Abstract
Liposome lipid peroxidation induced by cold atmospheric pressure plasma jet (CAPPJ) irradiation was investigated. The formation of thiobarbituric acid reactive substances (TBARS), an indicator of lipid peroxidation final products, as a function of irradiation was observed. Lipid radicals, peroxidation reaction intermediates generated by CAPPJ irradiation, were confirmed by increased NBD-pen fluorescence intensity. Additionally, lipid peroxidation products, liposomal phosphatidylcholine (PC) isomers, were analyzed by LC-MS/MS. Products specific to singlet oxygen (1O2) oxidation, 16:0/10-hydroperoxy-8E,12Z-octadecanoic acid (10-8E,12Z-HpODE) PC and 16:0/12-9E,13E-HpODE PC, were not detected, but radical oxidation specific products 16:0/13-9E,11E-HpODE PC and 16:0/9-10E,12E-HpODE PC were. This suggests that during CAPPJ irradiation, radicals, rather than 1O2, are the primary reactive species of lipid peroxidation. This is also supported by the β-carotene quenching of 1O2 not suppressing TBARS and lipid radical generation. Also, neither TBARS formation nor lipid radical generation were suppressed by SOD, indicating that the superoxide radical (O2•−) is not responsible for the lipid peroxidation reaction. As the CAPPJ irradiation of water produces large quantities of hydroxyl radical (•OH) and •OH scavengers decreased the amount of TBARS produced by CAPPJ irradiation, it is highly plausible that •OH is the primary species involved in CAPPJ-induced liposome lipid peroxidation.
Keywords: cold atmospheric pressure plasma jet, liposome lipid peroxidation, TBARS, NBD-pen, LC-MS/MS
Introduction
Plasma is a state of matter consisting of excited gas molecules, positively and negatively charged ions, free electrons, free radicals, and molecular fragments, emitting UV and visible light.(1,2) Plasma, with distinct characteristics setting it apart from ordinary neutral gases, is considered a fourth state of matter, in addition to solids, fluids, and gases. Plasma technology now enables plasma generation at near atmospheric pressure and room temperature, in a process referred to as cold atmospheric pressure plasma (CAP).
In addition to various physical and industrial applications, CAP is a promising tool for biomedical applications as it generates reactive species in a controlled manner. Plasma use in medicine has developed over the last decade, with applications including cancer cell apoptosis induction, wound healing, and skin disease damage regeneration.(3–5) Although the mechanisms behind plasma medicine’s mode of action remain unclear, the most widely accepted theory assumes the action of oxidative stress, caused by CAP generated reactive oxygen species (ROS).
Lipids are essential cell membrane components maintaining cellular structure and influencing cell function. However, lipids are vulnerable to ROS, with lipid oxidation implicated in various pathological conditions. Several human diseases are thought to involve lipid peroxidation, including atherosclerosis,(6) cancer,(7,8) diabetes,(9) and even neurodegenerative diseases(10) such as Alzheimer’s(11) and Parkinson’s disease.(12) Lipid peroxidation is a process in which free radical species, such as oxyl radicals, peroxyl radicals, and hydroxyl radicals, remove electrons from lipids to produce reactive intermediates that undergo further reactions. Products of lipid peroxidation chain reactions display high biological activity,(13) destroying DNA, proteins, and enzyme activity, as well as activating cell death pathways.(14)
CAP induces ROS formation, generating species including hydroxyl radicals (•OH), superoxide anion radicals (O2•−), and reactive nitrogen species (RNS), such as nitric oxide (•NO) and peroxynitrite (ONOO−).(15–18) Previously, we reported the generation of ROS such as •OH, •H, H2O2, and 1O2 in water by cold atmospheric pressure plasma jet (CAPPJ) irradiation.(19,20) These reactive species can cause lipid peroxidation. In fact, it was reported that when E. coli was irradiated with CAP, lipids were peroxidized and 1O2 and H2O2 are released into the culture medium and cells.(21) However, the identity of the primary responsible species for lipid peroxidation remains unclear.
As lipid peroxidation is likely a fundamental component of plasma medicine treatments, we attempted identification of the major active species responsible for CAPPJ irradiation induced liposome lipid peroxidation. During experimental evaluation of these effects, the inherent antioxidant systems of cells and cell membranes may greatly complicate experimental results. Therefore, phosphatidylcholine (PC) liposomes were used. This simple system contains only lipids, avoids potential issues related to cell antioxidant systems, better mimics the cell membrane aspects, and simplifies the interpretation of direct CAP reaction results.
Materials and Methods
Chemicals
Egg yolk PC was obtained from Nippon Oil & Fats Co. Ltd. 2,2,6-trimethyl-4-(4-nitrobenzo [1,2,5] oxadiazol-7-ylamino)-6-pentylpiperidine-1-oxyl (NBD-Pen) was purchased from COSMO Bio. Co. Ltd. (Tokyo, Japan). Xanthine oxidase was purchased from Oriental Yeast Co., Ltd. (Tokyo, Japan), and superoxide dismutase (SOD) from bovine erythrocyte was obtained from Sigma-Aldrich Co. (St. Louis, MO). Other reagents were obtained from Fujifilm Wako Pure Chemical Corporation (Osaka, Japan). All reagents were of the highest grade available and used without further purification.
