Abstract
Parkinson’s disease (PD) is a brain disorder in which neuronal cells responsible for the release of dopamine, a neurotransmitter that controls movement, are degenerated or impaired in the substantia nigra and basal ganglia. The disease typically affects people over the age of 5 and presents with a variety of motor and nonmotor dysfunctions, which are unique to each person. The impairment of the blood–brain barrier (BBB) and blood retinal barrier (BRB) due to age-related causes such as weakness of tight junctions or rare genetic factors allows several metabolic intermediates to reach and accumulate inside neurons such as Lewy bodies and α-synuclein, disrupting neuronal homeostasis and leading to genetic and epigenetic changes, e.g., damage to the DNA repair system. This perspective highlights the importance of blood barriers, such as the BBB and BRB, in the progression of PD, as the aggregation of Lewy bodies and α-synuclein disrupts neuronal homeostasis. Genetic and epigenetic factors, neuroinflammation, oxidative stress, and mitochondrial dysfunction play crucial roles in the progression of the disease. The implications of these findings are significant; identifying synaptic dysfunction could lead to earlier diagnosis and treatment, while developing targeted therapies focused on preserving synaptic function may slow or halt disease progression. Understanding the various genetic forms of PD could enable more personalized medicine approaches, and using patient-derived midbrain neurons for research may improve the accuracy of PD models due to the implications of an impaired BBB.
1. Introduction
Parkinson’s disease (PD) is a neurodegenerative disease that affects basal ganglia of the brain, causing impaired and uncontrollable movements. It is the second most common neurodegenerative disease after Alzheimer’s disease. PD is characterized by symptoms such as abnormal gait patterns, bradykinesia, changes in posture, and shortened strides.1 Vocal deficits, psychological disturbances, and loss of facial expression are also common signs of PD that have a significant effect on the quality of life. Other symptoms of Parkinson’s disease are depicted in Figure 1.2
Figure 1.
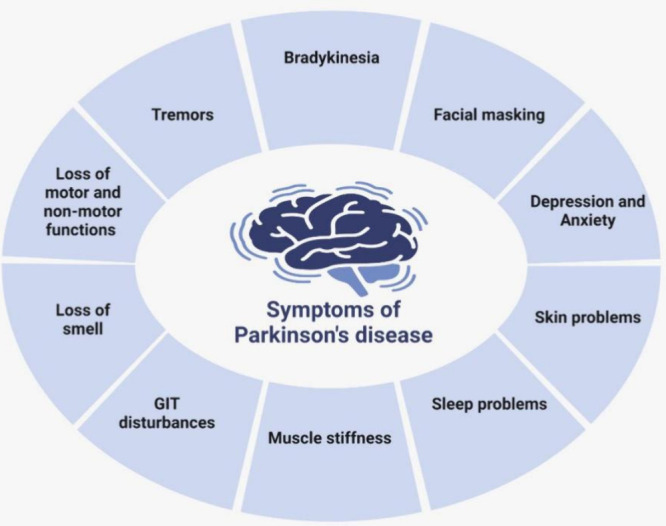
Symptoms of Parkinson’s disease, classifying them into motor and nonmotor symptoms. The loss of motor functions, tremors, muscle stiffness, loss of facial expression (facial masking), and bradykinesia (slow movement) are examples of motor symptoms. Nonmotor symptoms cover a variety of sensations, such as depression and anxiety, anosmia, dermatological issues, gastrointestinal (GIT) disruptions, and sleep difficulties. This detailed depiction showcases the intricate and diverse characteristics of Parkinson’s disease, underscoring the significance of a complete approach to diagnosing and treating the condition.
Parkinson’s disease is unique to each patient and has different symptoms, time of onset, and effects in each case. There are four types of Parkinson’s disease: primary parkinsonism, which occurs in people over the age of 60 due to the degeneration of dopamine producing brain cells and affects body movements only;3 dementia associated parkinsonism, where along with impaired motor function, dementia-like symptoms appear; atypical parkinsonism, which occurs in people under the age of 40 and carries its own unique symptoms;4 and multiple system atrophy, a rare type of parkinsonism that belongs to atypical parkinsonism and affects both motor as well as nonmotor functions such as heart rate, breathing, memory, and attention.5 Other atypical parkinsonism disorders such as dementia with Lewy bodies, progressive supra-nuclear palsy, and corticobasal degeneration are distinct forms of PD that require further research.6
The cause of Parkinson’s disease is unknown, but it is believed to be caused by a mix of hereditary factors such as mutations in genes like SNCA, LRRK2, and environmental factors such as exposure to toxins.7 PD is diagnosed clinically, as there is no specific test for the disease. Various diagnostic tools, including imaging techniques such as PET, SPECT, TCS, MRI, and thermal imaging, are used to accurately diagnose PD.8 In addition, a simple automated framework for PD detection has been proposed, which extracts geometric and texture features from facial visual information, providing a valuable tool for the clinical assessment and screening of PD.9 It is important to note that PD is a progressive disease that affects individuals, families, and society, and its prevalence is increasing worldwide.
2. Significance of Blood Barriers in Parkinson’s Disease
2.1. Role of Blood–Brain Barrier Impairment
The blood–brain barrier is a barricade between the brain and blood circulating in the body with its nutrients, pathogens, and other harmful substances. BBB plays a crucial role in the protection of the brain by maintaining homeostasis and providing protection against toxins and harmful substances by allowing only certain molecules to pass through. It is selectively permeable and allows lipid soluble molecules of size 400–600 Da only to pass through it.10
The blood–brain barrier is a crucial physiological barrier that separates the central nervous system (CNS) from peripheral circulation, protecting its microenvironment. Any type of dysfunction that may occur in the blood–brain barrier is directly associated with various neurological disorders.11 Understanding the regulation of BBB function under normal and inflammatory conditions is important. Recent advancements in the in vitro BBB models and cell-specific reporter mice have enhanced our understanding of the BBB dynamics. Strategies to control BBB structure and function have been suggested, and mathematical models have quantitatively correlated BBB anatomical structures with barrier functions. Chemical and physical stimuli can modulate BBB permeability12,13 (Figure 2).
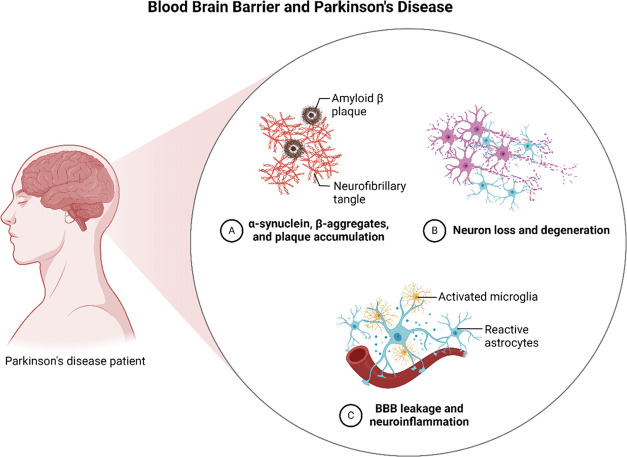
Figure 2.
