Abstract
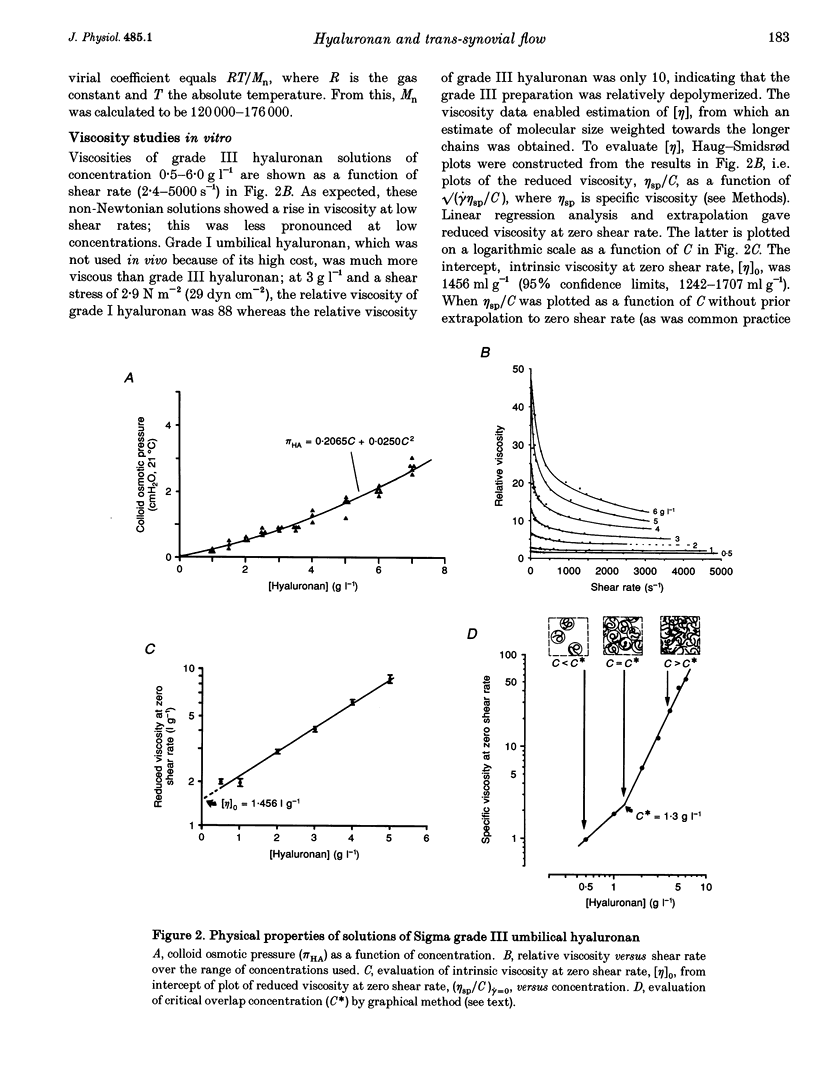
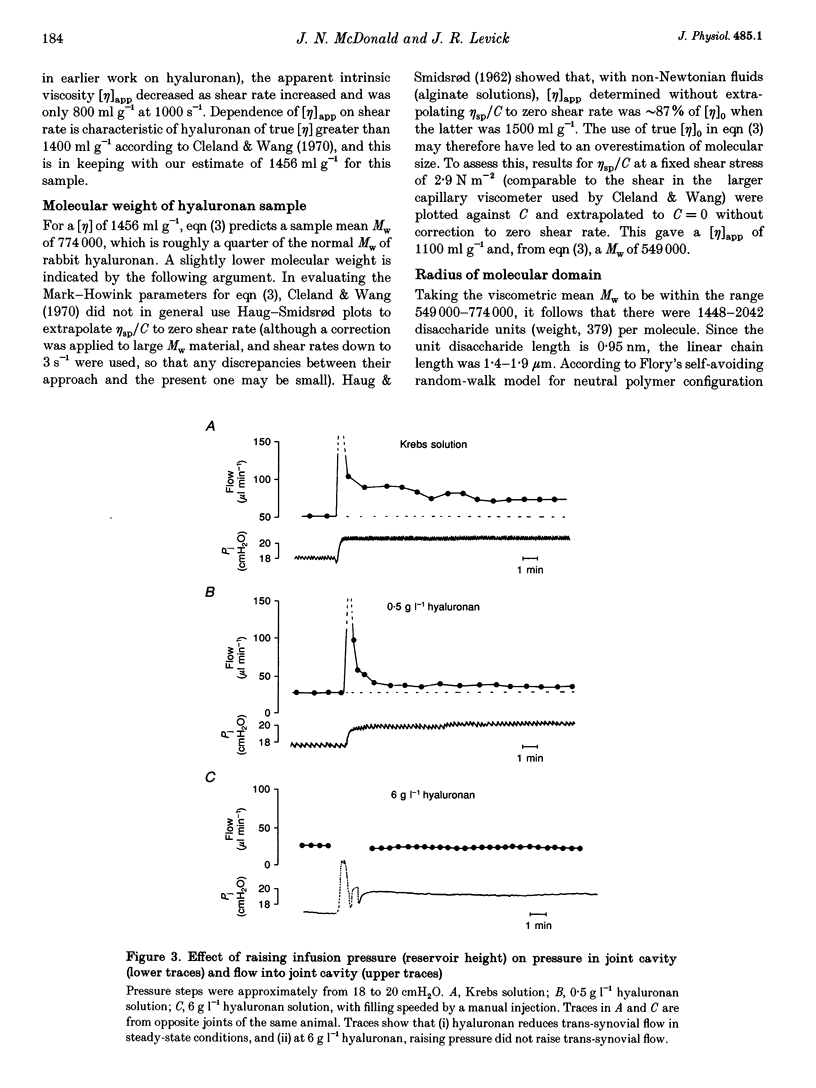
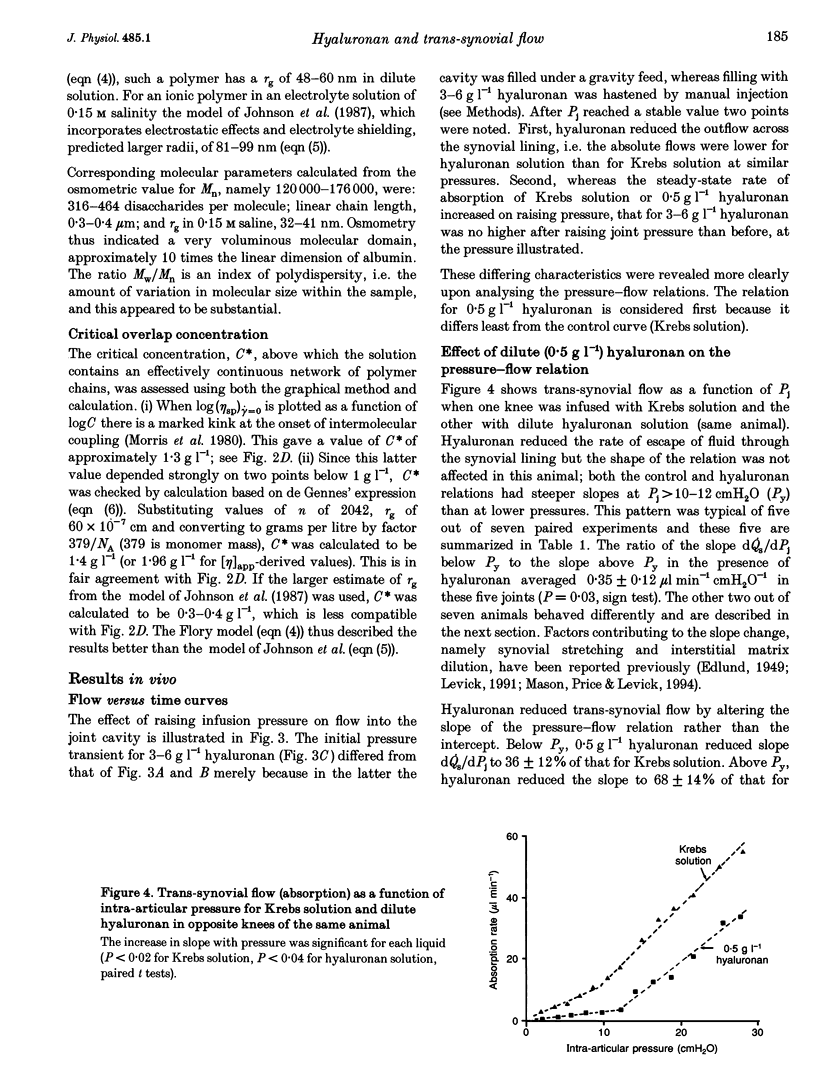
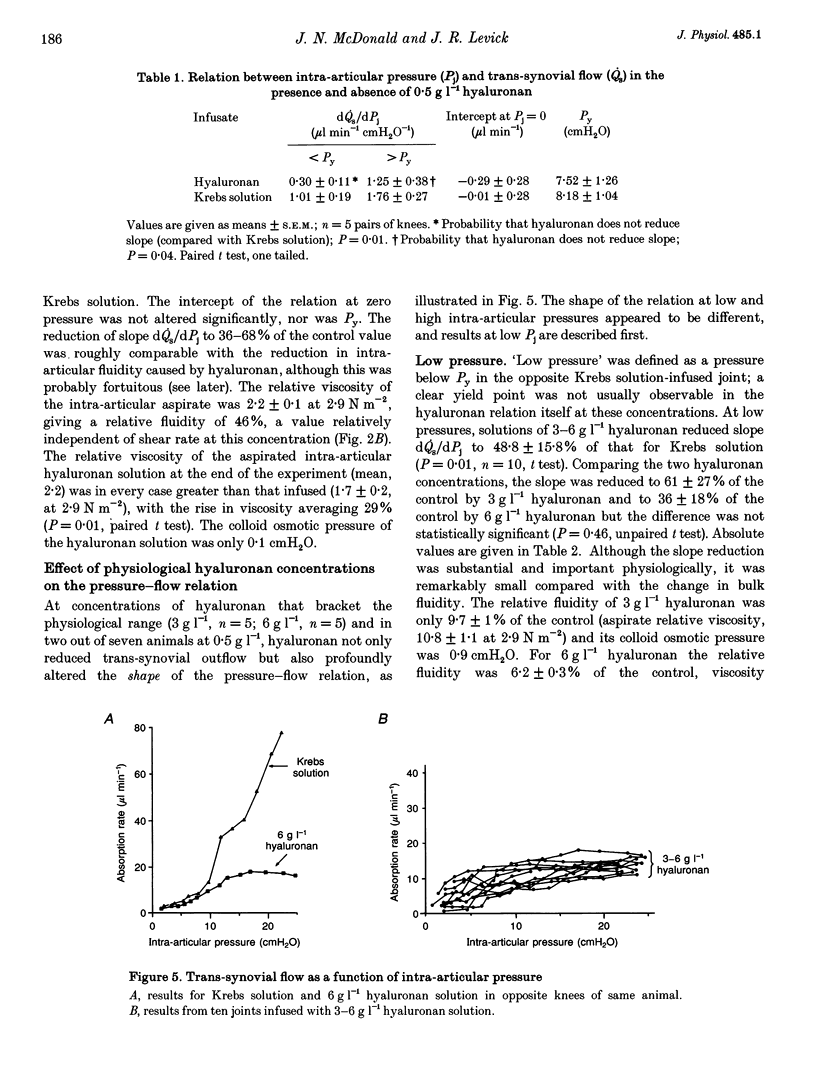
1. Hyaluronan is the major polysaccharide of synovial fluid, responsible for its high viscosity. The effect of hyaluronan on fluid transport across the synovial lining of the joint was investigated. Rate of fluid absorption from the joint cavity (Qs) was measured at intra-articular pressures (Pj) of up to 24 cmH2O in knees of anaesthetized rabbits, in the presence or absence of hyaluronan in intra-articular infusates. 