Abstract
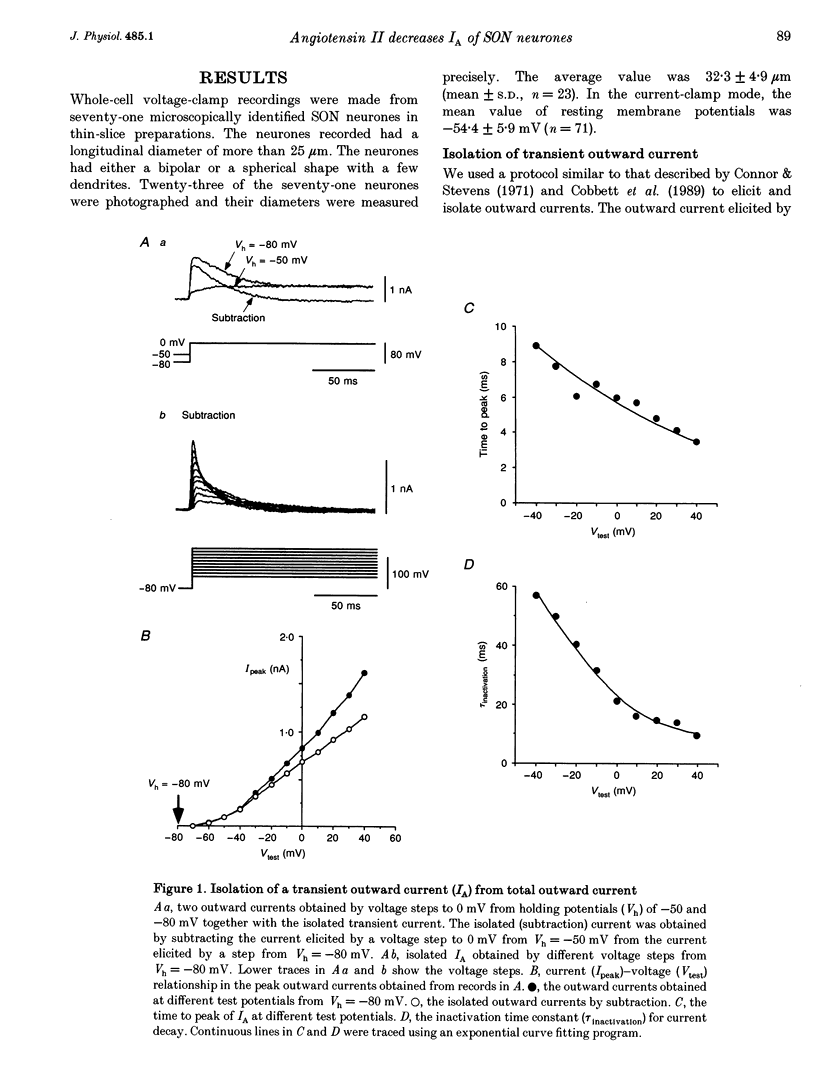
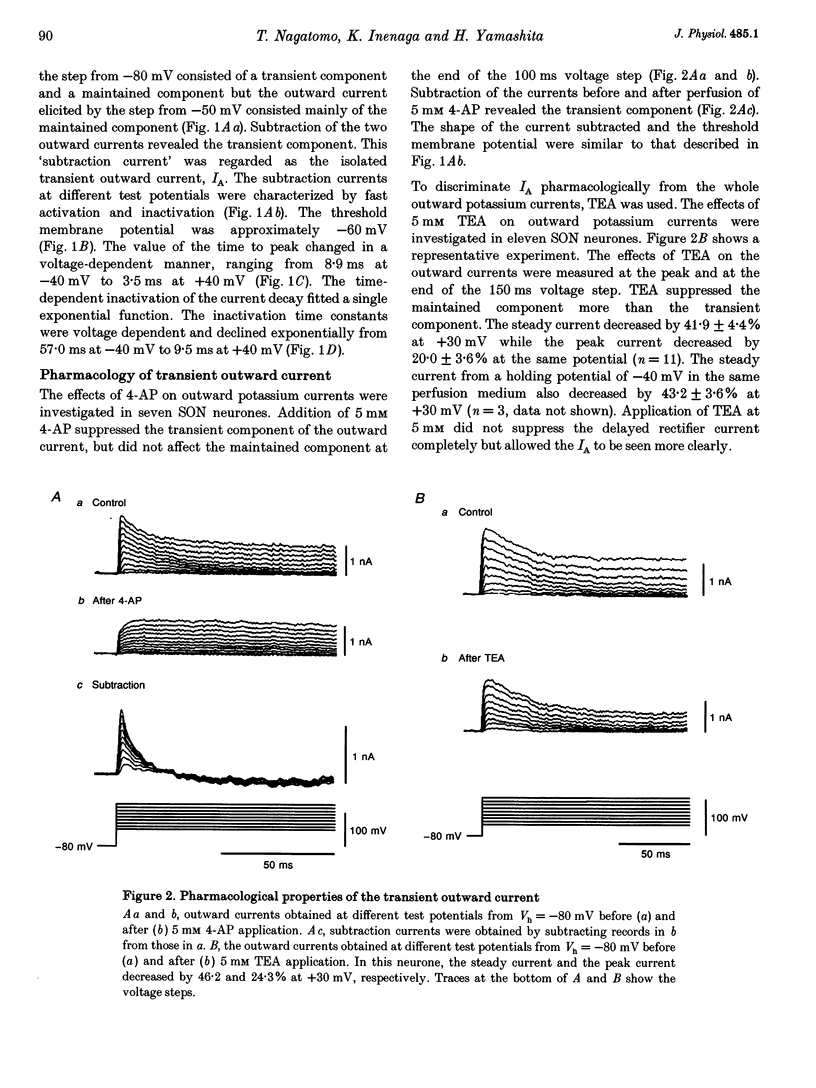
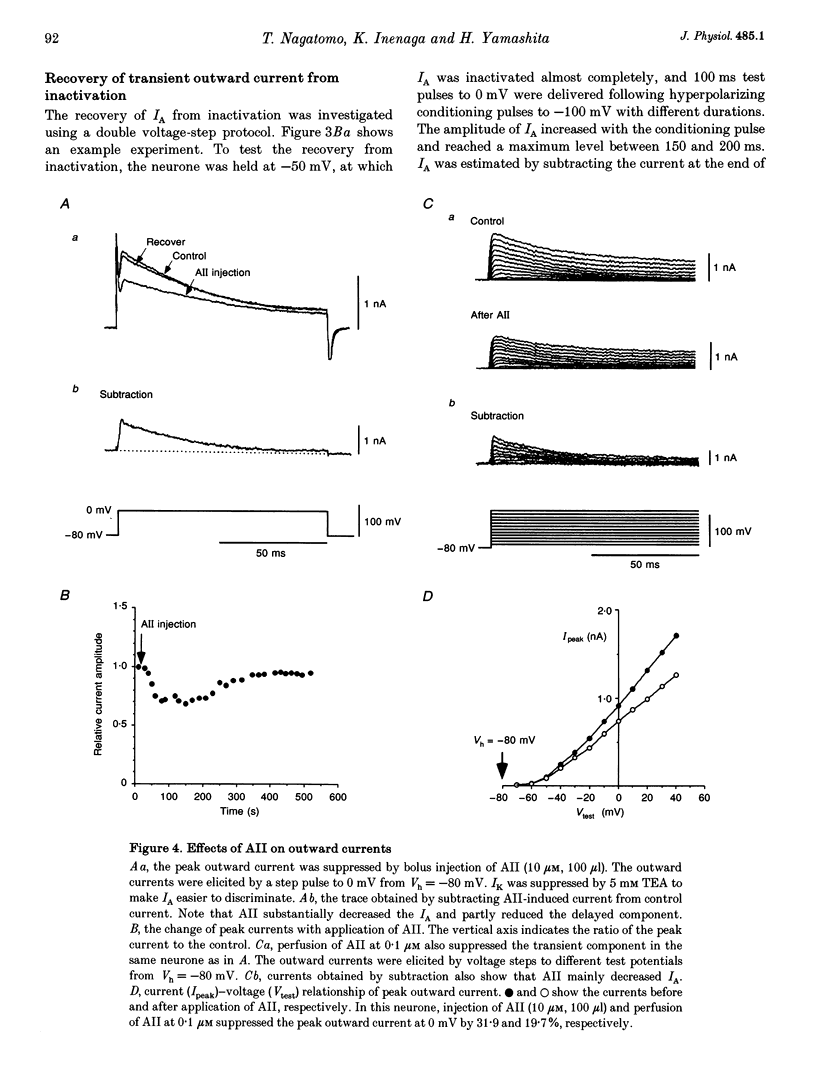
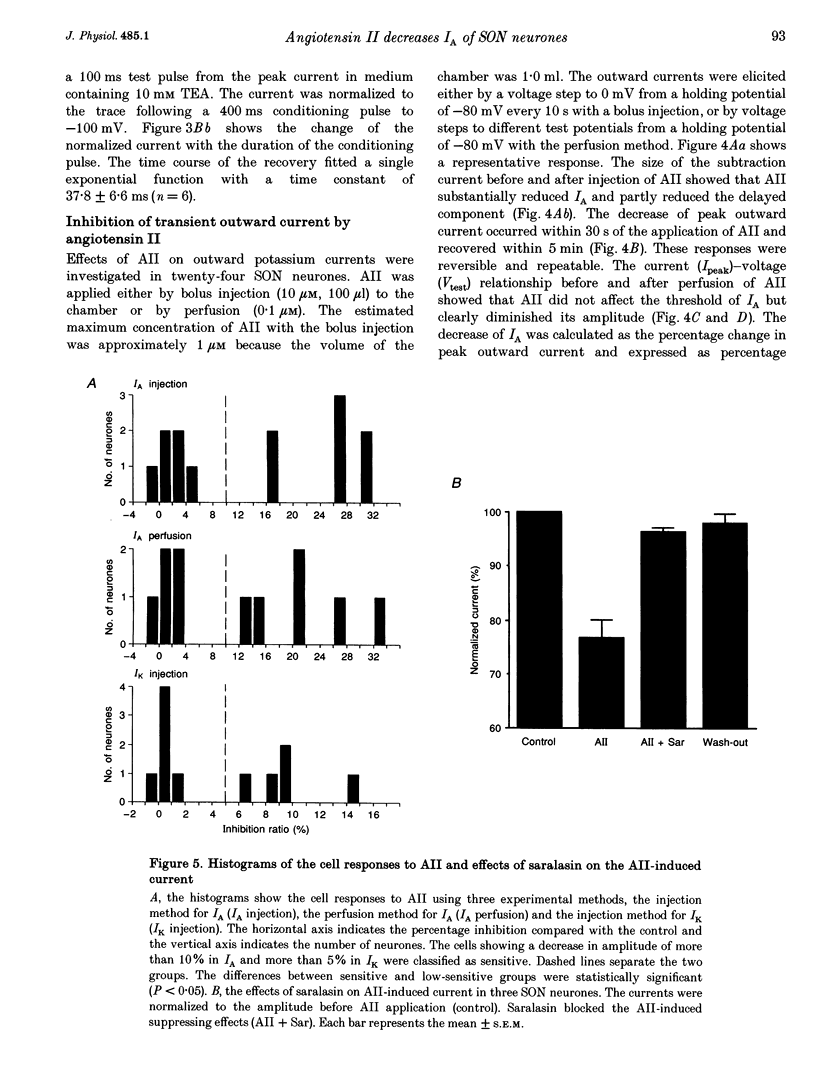
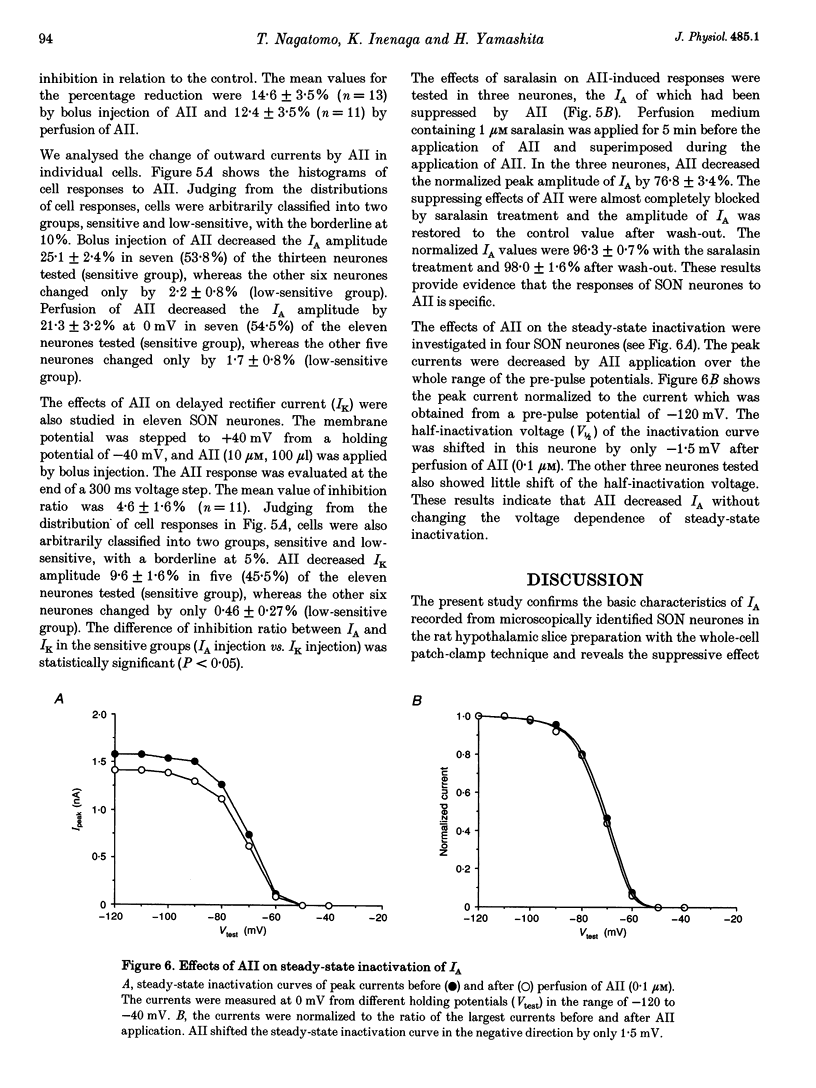
1. Outward potassium currents were recorded from microscopically identified supraoptic neurones of adult Wistar male rats using the whole-cell patch-clamp technique in thin-slice preparations. The basic characteristics of transient outward current (IA or A-current) and the effects of angiotensin II (AII) on the currents were studied. 2. IA was isolated by subtracting outward currents elicited by stepping from two different holding potentials to a test