Schematic diagram of the blood–brain barrier (BBB) and its function in Parkinson’s disease. The diagram illustrates the BBB (A) and its impairment in Parkinson’s disease (B), resulting in the buildup of harmful proteins such as amyloid-β plaques, neurofibrillary tangles, α-synuclein, and β-aggregates. Neuroinflammation is worsened by the presence of activated microglia and reactive astrocytes, leading to the loss and degeneration of neurons. The graphic emphasizes the crucial function of the blood–brain barrier (BBB) in obstructing the entry of hazardous compounds into the brain and the severe repercussions that occur when the BBB becomes permeable in Parkinson’s disease.
2.2. Role of Blood–Retina Barrier Impairment
The impaired visual ability in Parkinson’s disease is closely related to the blood–retinal barrier, which regulates the exchange of chemicals between the systemic circulation and retina.14 BRB is made up of two barriers, one inner barrier that is similar to BBB and is present in the inner retinal microvasculature and the other outer barrier, which is present at the retinal pigment epithelial cell layer.15 The outer barrier is the transportation of nutrients and solutes from blood vessels to the retina protecting it from blood-borne toxins. Both the BBB and the BRB are parts of the neurovascular unit and maintain the normal CNS by regulating the intake and transport of chemicals and solutes as well as steady intercellular interactions.16
BRB dysfunction is relevant to PD progression as the sleep disorders and depression cause sleep disturbances and autonomic dysfunction in early and prodromal PD.17 The aggregations of α–α-synuclein and degeneration of dopaminergic neuron, leading to motor and nonmotor symptoms, including sleep disorders, are major side effects of PD,18 which cause disturbances in BRB. The glymphatic system responsible for optimal sleep and removal of extracellular brain solutes, including α-synuclein, also faces deteriorations due to impaired BBB and enhances α-synuclein accumulation, which leads to dopamine neuron loss as well.19 All of these factors contribute to PD pathogenesis and progression.20
Disruption of the BRB, observed in various eye diseases, such as diabetic macular edema (DME), can lead to retinal edema and vision loss. Several mechanisms have been identified in the development and maintenance of the inner BRB, including the involvement of tight junction-related proteins and the Wnt signaling pathway.21,22 In DME, the breakdown of the BRB is associated with retinal inflammation and the dysregulation of angiogenic factors such as vascular endothelial growth factor (VEGF).23,24
2.3. Factors Causing Disruptions in the Blood–Brain Barrier
BBB dysfunction plays a significant role in the pathogenesis of Parkinson’s disease.25,26 Any damage to BBB allows immune cells and plasma proteins to enter the brain parenchyma, leading to neuro-inflammation.27 This neuro-inflammation, triggered by activated glial cells, contributes to neurotoxicity and neuronal dysfunction.28 The following section provides a detailed overview of a few factors that cause impairment of the BBB and accelerate the progression of PD.
2.4. Accumulation of Protein Aggregates in Brain Microenvironment
The impairment of the blood–brain barrier and reduced expression or weakness of tight junctions cause disruption in BBB integrity in Parkinson’s disease,29 which leads to increased vascular permeability and accumulation of Lewy bodies, Lewy body neutrites, and other proteins intermediates, e.g., oligomeric α-synuclein in neuronal cells in the basal ganglia, substantia nigra activated astrocytes.30 Neuronal Lewy bodies (LBs) and intrasynaptic aggregation of α-synuclein are hallmarks of brain lesions in PD.31 Mutations in genes involved in autophagy and lysosomal pathways, e.g., GBA and ATP13a2, have been linked to lysosomal dysfunction and increased α-synuclein levels.32 Abnormalities in ceramide metabolism, which are characteristic of PD, have been found in both brain tissue and the extracellular vesicle (EV) derived from cerebrospinal fluid (CSF). Mitochondrial dysfunction, influenced by mutations in PARK genes, has also been implicated in the formation of Lewy pathology and the aggregation of α-synuclein.33
Oligomeric α-synuclein plays a critical role in PD-associated BBB disruption, mediated by astrocyte-derived vascular endothelial growth factor A (VEGFA).34 α-Synuclein is responsible for nutrition and the supply of synaptic vesicles to neurons at presynaptic terminals. Their accumulation disrupts cellular homeostasis and causes neuronal death.35
High concentrations of homocysteine (Hcy) also induce changes in the homeostasis of the neuronal microenvironment. Homocysteine is an intermediate of methionine metabolism; imbalance in this metabolism can cause irreversible mental illness by damaging the DNA repair system and inducing epigenetic changes, which lead to apoptosis, oxidative stress, excitotoxicity, and other psychiatric disorders, e.g., bipolar disorder and schizophrenia.36 These conditions can be ameliorated by ingestion of vitamin B12 and folate, folic acid, and treatment with LCZ696, a novel antihypertensive agent with anti-inflammatory properties.37
Similarly, Sphingosine-1-phosphate receptor 2 (S1P2) mediates BBB disruption induced by lipopolysaccharide (LPS) accumulation, which causes systemic inflammation and reduction in tight junction protein expression. The inhibition of S1P2 attenuates neutrophil infiltration and downregulates occludin expression.38,39 Additionally, cabergoline, a dopamine D2 receptor agonist, protects BBB integrity against LPS-induced disruption by upregulating zonula occludens-1 (ZO-1).40 These findings suggest that BBB disruption is a common pathology in PD and can be influenced by factors such as α-synuclein, Hcy, S1P2, and inflammatory stimuli, highlighting potential therapeutic targets for maintaining BBB integrity in neurodegenerative diseases.
2.5. Dis-integrity of Tight Junctions
The breakdown of the blood–brain barrier can occur due to various mechanisms. One mechanism is the damage to the tight junctions, basement membrane, and adhesion molecules, which leads to the disruption of BBB integrity.41 Another mechanism is the increased transcytosis and weak tight junctions within autonomic nuclei, allowing the entrance of plasma constituents into the brain parenchyma.42
Furthermore, the age-related decline in BBB integrity may be associated with the manipulation of integrin function, as blocking β1 integrin has been shown to amplify hypoxia-induced vascular disruption and BBB breakdown.43 Understanding these mechanisms contributing to BBB breakdown is crucial for developing strategies to maintain the BBB integrity and prevent the entry of harmful substances into the brain.