2. Viscometry studies in vitro showed that the commercial hyaluronan used had a molecular weight of 549,000-774,000, a radius of gyration of 48-99 nm and a critical concentration for molecular overlap of 1.3 g l-1. 3. With intra-articular Krebs solution (control) or subnormal, subcritical concentrations of hyaluronan (0.5 g l-1), flow increased with pressure. Hyaluronan reduced the fluid escape rate by reducing slope dQs/dPj by 32-64% relative to Krebs solution. 4. At normal to high hyaluronan concentrations (3-6 g l-1) and low pressures, hyaluronan again reduced slope dQs/dPj, by 39-64%. The reduction in slope was slight, however, when compared with the reduction in bulk fluidity (1/relative viscosity). Fluidity at high shear rates was reduced to 6% of control values by 6 g l-1 hyaluronan. The effect on slope did not correlate significantly with the effect on fluidity. 5. At pressures above approximately 12 cmH2O, 3-6 g l-1 hyaluronan altered the shape of the pressure-flow relation: a flow plateau developed. In some joints raising pressure even reduced trans-synovial flow slightly. The pressure required to drive unit trans-synovial flow (an index of outflow resistance) increased 2.5-fold between 5 and 25 cmH2O in the presence of hyaluronan. By contrast, in the absence of hyaluronan the outflow resistance fell as pressure was raised. 6. It is suggested that the increasing resistance to flow in the presence of hyaluronan may be caused by partial molecular sieving of hyaluronan by the small