potential or by applying 4-aminopyridine (4-AP) at 5 mM. The isolated IA had a threshold for activation between -55 and -65 mV and was characterized by fast activation and inactivation. Values of the time to peak and the inactivation time constants for current decay at different test potentials were voltage dependent. 3. Normalized currents for activation and steady-state inactivation of IA were fitted to the Boltzmann function. The mid-points and the slope factors were, respectively, -35.0 and -14.3 +/- 0.40 mV (n = 5) for the activation curve, and -72.0 and 7.0 +/- 0.68 mV (n = 5) for the inactivation curve. 4. The time course of recovery from inactivation was best fitted to a single exponential function with the time constant of 37.8 +/- 6.6 ms (n = 6). 5. The effects of AII on IA and delayed rectifier current (IK) were investigated. According to their responses to AII, cells were classified into two groups, sensitive and low-sensitive. Bolus injection of AII (10 microM, 100 microliters) decreased the IA amplitude by 25.1 +/- 2.4% in seven (53.8%) of the thirteen neurones tested (sensitive group), whereas the other six neurones (low-sensitive group) changed by only 2.2 +/- 0.8%. Perfusion of AII (0.1 microM) decreased the IA amplitude by 21.3 +/- 3.1% in six (54.5%) of eleven neurones tested (sensitive group), whereas the other five neurones (low-sensitive group) changed only by 1.7 +/- 0.8%. Bolus injection of AII (10 microM, 100 microliters) decreased the IK amplitude 9.6 +/-1.6% mV in five (45.5%) of the eleven neurones tested (sensitive group), whereas the other six neurones (low-sensitive group) changed only by 0.46 +/- 0.27%. In the sensitive groups, the reduction of IA by AII was significantly larger than that of IK (P < 0.05). 6. Application of saralasin at 1 microM, an AII antagonist, blocked the effects of AII on IA. 7. These results suggest that the excitatory action of AII on supraoptic neurosecretory cells is mediated at least in part through suppression of IA.