3. Neuro-inflammation
Neuro-inflammation plays a significant role in the breakdown of the blood–brain barrier and the pathophysiology of Parkinson’s disease.27,44 It is caused by viral infection, stress, living conditions, autoimmune disorders or underlying inflammatory processes, e.g., activation of glial cells, such as microglia and astrocytes, and triggers the release of pro-inflammatory cytokines, leading to neurotoxicity and neuronal dysfunction.45 Inflammatory mediators released by perivascular cells, such as microglia and astrocytes, can disrupt the BBB and amplify neuro-inflammation.46 Additionally, infiltrating blood-borne immune cells, including neutrophils, monocytes, and T lymphocytes, increase BBB permeability and contribute to microvascular disorder and inflammation.47 The infiltration of these immune cells is not solely a consequence of BBB failure but is facilitated by various mediators produced by the neurovascular unit.48
Impairment of the BBB caused by any type of factor allows immune cells and plasma proteins to enter the brain parenchyma, amplifying neuro-inflammation.49 Increased permeability of the BBB to neurotoxic substances leads to PD pathogenesis,25,27,50 which causes neuro-inflammation, neurotoxicity, and neuronal dysfunction, which lead to the entry of immune cells or plasma proteins into the brain parenchyma.51 Additionally, chronic gut inflammation can lead to a leaky gut and systemic inflammation, which can further contribute to neuro-inflammation and neurodegeneration via BBB permeability.52 In PD, BBB disruption allows the trafficking of neurotoxic substances into the brain, contributing to the degeneration of dopaminergic neurons and the progression of the disease.53
In PD, abnormal aggregation of α-synuclein activates toll-like receptor 4 (TLR4), releasing pro-inflammatory cytokines and causing fatigue symptoms.54 Chronic peripheral inflammation and immune activation responses induce elevated levels of pro-inflammatory cytokines, which can cross the BBB and contribute to the occurrence of fatigue. Understanding the inflammatory mechanisms involved in BBB breakdown and neuro-inflammation can provide insights for the development of targeted treatments for PD. The knowledge of these underlying mechanisms of BBB impairment by inflammatory mediators released by perivascular cells is crucial for developing novel treatments for neuro-inflammatory diseases; e.g., modulating the gut microbiota through interventions like probiotics and fecal microbiota transplantation (FMT) may help restore gut dysbiosis, reduce inflammation, and potentially modulate the clinical phenotype of PD.
3.1. Oxidative Stress and Mitochondrial Dysfunction
Oxidative stress and mitochondrial dysfunction are seen in Parkinson’s disease and contribute to blood–brain barrier breakdown.55,56 Mitochondrial dysfunction, characterized by decreased ATP levels, disrupted mitochondrial morphology, and altered mitochondrial function, has been observed in neuronal cells exposed to particulate matter (PM) and lead (Pb).57,58 Additionally, PM exposure has been shown to increase oxidative stress and inflammatory cellular damage, leading to mitochondrial disruption and neurotoxic effects in neuronal cells.59
Mitochondrial alterations have also been observed in Rett syndrome (RTT), a neurodevelopmental disorder, and the absence of the MECP2 gene in RTT may lead to altered mitochondrial function and elevated levels of cellular oxidative stress.60 Additionally, mutations in the glucocerebrosidase (GBA) gene, which is associated with PD, can also contribute to mitochondrial dysfunction and altered lipid homeostasis. Furthermore, PGC-1α downregulation has been observed in animal and cellular models of neurodegenerative diseases, suggesting its role in the pathophysiology of PD.35 Therefore, the transcriptional coactivator peroxisome proliferator-activated receptor α-coactivator 1-alpha (PGC-1α) has been implicated in maintaining mitochondrial quality control and neuronal survival.
3.2. Other Associated Disorders That Enhance Progression of Parkinson’s Disease
The breakdown of the blood–brain barrier and dysfunction of tight junction proteins are important mechanisms under various neurological conditions. Implications of the blood–brain barrier (BBB) dysfunction in Parkinson’s disease (PD) pathogenesis include alterations in nutrient transport and waste clearance. The gut microbiota has been implicated in the pathogenesis of PD, and gut microbial dysbiosis may contribute to the loss of dopaminergic neurons through mitochondrial dysfunction.61 Similarly, endothelial dysfunction is considered an etiological factor in inflammatory bowel disease (IBD), and it can lead to structural and functional changes in the vascular endothelium, including alterations in nutrient transport and waste clearance.62 Inflammatory bowel disease (IBD) can cause loss-of-function mutations in the PTPN2 gene, which lead to increased intestinal permeability, that may also be relevant to BBB dysfunction in PD.27 Similarly, chronic inflammation in conditions like sleep apnea and Alzheimer’s disease can affect the integrity of the BBB, resulting in increased permeability and decreased expression of tight junction proteins.63
One study found that severe hypoglycemia leads to cognitive dysfunction in diabetic mice, and this is related to pericyte dysfunction and BBB destruction.64 Other health conditions, e.g., disruptions in circadian rhythm, ischemic stroke, and aneurysmal subarachnoid hemorrhage, lead to impaired BBB, weak tight junctions, inflammation, and oxidative stress in the brain65−67 (Figure 3).
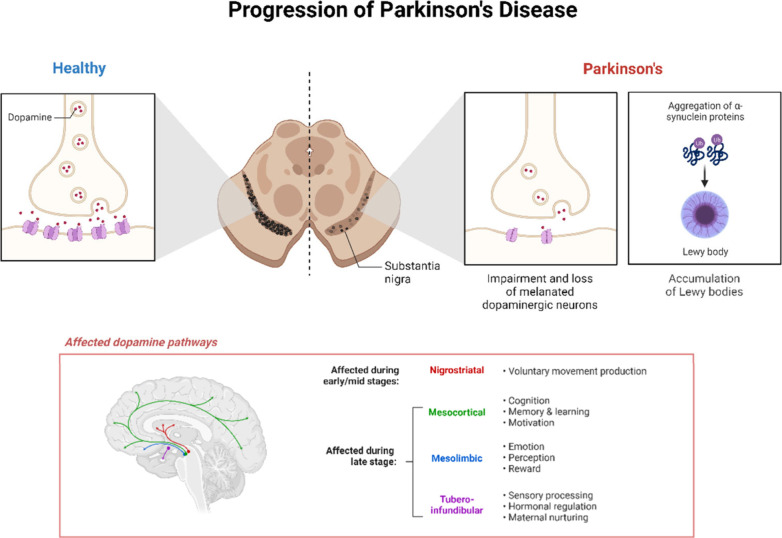
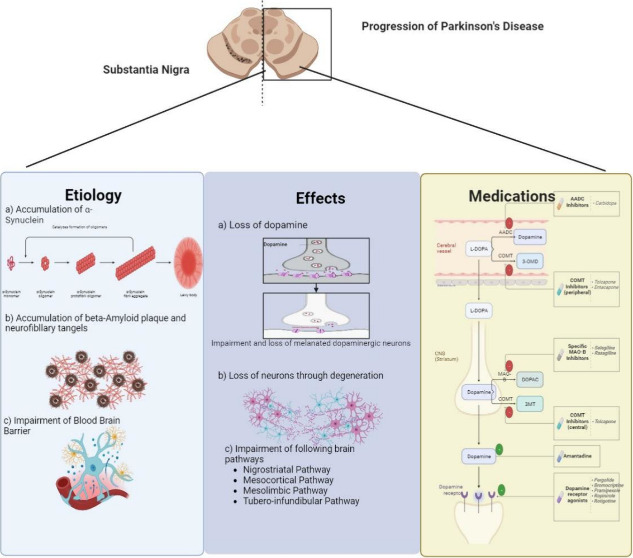
Figure 3.