Preparation of liposomes
The liposome (large unilamellar vesicles) samples were prepared as follows. Briefly, into a round bottom flask was added 3.8 ml of chloroform containing egg yolk PC (10 mg/ml) with the solvent subsequently removed by rotary evaporation. The residue was then dispersed in diethyl ether and KCl-Tris-HCl buffer (10 mM, pH 7.4) was added. The solution was sonicated, and the diethyl ether partially removed by rotary evaporation. The residue was then mixed using a vortex mixer, and diethyl ether further removed using a rotary evaporator producing the liposomes. To prepare sample suspensions, the resultant liposome suspension was diluted 50 times with KCl-Tris-HCl buffer.
CAPPJ irradiation of liposomes
CAPPJ was generated using a plasma head (TPN-20; NU global, Nagoya, Japan) and an electric regulating unit (PN-110TPG; NU global) with helium as the carrier gas. The helium gas flow rate (3 L/min) was regulated by a mass flow controller (CUBE GM2; Fcon Co., Ltd., Kochi, Japan). The 1-ml sample suspension was placed in a glass container with 2-cm inner diameter and 2-cm height and irradiated with CAPPJ at a distance of 1.5 cm between the sample surface and plasma jet tip.
Measurement of 2-thiobarbituric acid-reactive substances (TBARS)
The quantity of TBARS was measured to evaluate lipid peroxidation. Trichloroacetic acid (15%), thiobarbituric acid (TBA, 0.375%), and butylated hydroxytoluene (0.04%) were dissolved in hydrochloric acid aqueous solution (0.25 mol/L). The CAPPJ-irradiated liposome sample was added to the above mixed solution, heated for 15 min at 95°C, and then cooled to room temperature. TBARS were measured at 532 nm using a UV-VIS spectrophotometer (UV-mini 1240; SHIMADZU, Kyoto, Japan).
Measurement of lipid radicals
Lipid radicals generated by CAPPJ irradiation were detected using the NBD-pen fluorescent probe. NBD-pen is a fluorescence probe reported to primarily react with lipid radicals and minimally with ROS.(22) To 12.5 μl of the 2 mM NBD-pen solution was added 1 ml of the liposome sample suspension and, after mixing, was irradiated with CAPPJ. Then, 1.5 ml of KCl-Tris-HCl buffer was added, and the fluorescence intensity (ex 470 nm, em 530 nm) measured using a spectrofluorophotometer (RF-5300PC; SHIMADZU).
Analysis of lipid peroxidation isomers by LC-MS/MS method
To identify the major active species responsible for lipid peroxidation, PC hydroperoxide isomers were analyzed according to a previous report with minor modifications. Total lipids were extracted from liposomes using the Folch method, and the PC fraction purified via solid-phase extraction.(23) The obtained PC fractions were then analyzed using a 4000QTRAP system equipped with an Exion LC (SCIEX, Tokyo, Japan). PC hydroperoxide isomers were separated using a COSMOSIL column 5C18-MS-II (5 μm, 2.0 × 150 mm; Nacalai Tesque, Kyoto, Japan) with a binary gradient composed of solvent A (methanol) and solvent B (water). The gradient profile was as follows: 0 to 20 min, 90 to 100% B linear; 20 to 30 min, 100% B; 30 to 30.1 min, 100 to 90% B linear. A flow rate of 0.2 ml/min was used, and a column temperature of 40°C. Eluted column fractions were then mixed with 2 mM sodium acetate methanol solution (0.01 ml/min) to form the sodium adduct. PC hydroperoxide isomers were detected using multiple reaction monitoring.(24)
Statistical analysis
The statistical analysis of mean values was carried out using Welch’s t test. Differences with a p value <0.05 was considered statistically significant.
Results
Formation of TBARS by CAPPJ irradiation of liposomes
Liposomal suspensions were subjected to CAPPJ irradiation, and the products of lipid peroxidation measured using the TBA method. Lipid peroxidation products react with TBA forming TBARS, indicated by an absorption peak around 532 nm. With increased CAPPJ irradiation time, there is a concurrent increase in absorbance at 532 nm of the irradiated liposome reaction mixture, indicating an increase in TBARS concentration (Fig. 1).
Fig. 1.
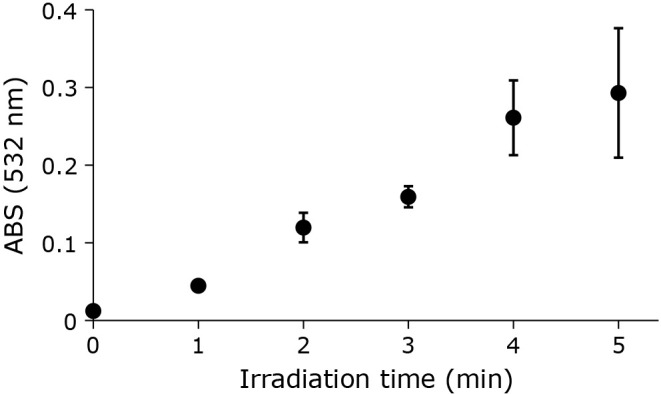
Time-dependent TBARS formation by CAPPJ irradiation of liposome suspension. The liposome suspension (1 ml) was CAPPJ irradiated at a distance of 1.5 cm. The helium gas flow rate was 3 L/min. After irradiation, TBARS formation was evaluated by measuring the absorbance at 532 nm. Error bar shows SD (n = 3).