Full text
PDF

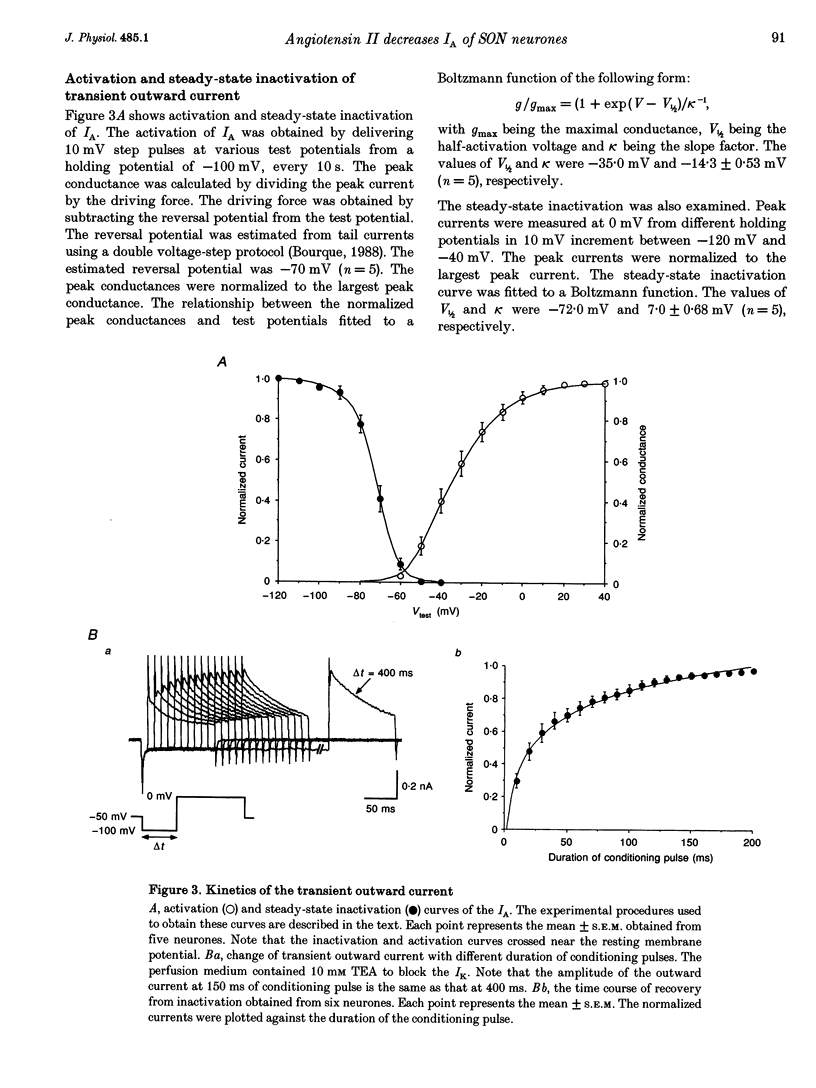


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belluzzi O., Sacchi O., Wanke E. A fast transient outward current in the rat sympathetic neurone studied under voltage-clamp conditions. J Physiol. 1985 Jan;358:91–108. doi: 10.1113/jphysiol.1985.sp015542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bkaily G., Peyrow M., Sculptoreanu A., Jacques D., Chahine M., Regoli D., Sperelakis N. Angiotensin II increases Isi and blocks IK in single aortic cell of rabbit. Pflugers Arch. 1988 Sep;412(4):448–450. doi: 10.1007/BF01907567. [DOI] [PubMed] [Google Scholar]
- Bourque C. W. Transient calcium-dependent potassium current in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 1988 Mar;397:331–347. doi: 10.1113/jphysiol.1988.sp017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauneis U., Vassilev P. M., Quinn S. J., Williams G. H., Tillotson D. L. ANG II blocks potassium currents in zona glomerulosa cells from rat, bovine, and human adrenals. Am J Physiol. 1991 May;260(5 Pt 1):E772–E779. doi: 10.1152/ajpendo.1991.260.5.E772. [DOI] [PubMed] [Google Scholar]
- Cobbett P., Legendre P., Mason W. T. Characterization of three types of potassium current in cultured neurones of rat supraoptic nucleus area. J Physiol. 1989 Mar;410:443–462. doi: 10.1113/jphysiol.1989.sp017543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbett P., Smithson K. G., Hatton G. I. Immunoreactivity to vasopressin- but not oxytocin-associated neurophysin antiserum in phasic neurons of rat hypothalamic paraventricular nucleus. Brain Res. 1986 Jan 1;362(1):7–16. doi: 10.1016/0006-8993(86)91392-2. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B., Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989 Sep;414(5):600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Konnerth A. Patch-clamping in slices of mammalian CNS. Trends Neurosci. 1990 Aug;13(8):321–323. doi: 10.1016/0166-2236(90)90137-y. [DOI] [PubMed] [Google Scholar]
- Kurtz A., Penner R. Angiotensin II induces oscillations of intracellular calcium and blocks anomalous inward rectifying potassium current in mouse renal juxtaglomerular cells. Proc Natl Acad Sci U S A. 1989 May;86(9):3423–3427. doi: 10.1073/pnas.86.9.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind R. W., Swanson L. W., Ganten D. Organization of angiotensin II immunoreactive cells and fibers in the rat central nervous system. An immunohistochemical study. Neuroendocrinology. 1985 Jan;40(1):2–24. doi: 10.1159/000124046. [DOI] [PubMed] [Google Scholar]