Sequential development of neuropathological changes in Parkinson’s disease, starting from a healthy brain and leading to the appearance of the disease. The accumulation of dopamine and the creation of Lewy bodies cause deterioration of dopaminergic neurons in the substantia nigra. This disrupts both motor and nonmotor pathways, such as the nigrostriatal, mesocortical, mesolimbic, and tubero-infundibular pathways, ultimately leading to the appearance of symptoms.
4. Diagnosis of Parkinson’s Disease
The diagnostic techniques include noninvasive imaging techniques, e.g., magnetic resonance imaging techniques, such as dynamic contrast-enhanced and dynamic susceptibility contrast MRI, and can be used to assess BBB integrity by detecting leakage of contrast agents. Other MRI techniques target different aspects of the BBB and use endogenous markers like water and glucose as contrast media.68 These techniques provide insights into the structural and biochemical changes associated with PD and can help identify biomarkers of disease progression. Other imaging techniques like position emission tomography (PET), single-photon emission computed tomography (SPECT), transcranial sonography (TCS), and thermal imaging can also be used to diagnose PD and assess autonomic dysfunction.69 These imaging techniques offer a more accurate and sensitive diagnostic tool for PD, improving the management and treatment of the disease. Further research and development of these noninvasive imaging techniques hold promise for better understanding and treatment of PD.
Olfactory dysfunction, such as hyposmia, can be a sensitive marker for early diagnosis of PD and may provide insights into the underlying mechanism of Lewy neurodegenerative diseases.70 Similarly, deficits in short-term visual memory in patients with REM sleep behavior disorder (RBD) may serve as a marker for early PD and could be used in clinical trials for novel disease interventions.71
5. Medications
The medications for PD available at the moment cannot completely cure the disease and are used to provide relief from symptoms associated with the disease. There are two types of medications available for PD: dopaminergic drugs that work through dopamine pathways and nondopaminergic drugs, e.g., cholinergic inhibitors that work on other pathways. Both of these types function in separate ways and enhance dopamine levels in the neurons as described in Table 1.
Table 1. Medications of Parkinson’s Disease, Their Mode of Action, and Side Effects.
| medications | mode of action | side effects | refs |
|---|---|---|---|
| Levodopa | crosses BBB and converts into dopamine by DOPA decarboxylase enzyme | anxiety, hallucinations, dyskinesia, and gastrointestinal disturbances | (72) |
| decarboxylase inhibitors | administered in combination with levodopa and blocks the conversion of levodopa outside the CNS into dopamine to reduce unwanted effects, e.g., bradykinesia | hallucinations, dizziness, trouble sleeping, and nausea | (73) |
| (a) Carbidopa | |||
| dopamine antagonists | activate dopaminergic pathways by binding to dopamine receptors | nausea, dry mouth, hallucinations, sleepiness, constipation, edema, and development of impulsive control disorder | (74), (75) |
| (a) Ropinirole | |||
| (b) Pramipexole | |||
| (c) Rotigotine | |||
| monoamine oxidase (MAO) inhibitors | MAO inhibitors reduce the breakdown of dopamine and enhance availability of the neurotransmitter | joint pain, fatigue, insomnia, dizziness, nightmares, hallucinations, headache, and indigestion | (76), (77) |
| (a) Selegiline | |||
| (b) Azilect | |||
| catechol-O-methyl transferase (COMT) inhibitors | COMT inhibitors reduce dopamine breakdown and help in controlling motor movements | amplification in dyskinesias, hepatotoxicity, sleepiness, gastrointestinal disturbances, hallucinations, chest pain, and urine discoloration | (78), (79) |
| (a) Opicapone | |||
| (b) Entacapone | |||
| (c) Tolacapone | |||
| amantadine | the mode of action of amantadine is not clear; however, it is believed that it may act as a weak glutamate antagonist; it reduces muscle stiffness, tremors, and fatigue and levodopa-induced dyskinesia | sweating, agitation, headache, gastrointestinal disturbances, swelling in legs, hallucinations, blurred vision, loss of concentration, nightmares, and loss of appetite | (80), (81) |
| (a) Symmetrel | |||
| cholinergic inhibitors | reduce activity of acetylcholine in basal ganglia and increases dopamine uptake and storage in neurons; it improves rigidity and tremors and restores balance between dopamine and acetylcholine | cognitive impairment, confusion in elderly, hallucinations, blurred vision, and gastrointestinal disturbances | (82), (83) |
| (a) Procyclidine | |||
| (b) Orphenadrine | |||
| (c) Benztropine | |||
| (d) Trihexyphenidyl |
5.1. Emerging Therapeutic Strategies for Parkinson’s Disease
Current therapies for PD include dopaminergic therapy and symptom management. However, these therapies have limitations and fail to address nonmotor and nondopaminergic aspects of the disease. There is a critical need for dopaminergic therapies with minimal side effects, treatment for nonmotor symptoms, and disease-modifying therapies. While existing treatments focus on restoring dopaminergic function and managing symptoms, they do not target the underlying neurodegenerative processes. Novel therapies are being explored to address cell death and disease progression. Astrocytes, which play a role in maintaining neuronal environment and exert neuroprotective effects, have been identified as a potential target for neuroprotection in PD.84 Experimental approaches targeting astrocytes have shown promise in preventing dopaminergic neurodegeneration. However, more research is needed to fully understand the implications and potential of targeting BBB integrity as a therapeutic strategy in PD.
Other potential therapies include restoring barrier function in Parkinson’s disease; e.g., defects in epithelial membrane barriers in the gut and cerebral vasculature can increase vulnerability to external factors involved in PD pathogenesis.85 Enteric glial cells (EGCs) play a major role in PD-related gastrointestinal disturbances and central disease development.86 Impairment of barrier permeability triggers dysfunctions of EGCs and reactive gliosis, leading to neuro-inflammation and pathological changes in the enteric nervous system.
Novel therapy approaches for PD aim to target symptoms, halt pathology, minimize neuronal loss, and moderate disease progression. The pathogenesis of PD involves α-synuclein aggregation, oxidative stress, ferroptosis, mitochondrial dysfunction, neuro-inflammation, and gut dysbiosis.87 Future interventions may include therapies that restore barrier function, avoid disruption of the intestinal epithelial barrier, and prevent reactive gliosis and neuro-inflammation. Additionally, noninvasive music therapy techniques have shown potential in improving motor, speech, and cognitive skills in PD patients.