Formation of lipid radicals by CAPPJ irradiation of liposomes
When liposomes premixed with NBD-pen were irradiated with CAPPJ, fluorescence induced by the reaction of NBD-pen with lipid radicals was detected. A time-dependent increase in fluorescence intensity was observed during CAPPJ irradiation (Fig. 2). No fluorescence intensity increase was observed when an NBD-pen solution without liposomes was irradiated (data not shown). These findings confirm the formation of lipid radicals during CAPPJ irradiation of the liposomal suspension. When only helium gas was blown onto the sample, no fluorescence intensity increase was observed (Fig. 2). Furthermore, no fluorescence intensity increase was observed when plasma gas flow was blocked by covering the sample container with a thin transparent quartz plate (Fig. 3). This confirmed that the observed reaction is not due to the light emitted by CAP, but is derived from the active species in the CAP gas.
Fig. 2.
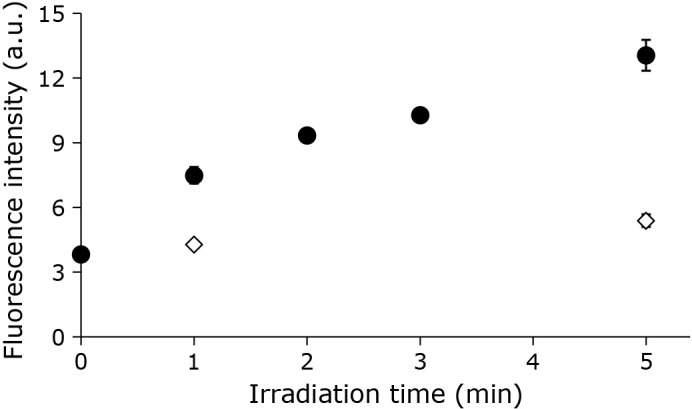
Lipid radical production by CAPPJ irradiation of liposome suspension. The liposome suspension (1 ml) containing NBD-pen, a fluorescent probe, was CAPPJ irradiated at a distance of 1.5 cm. The helium gas flow rate was 3 L/min. Sample fluorescence intensity was measured (ex 470 nm, em 530 nm) immediately after irradiation. Closed circles show the CAPPJ irradiated sample fluorescence intensity. Open diamonds show the fluorescence intensity without CAPPJ irradiation irradiated (Only helium gas used). Error bar shows SD (n = 3).
Fig. 3.
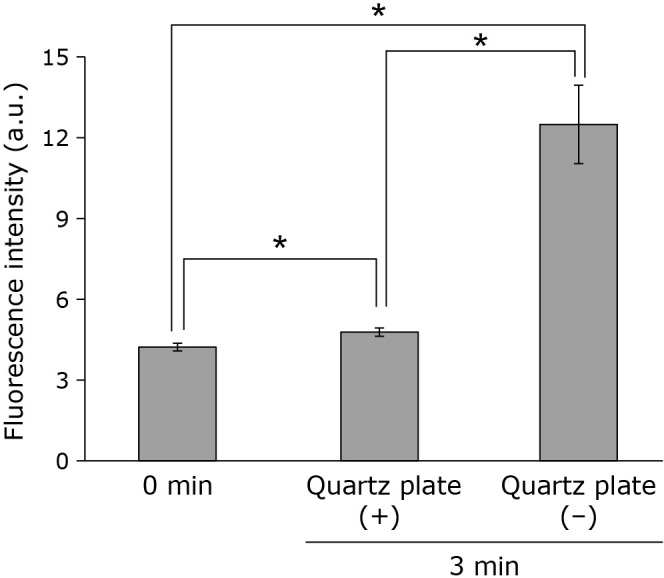
Requirement for the CAP gas flow to hit the sample for CAPPJ induced lipid radical production. The liposome suspension (1 ml) containing NBD-pen fluorescent probe was CAPPJ irradiated at a distance of 1.5 cm for 3 min. The sample was irradiated with a thin quartz plate placed on top of the glass container, allowing only UV and visible light to reach the sample while blocking gas flow [quartz plate (+)]. For comparison, fluorescence intensity without the thin quartz plate was measured [quartz plate (−)]. The helium gas flow rate was 3 L/min. Fluorescence was measured immediately after irradiation. Error bar shows SD (n = 3). * represents p<0.05.
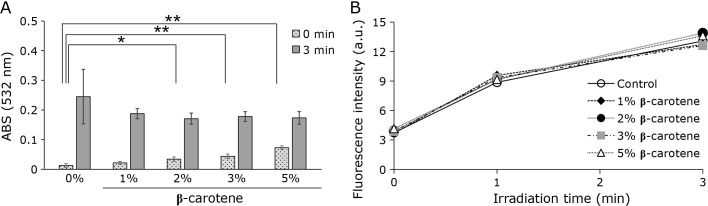
Effects of β-carotene (β-car) on the formation of TBARS and lipid radicals by CAPPJ irradiation of liposomes
Next, the ROS species involved in the CAPPJ irradiation induced liposome lipid peroxidation was investigated. First, the singlet oxygen (1O2) scavenger β-car was incorporated in the liposomal membranes. Liposomes containing β-car were irradiated with CAPPJ with TBARS formation measured at 532 nm. As shown in Fig. 4A, the increase in TBARS by CAPPJ irradiation was slightly diminished with increasing β-car concentration, but complete inhibition was not observed, even for the 5% β-car sample. The absorbance at 532 nm at 0 min (no CAPPJ treatment) was slightly elevated with increasing β-car amounts, presumably due to β-car absorbance. Upon CAPPJ irradiation of liposomes containing 1–5% β-Car for 1 min to 3 min, no lipid radical formation suppression was observed (Fig. 4B).
Fig. 4.