porosities of the synovial interstitial matrix, leading to accumulation of a resistive filter cake of hyaluronan chains at the tissue-cavity interface. Since hyaluronan impedes fluid escape when pressure is raised, it may serve to preserve synovial fluid volume in vivo, e.g. during sustained joint flexion.
Full text
PDF








Selected References
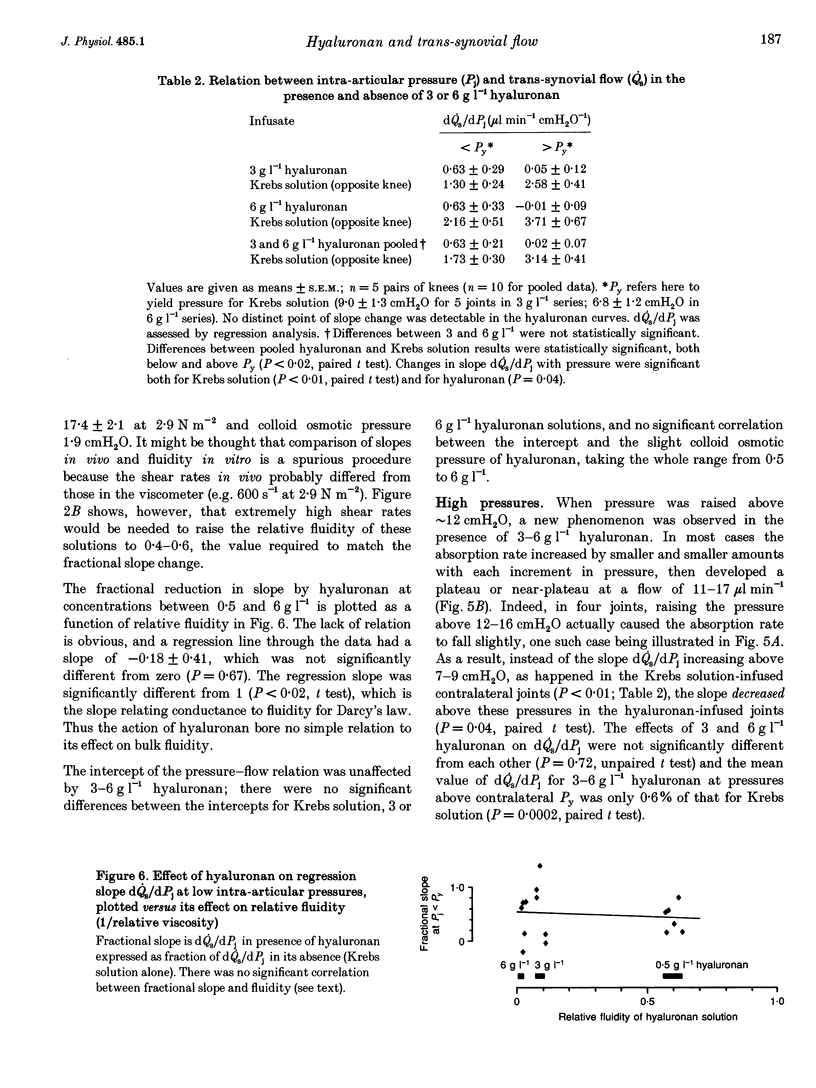
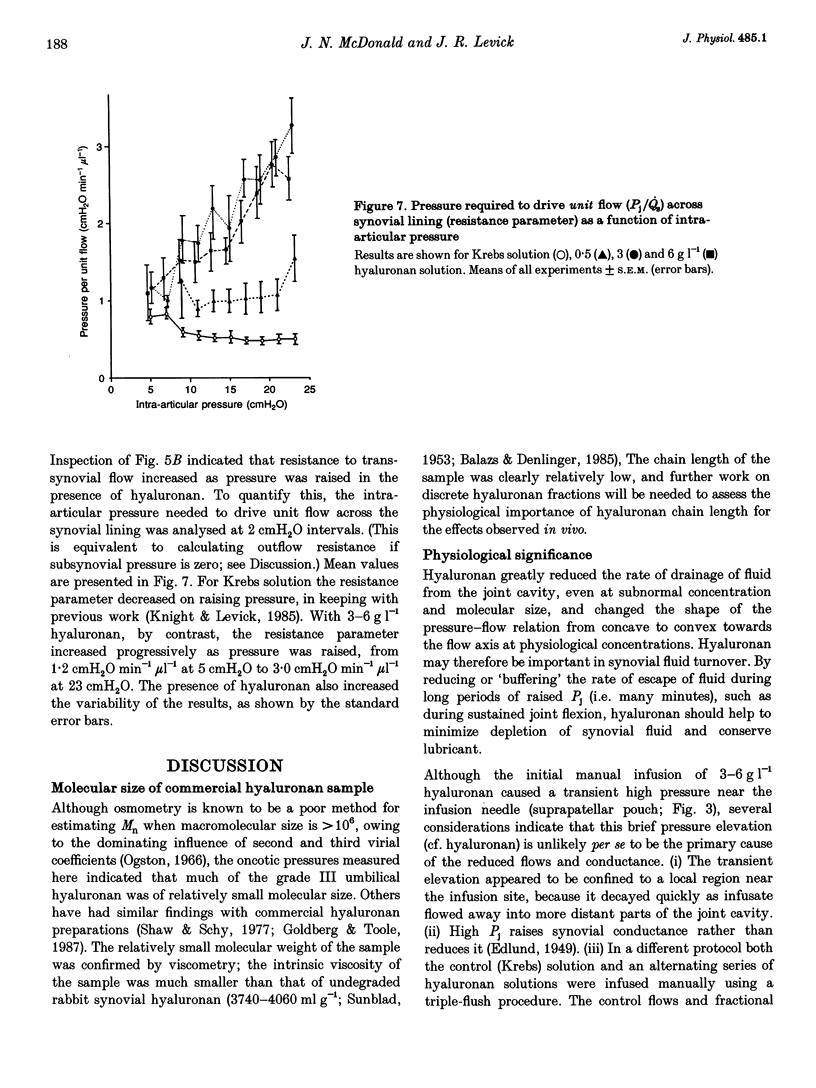
These references are in PubMed. This may not be the complete list of references from this article.