The in vitro BBB models are being used widely to study the barrier function of the BBB. These models simulate the working principle of BBB and can be used to quantify its permeability to water, ions, and solutes.11 Assessing the permeability of small molecules through the barrier is important for the development of central nervous system drugs. Other in silico and in vitro approaches, such as the parallel artificial membrane permeability assay (PAMPA-BBB) and computational methods, are also under investigations to predict BBB permeability in the early stages of drug discovery.88
Novel strategies, such as the use of cell-penetrating peptides (CPPs), are being explored to facilitate drug delivery across the BBB. CPPs have the potential to serve as shuttles for brain-specific drugs. Photobiomodulation (PBM) with near-infrared (NIR) light has shown potential as a noninvasive therapeutic approach for neurological disorders. NIR light irradiation has been found to increase the permeability of in vitro BBB models by affecting mitochondrial activity, reactive oxygen species (ROS) levels, and the expression of tight junction proteins.89 Nanoparticle-based drug delivery systems, such as solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs), have been investigated for their potential in facilitating drug transport across the BBB. Carriers have advantages of delivering hydrophilic and hydrophobic agents to the brain for the treatment of neurodegenerative diseases and brain cancers.90
Advances in drug delivery across the blood–brain barrier have significant therapeutic implications and future potential in Parkinson’s disease treatment. Nano drug delivery systems, such as solid lipid nanoparticles (SLNs), PLGA nanoparticles (NPs), and graphene oxide (GO) nanosheets, have shown promise in delivering therapeutic agents to the brain.90 These systems offer controlled drug delivery, a longer circulation time, target specificity, and reduced toxicity. Additionally, receptor-mediated transcytosis (RMT) has been utilized to transport nanoparticles across the BBB.91 The development of disease-modifying therapies and treatments for motor complications in PD is also progressing. Nanoparticles have the potential to improve the pharmacokinetics of conventional therapies and deliver better therapeutic agents.
6. Future Research Directions and Challenges
The therapeutic implications and future potential of research directions and challenges related to Parkinson’s disease are being explored. Medical imaging, such as magnetic resonance imaging (MRI), is being used to develop support systems for the diagnosis and prognosis of PD. The functional organization of thalamic inputs to the basal ganglia, including the intralaminar nuclei, is of particular interest in understanding motor and nonmotor functions in PD.92 Angiotensin-II AT1 receptor blockers (ARBs) show potential beneficial effects in PD patients, and further clinical trials are warranted.93 Emerging evidence suggests that disrupted oscillatory activity in cortico-basal ganglia-thalamo-cortical (CBGTC) and cerebellar networks can be partially corrected by applying deep brain stimulation (DBS).94 Similarly, intracerebro-ventricular administration of platelet-derived growth factor-BB has shown promising results in restoring function in Parkinson’s disease, including restoration of striatal dopamine transporter binding sites and expression of nigral tyrosine hydroxylase.95 The study of the placebo effect in PD has identified genuine psychologic placebo effects and nocebo responses, which have important implications for clinical trial design and drug dosage. Recent clinical trials have focused on novel strategies targeting α-synuclein and repurposing drugs for disease modification in PD, but results have been disappointing.96 Future research directions should continue to explore these therapeutic targets and address the challenges of the BBB in PD.
7. Conclusion
Parkinson’s disease is a slow and progressive neurodegenerative disorder. It begins around 10 years before the manifestation of symptoms and affects dopaminergic pathways only. It gradually creates an imbalance between two important neurotransmitters, dopamine and acetylcholine, leading to motor and nonmotor dysfunction. PD occurs due to genetic and environmental factors and depends on a person’s living conditions. It is directly connected to the blood–brain barrier as alterations to the BBB can accelerate PD progression. The weakening of the BBB gives rise to the accumulation of Lewy bodies, Lewy neurites, and protein aggregates, inducing neuro-inflammation that affects the BRB and causes impairment of vision, muscle rigidity, slow and involuntary movements, changes in speech, and other unique symptoms in each patient. Medications available for PD are used to relieve symptoms and slow disease progression. Therefore, it is essential to understand the mechanisms underlying PD and the effect of BBB impairment.
Regarding treatments for PD, significant developments have been made in the fields of regeneration, gene therapy, and stem cell approaches. However, there is a need for more drug repurposing, as they are safe to use and previously available medicines have limited effects. These medications can only slow progression, require constant dose increase, and lead to tolerance development in patients. Eventually, increased dose can result in side effects such as blurry vision, dementia, confusion, hallucinations, loss of cognitive function, loss of appetite, and other gastrointestinal disturbances.
Data Availability Statement
This is a review article, and all references supporting the findings discussed are available in the reference list.
Written informed consent was taken from all of the participants and their parents prior to enrollment into this research.
All authors mutually agreed for publication.
The authors declare no competing financial interest.
References
- Carvajal-Castaño H.; Lemos-Duque J.; Orozco-Arroyave J. Effective detection of abnormal gait patterns in Parkinson’s disease patients using kinematics, nonlinear, and stability gait features. Human Movement Science 2022, 81, 102891 10.1016/j.humov.2021.102891. [DOI] [PubMed] [Google Scholar]
- Hoffmeister J. D.; Kelm-Nelson C. A.; Ciucci M. R. Manipulation of vocal communication and anxiety through pharmacologic modulation of norepinephrine in the Pink1–/–rat model of Parkinson disease. Behavioural brain research 2022, 418, 113642 10.1016/j.bbr.2021.113642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetmann D.Types of Parkinson’s. 2021 May 18, 2021. [cited 2023 11 october, 2023]; available from https://www.healthline.com/health/parkinsons/types-of-parkinsons#takeaway.
- Post B.; et al. Young onset Parkinson’s disease: a modern and tailored approach. Journal of Parkinson’s disease 2020, 10 (s1), S29–S36. 10.3233/JPD-202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger K. A. Is Braak staging valid for all types of Parkinson’s disease?. Journal of neural transmission 2019, 126, 423–431. 10.1007/s00702-018-1898-9. [DOI] [PubMed] [Google Scholar]