Effect of β-car on lipid peroxidation by CAPPJ irradiation. (A) Liposomes containing various concentrations of β-car were CAPPJ irradiated for 3 min and TBARS formation measured by the absorbance at 532 nm. Error bar shows SD (n = 3). (B) Liposomes containing various concentrations of β-car were mixed with the NBD-pen fluorescent probe and samples irradiated with CAPPJ for 1 or 3 min. Fluorescence intensity was measured immediately after irradiation. Error bar shows SD (n = 3). * represents p<0.05 and ** represents p<0.01.
Isomers of lipid peroxides produced by CAPPJ irradiation
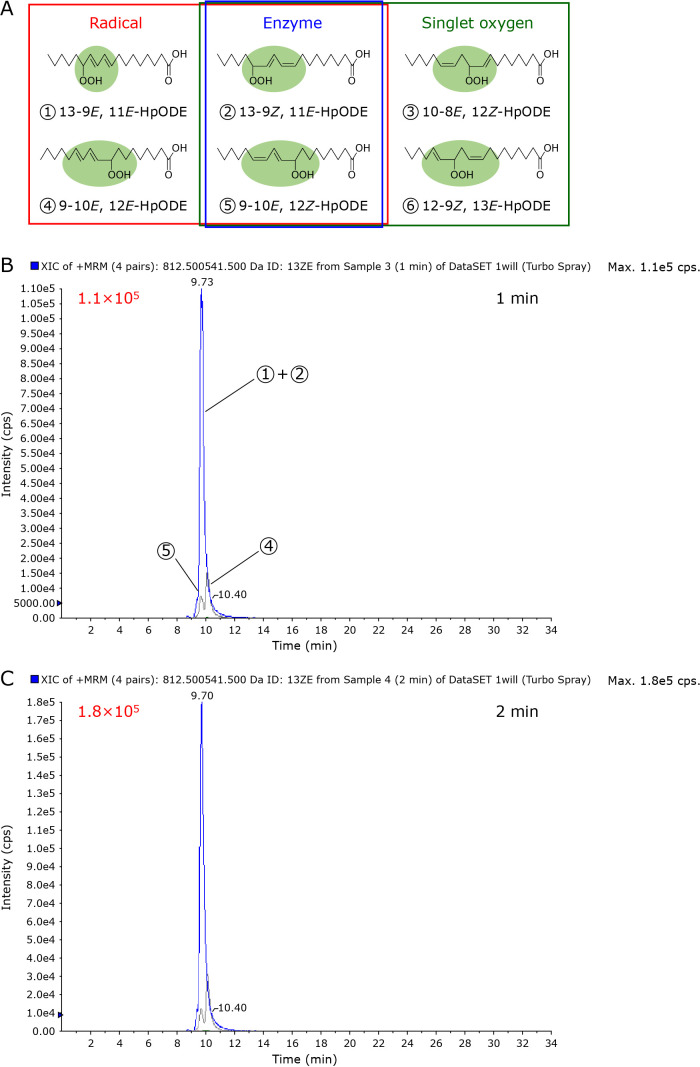
Lipid peroxidation results in the formation of different isomeric structures, which are produced by the lipid peroxidation mechanism. It is reported that 13-hydroperoxy-9E,11E-octadecadienoic acid (13-9E,11E-HpODE, ① in Fig. 5A), 13-9Z,11E-HpODE (② in Fig. 5A), 9-10E,12E-HpODE (④ in Fig. 5A) and 9-10E,12Z-HpODE (⑤ in Fig. 5A) are formed during the radical-induced lipid peroxidation of linoleic acid.(25,26) Upon 1O2-induced peroxidation, 13-9Z,11E-HpODE (②), 9-10E,12Z-HpODE (⑤), 10-8E,12Z-HpODE (③ in Fig. 5A), and 12-9Z,13E-HpODE (⑥ in Fig. 5A) are formed.(25,27) Therefore, 16:0/13-9E,11E-HpODE PC (①) and 16:0/9-10E,12E-HpODE PC (④) are specifically products of radical-induced PC peroxidation, while 16:0/10-8E,12Z-HpODE PC (③) and 16:0/12-9Z,13E-HpODE PC (⑥) are products of 1O2-induced PC peroxidation. As shown in Fig. 5B, CAPPJ irradiation of liposomes for 1 min formed large quantities of ① + ② (intensity 1.1 × 105) at retention time of 9.73 min. Small peaks of ④ and ⑤ were also detected. A similar pattern but larger chromatogram intensity was obtained by evaluation of the liposomes subjected to CAPPJ irradiation for 2 min. Products of 1O2-induced peroxidation, ③ and ⑥, could not be detected even after irradiation for 2 min. This shows clearly that the major CAPPJ irradiation induced peroxidation pathway is radical dependent.
Fig. 5.
Hydroperoxide isomers produced by CAPPJ irradiation induced lipid peroxidation. (A) General oxidation mechanisms of lipids and isomer structure. (B) LC–MS/MS chromatogram of lipid peroxides produced by 1 min of CAPPJ exposure. (C) LC–MS/MS chromatogram of the lipid peroxides produced by 2 min of CAPPJ exposure.
Effects of SOD on liposome peroxidation by CAPPJ irradiation
The above results show that 1O2 is unlikely to contribute to liposome lipid peroxidation, indicating that radical ROSs are involved in CAPPJ irradiation induced lipid peroxidation. As several radical species, such as O2•− and •OH, are produced by CAPPJ irradiation of water, the involvement of these ROS in lipid peroxidation were investigated. To examine the possibility of lipid peroxidation by O2•− produced by CAPPJ irradiation, TBARS formation and lipid radical formation in the presence of SOD was measured. As shown in Fig. 6, CAPPJ irradiation resulted in an absorbance increase, indicating TBARS formation and the continued generation of lipid radicals, despite the addition of SOD.