- Antonas K. N., Fraser J. R., Muirden K. D. Distribution of biologically labelled radioactive hyaluronic acid injected into joints. Ann Rheum Dis. 1973 Mar;32(2):103–111. doi: 10.1136/ard.32.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukland K., Reed R. K. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993 Jan;73(1):1–78. doi: 10.1152/physrev.1993.73.1.1. [DOI] [PubMed] [Google Scholar]
- BOLLET A. J. The intrinsic viscosity of synovial fluid hyaluronic acid. J Lab Clin Med. 1956 Nov;48(5):721–728. [PubMed] [Google Scholar]
- Barnett B., Dulfano M. J., Philippoff W., Han C. D. A method for determining the viscoelastic properties of bioogical fluids. Biorheology. 1970 Jun;7(1):55–67. doi: 10.3233/bir-1970-7104. [DOI] [PubMed] [Google Scholar]
- Brown T. J., Laurent U. B., Fraser J. R. Turnover of hyaluronan in synovial joints: elimination of labelled hyaluronan from the knee joint of the rabbit. Exp Physiol. 1991 Jan;76(1):125–134. doi: 10.1113/expphysiol.1991.sp003474. [DOI] [PubMed] [Google Scholar]
- Cleland R. L., Wang J. L. Ionic polysaccharides. 3. Dilute solution properties of hyaluronic acid fractions. Biopolymers. 1970;9(7):799–810. doi: 10.1002/bip.1970.360090706. [DOI] [PubMed] [Google Scholar]
- Cooke A. F., Dowson D., Wright V. Lubrication of synovial membrane. Ann Rheum Dis. 1976 Feb;35(1):56–59. doi: 10.1136/ard.35.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAY T. D. The permeability of interstitial connective tissue and the nature of the interfibrillary substance. J Physiol. 1952 May;117(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Dahl L. B., Dahl I. M., Engström-Laurent A., Granath K. Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann Rheum Dis. 1985 Dec;44(12):817–822. doi: 10.1136/ard.44.12.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J. R., Murdoch W. S., Curtain C. C., Watt B. J. Proteins retained with hyaluronic acid during ultrafiltration of synovial fluid. Connect Tissue Res. 1977;5(2):61–65. doi: 10.3109/03008207709152231. [DOI] [PubMed] [Google Scholar]
- Goldberg R. L., Toole B. P. Hyaluronate inhibition of cell proliferation. Arthritis Rheum. 1987 Jul;30(7):769–778. doi: 10.1002/art.1780300707. [DOI] [PubMed] [Google Scholar]
- Granger H. J., Laine S. H., Laine G. A. Osmotic pressure exerted by entangled polysaccharide chains. Microcirc Endothelium Lymphatics. 1985 Feb;2(1):85–105. [PubMed] [Google Scholar]
- Knight A. D., Levick J. R. Effect of fluid pressure on the hydraulic conductance of interstitium and fenestrated endothelium in the rabbit knee. J Physiol. 1985 Mar;360:311–332. doi: 10.1113/jphysiol.1985.sp015619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight A. D., Levick J. R., McDonald J. N. Relation between trans-synovial flow and plasma osmotic pressure, with an estimation of the albumin reflection coefficient in the rabbit knee. Q J Exp Physiol. 1988 Jan;73(1):47–65. doi: 10.1113/expphysiol.1988.sp003122. [DOI] [PubMed] [Google Scholar]
- Knox P., Levick J. R., McDonald J. N. Synovial fluid--its mass, macromolecular content and pressure in major limb joints of the rabbit. Q J Exp Physiol. 1988 Jan;73(1):33–45. doi: 10.1113/expphysiol.1988.sp003121. [DOI] [PubMed] [Google Scholar]
- LAURENT T. C., OGSTON A. G. THE INTERACTION BETWEEN POLYSACCHARIDES AND OTHER MACROMOLECULES. 4. THE OSMOTIC PRESSURE OF MIXTURES OF SERUM ALBUMIN AND HYALURONIC ACID. Biochem J. 1963 Nov;89:249–253. doi: 10.1042/bj0890249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURENT T. C., RYAN M., PIETRUSZKIEWICZ A. Fractionation of hyaluronic acid. The polydispersity of hyaluronic acid from the bovine vitreous body. Biochim Biophys Acta. 1960 Aug 26;42:476–485. doi: 10.1016/0006-3002(60)90826-x. [DOI] [PubMed] [Google Scholar]