- Brumm M. C. Parkinson’s Progression Markers Initiative: A Milestone-Based Strategy to Monitor PD Progression. medRxiv 2023, 13, 899. 10.3233/JPD-223433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D. K.; Tanner C. M.; Brundin P. Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clinics in geriatric medicine 2020, 36 (1), 1–12. 10.1016/j.cger.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindha A.; et al. Diagnosis of Parkinson Disease: Imaging and Non-Imaging Techniques. Techniques for Assessment of Parkinsonism for Diagnosis and Rehabilitation 2022, 61–78. 10.1007/978-981-16-3056-9_5. [DOI] [Google Scholar]
- Shi Q.; et al. A Chinese patient with the clinical features of Parkinson’s disease contains a single copy of octarepeat deletion in PRNP case report. Prion 2021, 15 (1), 121–125. 10.1080/19336896.2021.1946376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson M. A.; Banks W. A. Blood–brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. Journal of Cerebral Blood Flow & Metabolism 2013, 33 (10), 1500–1513. 10.1038/jcbfm.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu B. M. Quantification of In Vitro Blood-Brain Barrier Permeability. Vascular Tissue Engineering: Methods and Protocols 2022, 2375, 217–228. 10.1007/978-1-0716-1708-3_18. [DOI] [PubMed] [Google Scholar]
- Sá-Correia I.; Godinho C. P. Exploring the biological function of efflux pumps for the development of superior industrial yeasts. Curr. Opin. Biotechnol. 2022, 74, 32–41. 10.1016/j.copbio.2021.10.014. [DOI] [PubMed] [Google Scholar]
- Souza R. R.; Vargas V. Existence of Gibbs states and maximizing measures on a general one-dimensional lattice system with Markovian structure. Qualitative Theory of Dynamical Systems 2022, 21 (1), 5. 10.1007/s12346-021-00537-y. [DOI] [Google Scholar]
- Hayreh S. S.; Hayreh S. S. The Blood-Retinal Barrier. Ocular Vascular Occlusive Disorders 2015, 165–171. 10.1007/978-3-319-12781-1_9. [DOI] [Google Scholar]
- O’Leary F.; Campbell M. The blood–retina barrier in health and disease. FEBS Journal 2023, 290 (4), 878–891. 10.1111/febs.16330. [DOI] [PubMed] [Google Scholar]
- Tang L.; et al. Melatonin maintains inner blood–retinal barrier by regulating microglia via inhibition of PI3K/Akt/Stat3/NF-κB signaling pathways in experimental diabetic retinopathy. Frontiers in Immunology 2022, 13, 831660 10.3389/fimmu.2022.831660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet C. The Emergence and Progression of Motor Dysfunction in Individuals at Risk of Parkinson’s Disease. Movement Disorders 2023, 38, 1636. 10.1002/mds.29496. [DOI] [PubMed] [Google Scholar]
- Stewart C. B.; et al. Corrigendum: The longitudinal progression of autonomic dysfunction in Parkinson’s disease: a 7-year study. Frontiers in Neurology 2023, 14, 1218633 10.3389/fneur.2023.1218633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey A.; et al. Glymphatic System Dysfunction and Sleep Disturbance May contribute to the pathogenesis and progression of Parkinson’s Disease. International Journal of Molecular Sciences 2022, 23 (21), 12928. 10.3390/ijms232112928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X.; et al. TIMM8A is associated with dysfunction of immune cell in BRCA and UCEC for predicting anti-PD-L1 therapy efficacy. World Journal of Surgical Oncology 2022, 20 (1), 1–17. 10.1186/s12957-022-02736-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J.; et al. The disruption and hyperpermeability of blood-labyrinth barrier mediates cisplatin-induced ototoxicity. Toxicol. Lett. 2022, 354, 56–64. 10.1016/j.toxlet.2021.10.015. [DOI] [PubMed] [Google Scholar]
- Ding Y.; et al. Pseudogene RPL32P3 regulates the blood–tumor barrier permeability via the YBX2/HNF4G axis. Cell Death Discovery 2021, 7 (1), 367. 10.1038/s41420-021-00758-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.; et al. PHD2 attenuates high-glucose-induced blood retinal barrier breakdown in human retinal microvascular endothelial cells by regulating the Hif-1α/VEGF pathway. Inflammation Res. 2022, 71, 1–11. 10.1007/s00011-021-01518-2. [DOI] [PubMed] [Google Scholar]
- Yemanyi F.; et al. Wnt signaling in inner blood–retinal barrier maintenance. International Journal of Molecular Sciences 2021, 22 (21), 11877. 10.3390/ijms222111877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.; Yin Y.; Du L. Blood–brain barrier dysfunction in the pathogenesis of major depressive disorder. Cellular and Molecular Neurobiology 2022, 42, 1–21. 10.1007/s10571-021-01153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S.; et al. The pathogenesis and treatment of cardiovascular autonomic dysfunction in Parkinson’s disease: what we know and where to go. Aging and disease 2021, 12 (7), 1675. 10.14336/AD.2021.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata F.; et al. Blood-brain barrier dysfunction amplifies the development of neuroinflammation: understanding of cellular events in brain microvascular endothelial cells for prevention and treatment of BBB dysfunction. Frontiers in cellular neuroscience 2021, 15, 661838 10.3389/fncel.2021.661838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Q. L.; et al. Vascular PDGFR-alpha protects against BBB dysfunction after stroke in mice. Angiogenesis 2021, 24, 35–46. 10.1007/s10456-020-09742-w. [DOI] [PubMed] [Google Scholar]
- Lan G.; et al. Astrocytic VEGFA: An essential mediator in blood–brain-barrier disruption in Parkinson’s disease. Glia 2022, 70 (2), 337–353. 10.1002/glia.24109. [DOI] [PubMed] [Google Scholar]
- Li W.; et al. LCZ696 Possesses a Protective Effect Against Homocysteine (Hcy)-Induced Impairment of Blood–Brain Barrier (BBB) Integrity by Increasing Occludin, Mediated by the Inhibition of Egr-1. Neurotoxicity Research 2021, 39, 1981–1990. 10.1007/s12640-021-00414-1. [DOI] [PubMed] [Google Scholar]
- Li W.; et al. PARK genes link mitochondrial dysfunction and alpha-synuclein pathology in sporadic Parkinson’s disease. Frontiers in Cell and Developmental Biology 2021, 9, 612476 10.3389/fcell.2021.612476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamonaca G.; Volta M. Alpha-synuclein and LRRK2 in synaptic autophagy: linking early dysfunction to late-stage pathology in Parkinson’s disease. Cells 2020, 9 (5), 1115. 10.3390/cells9051115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrafa E.; et al. Mitochondrial Dysfunction in Peripheral Blood Mononuclear Cells as Novel Diagnostic Tools for Non-Alcoholic Fatty Liver Disease: Visualizing Relationships with Known and Potential Disease Biomarkers. Diagnostics 2023, 13 (14), 2363. 10.3390/diagnostics13142363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon M.-T.; et al. Emerging pathogenic role of peripheral blood factors following BBB disruption in neurodegenerative disease. Ageing Research Reviews 2021, 68, 101333 10.1016/j.arr.2021.101333. [DOI] [PubMed] [Google Scholar]
- Johnson M. E.; et al. Heterozygous GBA D409V and ATP13a2 mutations do not exacerbate pathological α-synuclein spread in the prodromal preformed fibrils model in young mice. Neurobiology of Disease 2021, 159, 105513 10.1016/j.nbd.2021.105513. [DOI] [PubMed] [Google Scholar]
- Fan X.; et al. Role of homocysteine in the development and progression of Parkinson’s disease. Annals of Clinical and Translational Neurology 2020, 7 (11), 2332–2338. 10.1002/acn3.51227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang P.; et al. The S1P2 receptor regulates blood-brain barrier integrity and leukocyte extravasation with implications for neurodegenerative disease. Neurochem. Int. 2021, 146, 105018 10.1016/j.neuint.2021.105018. [DOI] [PubMed] [Google Scholar]