Fig. 6.
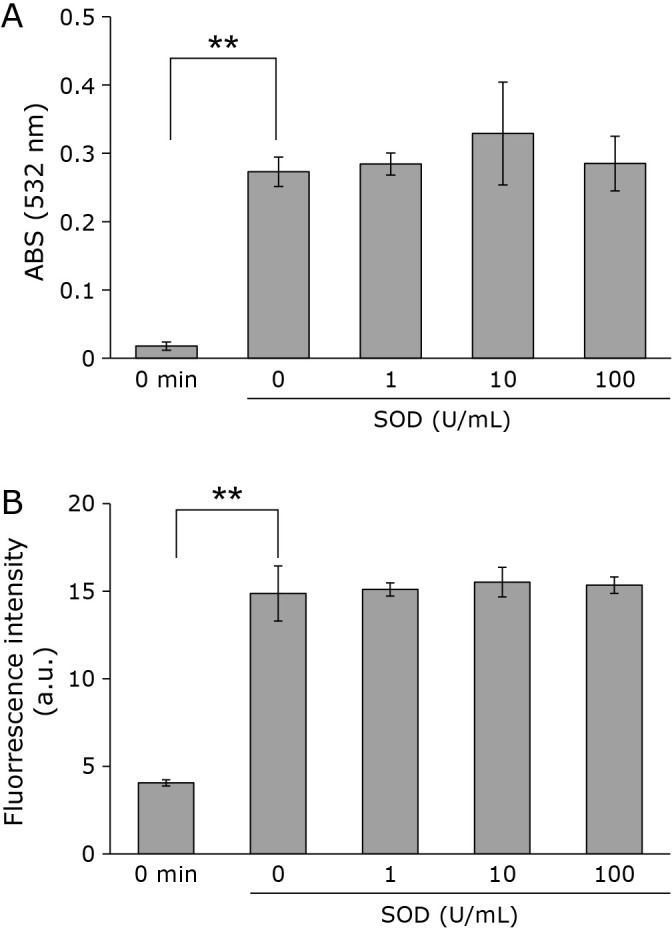
Lipid peroxidation by CAPPJ irradiation in the presence of SOD. SOD of various concentrations was added to the liposome suspension and TBARS (A) with the measured fluorescence intensity (B) of samples after 3 min of CAPPJ irradiation. Error bar shows SD (n = 3). ** represents p<0.01.
Effects of chemically generated O2•− on liposome peroxidation
To confirm that O2•− does not contribute to liposome lipid peroxidation, the hypoxanthine-xanthine oxidase (Hyp-XOD) system to generate O2•− was used. Upon combining the liposome suspension and Hyp-XOD reaction solution, no increase in absorption indicative of an increase in TBARS was observed (Fig. 7A). Although slight increase in the NBD-pen fluorescence was observed (Fig. 7B), the change does not indicate the lipid radical formation, because similar slight increase was also observed in the control experiment of Fig. 2 (containing no liposomes). Thus, as lipid peroxidation was not observed using O2•− chemically generated outside the liposome membrane, O2•− is not involved in CAPPJ irradiation induced lipid peroxidation.
Fig. 7.
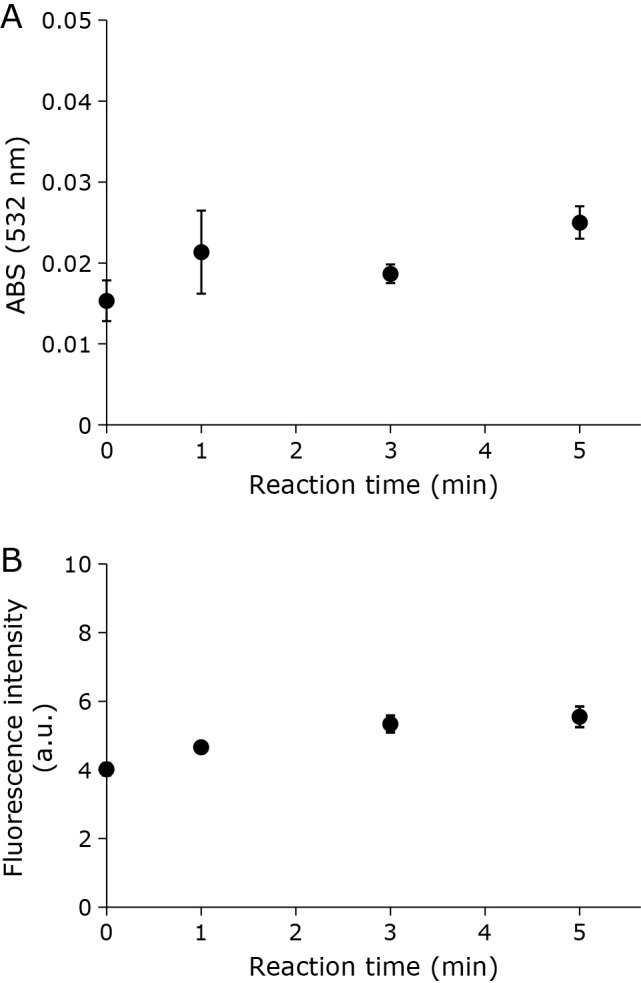
Effect of chemically generated •O2− on liposome lipid peroxidation. The liposome suspension combined with the O2•− generating system (Hyp-XOD). (A) TBARS was measured after reaction. (B) NDB-pen was also added to the liposome suspension, and fluorescence intensity measured after reaction. Error bar shows SD (n = 3).
