi: 10.1111/j.1460-9568.1992.tb00168.x. [DOI] [PubMed] [Google Scholar]
- Marchetti C., Carignani C., Robello M. Voltage-dependent calcium currents in dissociated granule cells from rat cerebellum. Neuroscience. 1991;43(1):121–133. doi: 10.1016/0306-4522(91)90422-k. [DOI] [PubMed] [Google Scholar]
- Marescaux C., Vergnes M., Bernasconi R. GABAB receptor antagonists: potential new anti-absence drugs. J Neural Transm Suppl. 1992;35:179–188. doi: 10.1007/978-3-7091-9206-1_12. [DOI] [PubMed] [Google Scholar]
- Mintz I. M., Adams M. E., Bean B. P. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992 Jul;9(1):85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- Mintz I. M., Bean B. P. GABAB receptor inhibition of P-type Ca2+ channels in central neurons. Neuron. 1993 May;10(5):889–898. doi: 10.1016/0896-6273(93)90204-5. [DOI] [PubMed] [Google Scholar]
- Pape H. C., Budde T., Mager R., Kisvárday Z. F. Prevention of Ca(2+)-mediated action potentials in GABAergic local circuit neurones of rat thalamus by a transient K+ current. J Physiol. 1994 Aug 1;478(Pt 3):403–422. doi: 10.1113/jphysiol.1994.sp020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfrieger F. W., Veselovsky N. S., Gottmann K., Lux H. D. Pharmacological characterization of calcium currents and synaptic transmission between thalamic neurons in vitro. J Neurosci. 1992 Nov;12(11):4347–4357. doi: 10.1523/JNEUROSCI.12-11-04347.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potier B., Dutar P. Presynaptic inhibitory effect of baclofen on hippocampal inhibitory synaptic transmission involves a pertussis toxin-sensitive G-protein. Eur J Pharmacol. 1993 Feb 16;231(3):427–433. doi: 10.1016/0014-2999(93)90120-7. [DOI] [PubMed] [Google Scholar]
- Regan L. J., Sah D. W., Bean B. P. Ca2+ channels in rat central and peripheral neurons: high-threshold current resistant to dihydropyridine blockers and omega-conotoxin. Neuron. 1991 Feb;6(2):269–280. doi: 10.1016/0896-6273(91)90362-4. [DOI] [PubMed] [Google Scholar]
- Scholz K. P., Miller R. J. GABAB receptor-mediated inhibition of Ca2+ currents and synaptic transmission in cultured rat hippocampal neurones. J Physiol. 1991 Dec;444:669–686. doi: 10.1113/jphysiol.1991.sp018900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. H., Wootton J. F., Dolphin A. C. Modulation of neuronal T-type calcium channel currents by photoactivation of intracellular guanosine 5'-O(3-thio) triphosphate. Neuroscience. 1990;38(2):285–294. doi: 10.1016/0306-4522(90)90028-3. [DOI] [PubMed] [Google Scholar]
- Steriade M., Llinás R. R. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988 Jul;68(3):649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Momiyama A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993 Nov 11;366(6451):156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- Tatebayashi H., Ogata N. Kinetic analysis of the GABAB-mediated inhibition of the high-threshold Ca2+ current in cultured rat sensory neurones. J Physiol. 1992 Feb;447:391–407. doi: 10.1113/jphysiol.1992.sp019008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. M., Gähwiler B. H. Comparison of the actions of baclofen at pre- and postsynaptic receptors in the rat hippocampus in vitro. J Physiol. 1992;451:329–345. doi: 10.1113/jphysiol.1992.sp019167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toselli M., Taglietti V. Baclofen inhibits high-threshold calcium currents with two distinct modes in rat hippocampal neurons. Neurosci Lett. 1993 Dec 24;164(1-2):134–136. doi: 10.1016/0304-3940(93)90875-l. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Lipscombe D., Madison D. V., Bley K. R., Fox A. P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988 Oct;11(10):431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Williams S. R., Turner J. P., Crunelli V. Gamma-hydroxybutyrate promotes oscillatory activity of rat and cat thalamocortical neurons by a tonic GABAB, receptor-mediated hyperpolarization. Neuroscience. 1995 May;66(1):133–141. doi: 10.1016/0306-4522(94)00604-4. [DOI] [PubMed] [Google Scholar]
- Wojcik W. J., Travagli R. A., Costa E., Bertolino M. Baclofen inhibits with high affinity an L-type-like voltage-dependent calcium channel in cerebellar granule cell cultures. Neuropharmacology. 1990 Oct;29(10):969–972. doi: 10.1016/0028-3908(90)90150-p. [DOI] [PubMed] [Google Scholar]
- Zhu X. Z., Chuang D. M. Modulation of calcium uptake and D-aspartate release by GABAB receptors in cultured cerebellar granule cells. Eur J Pharmacol. 1987 Sep 23;141(3):401–408. doi: 10.1016/0014-2999(87)90557-7. [DOI] [PubMed] [Google Scholar]