- You L.; Jiang H. Cabergoline possesses a beneficial effect on blood-brain barrier (BBB) integrity against lipopolysaccharide (LPS). Bioengineered 2021, 12 (1), 8358–8369. 10.1080/21655979.2021.1987066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motyl J. A.; et al. Recent insights into the interplay of alpha-synuclein and sphingolipid signaling in Parkinson’s disease. International Journal of Molecular Sciences 2021, 22 (12), 6277. 10.3390/ijms22126277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique Y. H.; Idrisi M.; Shahid M. Effect of Cabergoline on Cognitive Impairments in Transgenic Drosophila Model of Parkinson’s Disease. Letters in Drug Design & Discovery 2020, 17 (10), 1261–1269. 10.2174/1570180817999200514100917. [DOI] [Google Scholar]
- Mishra A.; et al. Strategies facilitating the permeation of nanoparticles through blood-brain barrier: An insight towards the development of brain-targeted drug delivery system. Journal of Drug Delivery Science and Technology 2023, 86, 104694. 10.1016/j.jddst.2023.104694. [DOI] [Google Scholar]
- Raquel H. A. Blood-brain barrier lesion–a novel determinant of autonomic imbalance in heart failure and the effects of exercise training. Clinical Science 2023, 137, 1049. 10.1042/CS20230489. [DOI] [PubMed] [Google Scholar]
- Dai Z. The role of Circadian rhythm in blood-brain barrier permeability. Highlights in Science, Engineering and Technology 2023, 54, 448–454. 10.54097/hset.v54i.9809. [DOI] [Google Scholar]
- Tan X.-x.; Qiu L.-L.; Sun J. Research progress on the role of inflammatory mechanisms in the development of postoperative cognitive dysfunction. BioMed. research international 2021, 2021, 1. 10.1155/2021/3883204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buruş A.; Çeltikçi B.; Aksoy Y. The Inflammatory Processes Driven by Gut Microbiota. Acta Medica 2021, 52 (3), 171–179. 10.32552/2021.ActaMedica.548. [DOI] [Google Scholar]
- Sun T.; et al. Focused ultrasound with anti-pGlu3 Aβ enhances efficacy in Alzheimer’s disease-like mice via recruitment of peripheral immune cells. J. Controlled Release 2021, 336, 443–456. 10.1016/j.jconrel.2021.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y.-m.; et al. Immune cells in the BBB disruption after acute ischemic stroke: targets for immune therapy?. Frontiers in Immunology 2021, 12, 678744 10.3389/fimmu.2021.678744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka G.; et al. Links between immune cells from the periphery and the brain in the pathogenesis of epilepsy: a narrative review. International journal of molecular sciences 2021, 22 (9), 4395. 10.3390/ijms22094395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; et al. Possible inflammatory mechanisms and predictors of Parkinson’s disease patients with fatigue (Brief Review). Clinical Neurology and Neurosurgery 2021, 208, 106844 10.1016/j.clineuro.2021.106844. [DOI] [PubMed] [Google Scholar]
- Herrera E. A.; González-Candia A. Gestational hypoxia and blood-brain barrier permeability: early origins of cerebrovascular dysfunction induced by epigenetic mechanisms. Frontiers in Physiology 2021, 12, 717550 10.3389/fphys.2021.717550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.; et al. Procyanidin B2 attenuates neurological deficits and blood–brain barrier disruption in a rat model of cerebral ischemia. Molecular nutrition & food research 2015, 59 (10), 1930–1941. 10.1002/mnfr.201500181. [DOI] [PubMed] [Google Scholar]
- Mou Y.; et al. Gut microbiota interact with the brain through systemic chronic inflammation: Implications on neuroinflammation, neurodegeneration, and aging. Frontiers in immunology 2022, 13, 796288 10.3389/fimmu.2022.796288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier B.; Daneman R.; Ransohoff R. M. Development, maintenance and disruption of the blood-brain barrier. Nature medicine 2013, 19 (12), 1584–1596. 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithaiwala M. N.; et al. Neuroinflammation and the kynurenine pathway in CNS disease: molecular mechanisms and therapeutic implications. Cells 2021, 10 (6), 1548. 10.3390/cells10061548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panes J. D.; et al. Deciphering the role of PGC-1α in neurological disorders: from mitochondrial dysfunction to synaptic failure. Neural Regeneration Research 2022, 17 (2), 237. 10.4103/1673-5374.317957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S.; et al. Targeted antioxidant delivery modulates mitochondrial functions, ameliorates oxidative stress and preserve sperm quality during cryopreservation. Theriogenology 2022, 179, 22–31. 10.1016/j.theriogenology.2021.11.013. [DOI] [PubMed] [Google Scholar]
- Lin C.-H.; et al. Exposure to PM2. 5 induces neurotoxicity, mitochondrial dysfunction, oxidative stress and inflammation in human SH-SY5Y neuronal cells. Neurotoxicology 2022, 88, 25–35. 10.1016/j.neuro.2021.10.009. [DOI] [PubMed] [Google Scholar]
- Wei Q.; et al. Oxidative stress-mediated particulate matter affects the risk of relapse in schizophrenia patients: Air purification intervention-based panel study. Environ. Pollut. 2022, 292, 118348 10.1016/j.envpol.2021.118348. [DOI] [PubMed] [Google Scholar]
- Ye F.; et al. CypD deficiency confers neuroprotection against mitochondrial abnormality caused by lead in SH-SY5Y cell. Toxicol. Lett. 2020, 323, 25–34. 10.1016/j.toxlet.2019.12.025. [DOI] [PubMed] [Google Scholar]
- Torres-Vergara P.; Escudero C.; Penny J. Drug transport at the brain and endothelial dysfunction in preeclampsia: implications and perspectives. Frontiers in Physiology 2018, 9, 1502. 10.3389/fphys.2018.01502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago J. L.; et al. Characterization of permeability barrier dysfunction in a murine model of cutaneous field cancerization following chronic UV-B irradiation: Implications for the pathogenesis of skin cancer. Cancers 2021, 13 (16), 3935. 10.3390/cancers13163935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulyakova N.; et al. Mitochondrial dysfunction in the pathogenesis of Rett syndrome: implications for mitochondria-targeted therapies. Frontiers in cellular neuroscience 2017, 11, 58. 10.3389/fncel.2017.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; et al. Heat shock proteins took part in oxidative stress-mediated inflammatory injury via NF-κB pathway in excess manganese-treated chicken livers. Ecotoxicology and Environmental Safety 2021, 226, 112833 10.1016/j.ecoenv.2021.112833. [DOI] [PubMed] [Google Scholar]
- Lin L.; et al. Severe Hypoglycemia Contributing to Cognitive Dysfunction in Diabetic Mice Is Associated With Pericyte and Blood–Brain Barrier Dysfunction. Frontiers in Aging Neuroscience 2021, 13, 775244 10.3389/fnagi.2021.775244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamil Al-Obaidi M. M.; M.N.M Desa A review of the mechanisms of blood–brain barrier disruption during COVID-19 infection. Journal of Neuroscience Research 2023, 101, 1687. 10.1002/jnr.25232. [DOI] [PubMed] [Google Scholar]
- Xu X.TDP43 CFTS Affect Brain Endothelial Cell Functions by Regulating YAP and Tight Junction Proteins in Cerebral Ischemic Injury, 2021.