Effects of •OH on liposome peroxidation by CAPPJ irradiation
Next, the possibility that •OH induced liposome lipid peroxidation was investigated using •OH scavengers of various reaction rates. As shown in Fig. 8, most of •OH scavengers suppressed the formation of TBARS. No effect was observed by mannitol probably due to its smaller rate constant of the reaction with •OH in addition to its low solubility in membrane lipid phase.
Fig. 8.
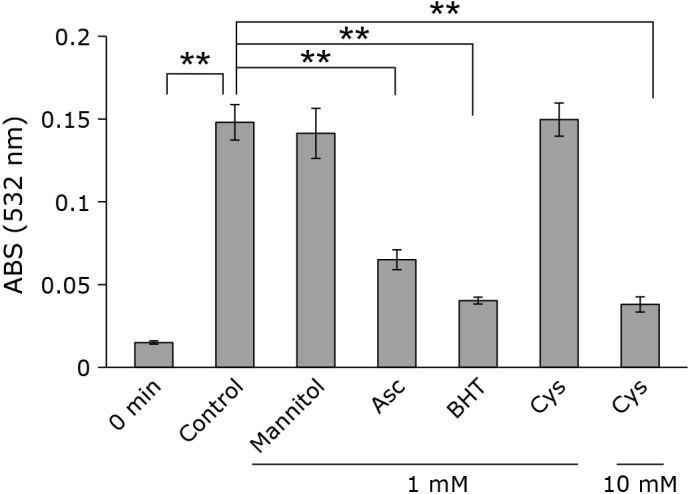
Effect of •OH sucavenger on lipid peroxidation by CAPPJ irradiation. CAPPJ was irradiated to the liposome suspension containing a •OH scavenger such as mannitol, ascorbic acid (Asc), buthylhydroxyltoluene (BHT), or cysteine (Cys) for 3 min and TBARS was measured. Error bar shows SD (n = 3). ** represents p<0.01.
Discussion
Previous studies reported CAP irradiation results in an increase in TBARS,(28) but the detailed mechanisms of CAP irradiation induced lipid peroxidation remain unclear. In the present study, the CAP irradiation induced lipid peroxidation of liposomes was investigated by measuring lipid radicals, reaction intermediates, and TBARS, end products. As shown in Fig. 1 and 2, CAPPJ irradiation of liposomes was confirmed to increase both TBARS and lipid radicals.
Lipids are peroxidized by (1) enzymatic oxidation by enzymes such as lipoxygenase, (2) autoxidation by radicals, and (3) photo-oxidation mediated by 1O2 with each oxidation process producing characteristic isomer products.(25–27) In the peroxidation of PC containing a linoleic acid fatty acyl chain, radical oxidation produces 16:0/9-10E,12Z-HpODE PC, 16:0/9-10E,12E-HpODE PC, 16:0/13-9Z,11E-HpODE PC and 16:0/13-9E,11E-HpODE PC. Oxidation with 1O2 produces 16:0/9-10E,12Z-HpODE PC, 16:0/10-8E,12Z-HpODE PC, 16:0/12-9Z,13E-HpODE PC, and 16:0/13-9Z,11E-HpODE PC. Therefore, the isomeric structures of lipid peroxidation products provide information elucidating lipid peroxidation pathways. Positive mode MS/MS analysis with alkali metal ions is reported for hydroperoxide isomer analysis, achieving high sensitivity and selectivity.(23,29,30) In the present study, the HpODE isomers produced by CAPPJ irradiation were analyzed using a similar method. As shown in Fig. 5,13-HpODE and 9-HpODE was detected in the CAPPJ-irradiated sample. Furthermore, β-car, a 1O2 scavenger, did not inhibit CAPPJ irradiation induced formation of lipid-derived radicals (Fig. 4). These findings indicate that CAPPJ irradiation induced lipid peroxidation is caused by radicals rather than 1O2.
CAPPJ irradiation of water generates numerous ROSs, and we previously reported the CAPPJ irradiation induced generation of •OH, 1O2, H2O2, and O2•−(19,20) under conditions used in this study (helium gas flow rate was 3 L/min, and the distance from sample surface to plasma jet tip was 1.5 cm). Next, whether the CAPPJ irradiation generated O2•− was involved in liposome lipid peroxidation was evaluated. Upon liposome irradiation by CAPPJ in the presence of SOD, no reduction in the production of lipid TBARS or lipid radicals was observed (Fig. 6). Additionally, when the Hyp-XOD system was used to chemically generate O2•−, the liposomes showed no increase in lipid radical or TBARS formation (Fig. 7), supporting the findings that O2•− does not initiate lipid peroxidation.(31)
In this study, the involvement of CAPPJ irradiation generated •NO and ONOO− in liposome lipid peroxidation was not investigated. As CAP irradiation induced nitrogen oxides (NOx) formation is reported,(15,32) their involvement in lipid peroxidation cannot be ruled out. However, •NO is insufficiently reactive to extract a bis-allylic hydrogen from an unsaturated fatty acid,(33) instead potentially acting as an antioxidant, preventing lipid chain reactions by reacting with lipid radicals.(34,35) Another nitrogenous radical, formed by the reaction of •NO and O2•−, is ONOO−. Upon addition of SOD, which reduces O2•− thus limiting ONOO− formation, no CAPPJ irradiation induced lipid peroxidation inhibition was observed (Fig. 6). This finding suggests that ONOO− involvement in lipid peroxidation during CAPPJ irradiation is unlikely.