- Mendelsohn F. A., Quirion R., Saavedra J. M., Aguilera G., Catt K. J. Autoradiographic localization of angiotensin II receptors in rat brain. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1575–1579. doi: 10.1073/pnas.81.5.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T. H., Misgeld U., Swandulla D. Ionic currents in cultured rat hypothalamic neurones. J Physiol. 1992 May;450:341–362. doi: 10.1113/jphysiol.1992.sp019130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numann R. E., Wadman W. J., Wong R. K. Outward currents of single hippocampal cells obtained from the adult guinea-pig. J Physiol. 1987 Dec;393:331–353. doi: 10.1113/jphysiol.1987.sp016826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuya S., Inenaga K., Kaneko T., Yamashita H. Angiotensin II sensitive neurons in the supraoptic nucleus, subfornical organ and anteroventral third ventricle of rats in vitro. Brain Res. 1987 Jan 27;402(1):58–67. doi: 10.1016/0006-8993(87)91047-x. [DOI] [PubMed] [Google Scholar]
- Phillips M. I. Functions of angiotensin in the central nervous system. Annu Rev Physiol. 1987;49:413–435. doi: 10.1146/annurev.ph.49.030187.002213. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982 Apr;7(4):773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Tsuda Y., Oyama Y., Carpenter D. O., Akaike N. Effects of Ca2+ on the transient outward current of single isolated Helix central neurones. Br J Pharmacol. 1988 Oct;95(2):526–530. doi: 10.1111/j.1476-5381.1988.tb11673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H., Inenaga K., Kawata M., Sano Y. Phasically firing neurons in the supraoptic nucleus of the rat hypothalamus: immunocytochemical and electrophysiological studies. Neurosci Lett. 1983 May 27;37(1):87–92. doi: 10.1016/0304-3940(83)90509-8. [DOI] [PubMed] [Google Scholar]
- Yang C. R., Phillips M. I., Renaud L. P. Angiotensin II receptor activation depolarizes rat supraoptic neurons in vitro. Am J Physiol. 1992 Dec;263(6 Pt 2):R1333–R1338. doi: 10.1152/ajpregu.1992.263.6.R1333. [DOI] [PubMed] [Google Scholar]