- Voirin A.-C.; Perek N.; Roche F. Inflammatory stress induced by a combination of cytokines (IL-6, IL-17, TNF-α) leads to a loss of integrity on bEnd. 3 endothelial cells in vitro BBB model. Brain Res. 2020, 1730, 146647 10.1016/j.brainres.2020.146647. [DOI] [PubMed] [Google Scholar]
- Tolosa E.; et al. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurology 2021, 20 (5), 385–397. 10.1016/S1474-4422(21)00030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei J.; Desrosiers C.; Frasnelli J. Machine learning for the diagnosis of Parkinson’s disease: a review of literature. Frontiers in aging neuroscience 2021, 13, 633752 10.3389/fnagi.2021.633752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui X. Hyposmia as a predictive marker of Parkinson’s disease: a systematic review and meta-analysis. BioMed. research international 2019, 2019, 1. 10.1155/2019/3753786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde S.; et al. Predictive markers for Parkinson’s disease using deep neural nets on neuromelanin sensitive MRI. NeuroImage: Clinical 2019, 22, 101748 10.1016/j.nicl.2019.101748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saranza G.; Lang A. E. Levodopa challenge test: indications, protocol, and guide. Journal of Neurology 2021, 268 (9), 3135–3143. 10.1007/s00415-020-09810-7. [DOI] [PubMed] [Google Scholar]
- Antonini A.; et al. The long-term impact of levodopa/carbidopa intestinal gel on ‘off’-time in patients with advanced Parkinson’s disease: a systematic review. Advances in Therapy 2021, 38 (6), 2854–2890. 10.1007/s12325-021-01747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson S. H.; et al. Dopamine Agonists in Parkinson’s Disease: Impact of D1-like or D2-like Dopamine Receptor Subtype Selectivity and Avenues for Future Treatment. Clinical Parkinsonism & Related Disorders 2023, 9, 100212. 10.1016/j.prdoa.2023.100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan X.; et al. Comparative efficacy and safety of dopamine agonists in advanced Parkinson’s disease with motor fluctuations: a systematic review and network meta-analysis of double-blind randomized controlled trials. Frontiers in Neuroscience 2021, 15, 728083 10.3389/fnins.2021.728083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R.; et al. Long-term effectiveness of adjuvant treatment with catechol-O-methyltransferase or monoamine oxidase B inhibitors compared with dopamine agonists among patients with Parkinson disease uncontrolled by levodopa therapy: the PD MED randomized clinical trial. JAMA neurology 2022, 79 (2), 131–140. 10.1001/jamaneurol.2021.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binde C. D.; et al. Comparative effectiveness of dopamine agonists and monoamine oxidase type-B inhibitors for Parkinson’s disease: a multiple treatment comparison meta-analysis. European journal of clinical pharmacology 2020, 76, 1731–1743. 10.1007/s00228-020-02961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrales-Macias V.; et al. Effects of a new natural catechol-O-methyl transferase inhibitor on two in vivo models of parkinson’s disease. ACS Chem. Neurosci. 2022, 13 (23), 3303–3313. 10.1021/acschemneuro.2c00356. [DOI] [PubMed] [Google Scholar]
- Song Z.; et al. Different catechol-o-methyl transferase inhibitors in Parkinson’s disease: a bayesian network meta-analysis. Frontiers in Neurology 2021, 12, 707723 10.3389/fneur.2021.707723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A. A. Opicapone, a Novel Catechol-O-methyl Transferase Inhibitor, for Treatment of Parkinson’s Disease “Off” Episodes. Health Psychol. Res. 2022, 10 (2), 1. 10.52965/001c.36074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmol S.; et al. Amantadine revisited: a contender for initial treatment in Parkinson’s disease?. CNS drugs 2021, 35, 1141–1152. 10.1007/s40263-021-00862-5. [DOI] [PubMed] [Google Scholar]
- Bohnen N. I.; et al. Cholinergic system changes in Parkinson’s disease: emerging therapeutic approaches. Lancet Neurology 2022, 21 (4), 381–392. 10.1016/S1474-4422(21)00377-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquini J.; Brooks D. J.; Pavese N. The cholinergic brain in Parkinson’s disease. Movement Disorders Clinical Practice 2021, 8 (7), 1012–1026. 10.1002/mdc3.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindeya Gebreyesus H.; Gebrehiwot Gebremichael T. The potential role of astrocytes in Parkinson’s disease (PD). Medical Sciences 2020, 8 (1), 7. 10.3390/medsci8010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X. Impaired tissue barriers as potential therapeutic targets for Parkinson’s disease and amyotrophic lateral sclerosis. Metabolic Brain Disease 2018, 33, 1031–1043. 10.1007/s11011-018-0239-x. [DOI] [PubMed] [Google Scholar]
- Clairembault T.; et al. Enteric glial cells: new players in Parkinson’s disease?. Movement Disorders 2015, 30 (4), 494–498. 10.1002/mds.25979. [DOI] [PubMed] [Google Scholar]
- Žerovnik E.; Ventura S.; Jerala N. K.. Inflammation, Oxidative Stress and Protein Aggregation; Any Links? MDPI, 2020; p 2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radan M.; et al. Application of in vitro PAMPA technique and in silico computational methods for blood-brain barrier permeability prediction of novel CNS drug candidates. European Journal of Pharmaceutical Sciences 2022, 168, 106056 10.1016/j.ejps.2021.106056. [DOI] [PubMed] [Google Scholar]
- Zhou T.; Ohulchanskyy T. Y.; Qu J. Effect of NIR light on the permeability of the blood-brain barriers in in vitro models. Biomedical Optics Express 2021, 12 (12), 7544–7555. 10.1364/BOE.438445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri M.; et al. Engineered solid lipid nanoparticles and nanostructured lipid carriers as new generations of blood–brain barrier transmitters. ACS Chem. Neurosci. 2021, 12 (24), 4475–4490. 10.1021/acschemneuro.1c00540. [DOI] [PubMed] [Google Scholar]
- Tashima T. Smart strategies for therapeutic agent delivery into brain across the blood–brain barrier using receptor-mediated transcytosis. Chem. Pharm. Bull. 2020, 68 (4), 316–325. 10.1248/cpb.c19-00854. [DOI] [PubMed] [Google Scholar]
- Benarroch E. What Is the Role of the Intralaminar Thalamic Input to the Striatum and Its Potential Implications in Parkinson Disease?. Neurology 2023, 101 (3), 118–123. 10.1212/WNL.0000000000207610. [DOI] [PubMed] [Google Scholar]
- Udovin L.; et al. Effects of angiotensin type 1 receptor antagonists on Parkinson’s disease progression: An exploratory study in the PPMI database. Parkinsonism & Related Disorders 2021, 86, 34–37. 10.1016/j.parkreldis.2021.03.007. [DOI] [PubMed] [Google Scholar]
- Paff M.; et al. Update on current technologies for deep brain stimulation in Parkinson’s disease. Journal of movement disorders 2020, 13 (3), 185. 10.14802/jmd.20052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D.; et al. Insights Into the Role of Platelet-Derived Growth Factors: Implications for Parkinson’s Disease Pathogenesis and Treatment. Frontiers in Aging Neuroscience 2022, 14, 890509 10.3389/fnagi.2022.890509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahoor I.; Shafi A.; Haq E. Pharmacological treatment of Parkinson’s disease. Exon Publications 2018, 129–144. 10.15586/codonpublications.parkinsonsdisease.2018.ch7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This is a review article, and all references supporting the findings discussed are available in the reference list.