•OH scavengers decreased the amount of TBARS produced by CAPPJ irradiation (Fig. 8). We previously reported that CAPPJ irradiation of water generates at least 270 μM •OH and about 17 μM 1O2 in 1 min under helium gas flow rates of 5 L/min.(19,20) Thus, the amount of •OH generated by CAPPJ irradiation of water is 15 times higher than that of 1O2. Furthermore, we found that the amount of NO2 generated by 1-min irradiation of CAPPJ to water is around several μM (unpublished). Also, •OH is very reactive towards all biological molecules, usually initiating free-radical chain reactions.(36) Considering all together, we can say that •OH is the primary species driving lipid peroxidation during CAPPJ irradiation.
In conclusion, this study’s findings eliminate 1O2 and O2•− as species responsible for lipid peroxidation under CAPPJ irradiation. Furthermore, as •NO and ONOO− are also unlikely to be responsible for lipid peroxidation and •OH scavengers decreased the amount of TBARS produced by CAPPJ irradiation, •OH may be the primary responsible species for liposome lipid peroxidation during CAPPJ irradiation.
Author Contributions
TT contributed to the study design, data acquisition, and manuscript drafting. KS collected data. SK and KN performed LC-MS/MS experiments. KT and AO contributed to critical revision of the manuscript. KA supervised the study, data interpretation and critical revision of the manuscript.
Acknowledgments
We thank Dr. Ken-ichi Yamada of Kyushu University for suggesting the use of NBD-pen. We also thank Kenta Ueno, Shiori Fukuda, Kana Kakiuchi, and Ako Fukutomi for their contribution to basic experiments related to this study. This work was supported in part by JSPS KAKENHI Gran Number 16K08203 to KA. A part of this research is based on the Cooperative Research Project of Research Center for Biomedical Engineering.
Abbreviations
- β-car
β-carotene
- CAP
cold atmospheric pressure plasma
- CAPPJ
cold atmospheric pressure plasma jet
- HpODE
hydroperoxyl octadecadienoic acid
- Hyp-XOD
hypoxanthine-xanthine oxidase
- NBD-Pen
2,2,6-trimethyl-4-(4-nitrobenzo [1,2,5] oxadiazol-7-ylamino)-6-pentylpiperidine-1-oxyl
- PC
phosphatidylcholine
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TBA
thiobarbituric acid
- TBARS
2-thiobarbituric acid-reactive substances
Conflict of Interest
We received no financial support or other benefits from commercial sources for the work reported in the manuscript. None of the authors have financial interests that could create a potential conflict of interest or the appearance of a conflict of interest with regard to this work.
References
- 1.Volkov AG, Hairston JS, Taengwa G, Roberts J, Liburd L, Patel D. Redox reactions of biologically active molecules upon cold atmospheric pressure plasma treatment of aqueous solutions. Molecules 2022; 27: 7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reuter S, von Woedtke T, Weltmann KD. The kINPen—a review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J Phys D Appl Phys 2018; 51: 233001. [Google Scholar]
- 3.Brun P, Brun P, Vono M, et al. Disinfection of ocular cells and tissues by atmospheric-pressure cold plasma. PLoS One 2012; 7: e33245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn HJ, Kim KI, Kim G, Moon E, Yang SS, Lee JS. Atmospheric-pressure plasma jet induces apoptosis involving mitochondria via generation of free radicals. PLoS One 2011; 6: e28154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmidt A, Wende K, Bekeschus S, et al. Non-thermal plasma treatment is associated with changes in transcriptome of human epithelial skin cells. Free Radic Res 2013; 47: 577–592. [DOI] [PubMed] [Google Scholar]
- 6.Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med 1996; 20: 707–727. [DOI] [PubMed] [Google Scholar]
- 7.Hammad LA, Wu G, Saleh MM, et al. Elevated levels of hydroxylated phosphocholine lipids in the blood serum of breast cancer patients. Rapid Commun Mass Spectrom 2009; 23: 863–876. [DOI] [PubMed] [Google Scholar]
- 8.Van der Paal J, Neyts EC, Verlackt CCW, Bogaerts A. Effect of lipid peroxidation on membrane permeability of cancer and normal cells subjected to oxidative stress. Chem Sci 2016; 7: 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal 2009; 2: re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonian NA, Coyle JT. Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol 1996; 36: 83–106. [DOI] [PubMed] [Google Scholar]
- 11.Montine TJ, Montine KS, McMahan W, Markesbery WR, Quinn JF, Morrow JD. F2-isoprostanes in Alzheimer and other neurodegenerative diseases. Antioxid Redox Signal 2005; 7: 269–275. [DOI] [PubMed] [Google Scholar]
- 12.Porter FD, Scherrer DE, Lanier MH, et al. Cholesterol oxidation products are sensitive and specific blood-based biomarkers for Niemann-Pick C1 disease. Sci Transl Med 2010; 2: 56ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarkovic K, Jakovcevic A, Zarkovic N. Contribution of the HNE-immunohistochemistry to modern pathological concepts of major human diseases. Free Radic Biol Med 2017; 111: 110–126. [DOI] [PubMed] [Google Scholar]