- Levick J. R. A two-dimensional morphometry-based model of interstitial and transcapillary flow in rabbit synovium. Exp Physiol. 1991 Nov;76(6):905–921. doi: 10.1113/expphysiol.1991.sp003553. [DOI] [PubMed] [Google Scholar]
- Levick J. R. An analysis of the interaction between interstitial plasma protein, interstitial flow, and fenestral filtration and its application to synovium. Microvasc Res. 1994 Jan;47(1):90–125. doi: 10.1006/mvre.1994.1007. [DOI] [PubMed] [Google Scholar]
- Levick J. R. Flow through interstitium and other fibrous matrices. Q J Exp Physiol. 1987 Oct;72(4):409–437. doi: 10.1113/expphysiol.1987.sp003085. [DOI] [PubMed] [Google Scholar]
- Levick J. R., McDonald J. N. Microfibrillar meshwork of the synovial lining and associated broad banded collagen: a clue to identity. Ann Rheum Dis. 1990 Jan;49(1):31–36. doi: 10.1136/ard.49.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick J. R. The influence of hydrostatic pressure on trans-synovial fluid movement and on capsular expansion in the rabbit knee. J Physiol. 1979 Apr;289:69–82. doi: 10.1113/jphysiol.1979.sp012725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. N., Levick J. R. Effect of extravascular plasma protein on pressure-flow relations across synovium in anaesthetized rabbits. J Physiol. 1993 Jun;465:539–559. doi: 10.1113/jphysiol.1993.sp019692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. N., Levick J. R. Hyaluronan reduces fluid escape rate from rabbit knee joints disparately from its effect on fluidity. Exp Physiol. 1994 Jan;79(1):103–106. doi: 10.1113/expphysiol.1994.sp003736. [DOI] [PubMed] [Google Scholar]
- Morris E. R., Rees D. A., Welsh E. J. Conformation and dynamic interactions in hyaluronate solutions. J Mol Biol. 1980 Apr;138(2):383–400. doi: 10.1016/0022-2836(80)90294-6. [DOI] [PubMed] [Google Scholar]
- OGSTON A. G., SHERMAN T. F. Effects of hyaluronic acid upon diffusion of solutes and flow of solvent. J Physiol. 1961 Apr;156:67–74. doi: 10.1113/jphysiol.1961.sp006658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogston A. G. On water binding. Fed Proc. 1966 May-Jun;25(3):986–989. [PubMed] [Google Scholar]
- Ogston A. G., Stanier J. E. On the state of hyaluronic acid in synovial fluid. Biochem J. 1950 Mar;46(3):364–376. doi: 10.1042/bj0460364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker K. H., Winlove C. P. The macromolecular basis of the hydraulic conductivity of the arterial wall. Biorheology. 1984;21(1-2):181–196. doi: 10.3233/bir-1984-211-221. [DOI] [PubMed] [Google Scholar]
- Pedley T. J., Fischbarg J. The development of osmotic flow through an unstirred layer. J Theor Biol. 1978 Feb 20;70(4):427–447. doi: 10.1016/0022-5193(78)90251-5. [DOI] [PubMed] [Google Scholar]
- Radin E. L., Paul I. L., Swann D. A., Schottstaedt E. S. Lubrication of synovial membrane. Ann Rheum Dis. 1971 May;30(3):322–325. doi: 10.1136/ard.30.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. J., Unsworth A., Mian N. Modes of lubrication in human hip joints. Ann Rheum Dis. 1982 Jun;41(3):217–224. doi: 10.1136/ard.41.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUNBLAD L. Studies on hyaluronic acid in synovial fluids. Acta Soc Med Ups. 1953 Apr 29;58(3-4):113–238. [PubMed] [Google Scholar]
- Shaw M., Schy A. Exclusion in hyaluronate gels. Biophys J. 1977 Jan;17(1):47–55. doi: 10.1016/S0006-3495(77)85626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedgui A., Lever M. J. The interaction of convection and diffusion in the transport of 131I-albumin within the media of the rabbit thoracic aorta. Circ Res. 1985 Dec;57(6):856–863. doi: 10.1161/01.res.57.6.856. [DOI] [PubMed] [Google Scholar]