- 14.Łucaj W, Gęgotek A, Skrzydlewska E. Antioxidants and HNE in redox homeostasis. Free Radic Biol Med 2017; 111: 87–101. [DOI] [PubMed] [Google Scholar]
- 15.Takamatsu T, Uehara K, Sasaki Y, et al. Investigation of reactive species using various gas plasmas. RSC Adv 2014; 4: 39901–39905. [Google Scholar]
- 16.Kawano H, Takamatsu T, Matsumura Y, Miyahara H, Iwasawa A, Okino A. Influence of gas temperature in atmospheric non-equilibrium plasma on bactericidal effect. Biocontrol Sci 2018; 23: 167–175. [DOI] [PubMed] [Google Scholar]
- 17.Kurake N, Tanaka H, Ishikawa K, et al. Effects of •OH and •NO radicals in the aqueous phase on H2O2 and NO2− generated in plasma-activated medium. J Phys D Appl Phys 2017; 50: 155202. [Google Scholar]
- 18.Rehman MU, Jawaid P, Uchiyama H, Kondo T. Comparison of free radicals formation induced by cold atmospheric plasma, ultrasound, and ionizing radiation. Arch Biochem Biophys 2016; 605: 19–25. [DOI] [PubMed] [Google Scholar]
- 19.Anzai K, Aoki T, Koshimizu S, Takaya R, Tsuchida K, Takajo T. Formation of reactive oxygen species by irradiation of cold atmospheric pressure plasma jet to water depends on the irradiation distance. J Clin Biochem Nutr 2019; 64: 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takajo T, Nagahama H, Zuinen K, Tsuchida K, Okino A, Anzai K. Evaluation of cold atmospheric pressure plasma irradiation of water as a method of singlet oxygen generation. J Clin Biochem Nutr 2023; 73: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joshi SG, Cooper M, Yost A, et al. Nonthermal dielectric-barrier discharge plasma-induced inactivation involves oxidative DNA damage and membrane lipid peroxidation in Escherichia coli. Antimicrob Agents Chemother 2011; 55: 1053–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada K, Mito F, Matsuoka Y, et al. Fluorescence probes to detect lipid-derived radicals. Nat Chem Biol 2016; 12: 608–613. [DOI] [PubMed] [Google Scholar]
- 23.Kato S, Nakagawa K, Suzuki Y, et al. Liquid chromatography-tandem mass spectrometry determination of human plasma 1-palmitoyl-2-hydroperoxyoctadecadienoyl-phosphatidylcholine isomers via promotion of sodium adduct formation. Anal Biochem 2015; 471: 51–60. [DOI] [PubMed] [Google Scholar]
- 24.Kato S, Osuka Y, Khalifa S, Obama T, Itabe H, Nakagawa K. Investigation of lipoproteins oxidation mechanisms by the analysis of lipid hydroperoxide isomers. Antioxidants (Basel) 2021; 10: 1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niki E, Yoshida Y, Saito Y, Noguchi N. Lipid peroxidation: mechanisms, inhibition, and biological effects. Biochem Biophys Res Commun 2005; 338: 668–676. [DOI] [PubMed] [Google Scholar]
- 26.Gupta KK, Gupta VK, Shirasaka T. An update on fetal alcohol syndrome-pathogenesis, risks, and treatment. Alcohol Clin Exp Res 2016; 40: 1594–1602. [DOI] [PubMed] [Google Scholar]
- 27.Frankel EN. Chemistry of free radical and singlet oxidation of lipids. Prog Lipid Res 1984; 23: 197–221. [DOI] [PubMed] [Google Scholar]
- 28.Okazaki Y, Wang Y, Tanaka H, et al. Direct exposure of non-equilibrium atmospheric pressure plasma confers simultaneous oxidative and ultraviolet modifications in biomolecules. J Clin Biochem Nutr 2014; 55: 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagawa K, Kato S, Miyazawa T. Determination of phosphatidylcholine hydroperoxide (PCOOH) as a marker of membrane lipid peroxidation. J Nutr Sci Vitaminol (Tokyo) 2015; 61 Suppl: S78–S80. [DOI] [PubMed] [Google Scholar]
- 30.Ito J, Mizuochi S, Nakagawa K, Kato S, Miyazawa T. Tandem mass spectrometry analysis of linoleic and arachidonic acid hydroperoxides via promotion of alkali metal adduct formation. Anal Chem 2015; 87: 4980–4987. [DOI] [PubMed] [Google Scholar]
- 31.Bielski BH, Arudi RL, Sutherland MW. A study of the reactivity of HO2/O2− with unsaturated fatty acids. J Biol Chem 1983; 258: 4759–4761. [PubMed] [Google Scholar]
- 32.Girard F, Peret M, Dumont N, et al. Correlations between gaseous and liquid phase chemistries induced by cold atmospheric plasmas in a physiological buffer. Phys Chem Chem Phys 2018; 20: 9198–9210. [DOI] [PubMed] [Google Scholar]
- 33.Darley-Usmar VM, Hogg N, O'Leary VJ, Wilson MT, Moncada S. The simultaneous generation of superoxide and nitric oxide can initiate lipid peroxidation in human low density lipoprotein. Free Radic Res Commun 1992; 17: 9–20. [DOI] [PubMed] [Google Scholar]
- 34.Hogg N, Kalyanaraman B, Joseph J, Struck A, Parthasarathy S. Inhibition of low-density lipoprotein oxidation by nitric oxide. Potential role in atherogenesis. FEBS Lett 1993; 334: 170–174. [DOI] [PubMed] [Google Scholar]
- 35.Rubbo H, Radi R, Trujillo M, et al. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem 1994; 269: 26066–26075. [PubMed] [Google Scholar]
- 36.Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem 1995; 41: 1819–1828. [PubMed] [Google Scholar]