Abstract
BACKGROUND
Aneurysmal bone cysts (ABCs) are rare, benign, yet locally aggressive lesions that contain blood-filled channels that rarely occur in the thoracic spine of adults. The literature on the treatment of spinal ABCs is sparse, but the consensus is to achieve gross-total resection (GTR) due to these lesions being locally aggressive and to prevent recurrence.
OBSERVATIONS
This report describes a 35-year-old female admitted with back pain and right T5 dermatome radiculopathy without any inciting events. Magnetic resonance imaging revealed a 3.0 × 4.3 × 4.0–cm solid, enhancing, multicystic lesion with multiple fluid levels centered in the right posteromedial chest wall, involving the right fifth rib and costovertebral junction. Because of the high suspicion for an ABC, later found to be secondary to an osteoblastoma, surgical intervention was planned via preoperative embolization and T4–6 fusion with right T5 laminectomy and costotransversectomy to obtain GTR.
LESSONS
This case of an ABC secondary to osteoblastoma of the spine showcases a strategy for unique surgical management, given the limited information on treatment considerations for secondary ABCs.
Keywords: aneurysmal bone cysts, thoracic spine, costotransversectomy, embolization, multicystic mass, resection
ABBREVIATIONS: ABC = aneurysmal bone cyst, CT = computed tomography, GTR = gross-total resection, MRI = magnetic resonance imaging.
Aneurysmal bone cysts (ABCs) are benign lesions containing blood-filled channels that can be found in any bone of the body and can protrude into surrounding structures. They are a rare and difficult pathology for clinicians to identify accurately, with an incidence of 0.14 per 100,000, making up only 1%–2.5% of all bone tumors.1–3 Although commonly presenting in the metaphysis of long bones, ABCs rarely present within the spine and do so typically along the posterior column of the thoracolumbar spine.4–8 Even though benign, these lesions are locally aggressive and necessitate surgical intervention when involving neural elements or instability of the spine.2, 5, 6
ABCs can be either primary or secondary lesions. Secondary ABCs make up one-third of all ABCs and are derived from lesions such as osteoblastoma, giant cell tumor, and chondroblastomas.6 Osteoblastomas are also rare, benign, yet locally aggressive bone tumors, comprising about 1% of all bone tumors.9 When involving the spine, they are most commonly located in the posterior elements of the vertebral column, and management involves gross-total resection (GTR).9
This case involves a rare ABC in the thoracic vertebrae, which was found to be caused by an osteoblastoma in a 35-year-old woman, requiring a multidisciplinary surgical approach.
Illustrative Case
History and Examination
A 35-year-old right-handed female without a significant past medical/surgical history was admitted for the 3-month onset of subjective right arm weakness/numbness, upper back pain, and right T5 distribution radiculopathy. Magnetic resonance imaging (MRI) of the thoracic spine revealed a 3.0 × 4.3 × 4.0–cm, solid, enhancing, multicystic mass with multiple fluid levels, centered in the right posteromedial chest wall, involving the right fifth rib and costovertebral junction (Fig. 1). There was no obvious encroachment into the spinal canal or compression of the thecal sac, but intimate involvement of the right T5 nerve root and the respective neural foramen. Computed tomography (CT) of the thoracic spine showed an expansile, destructive, lytic chest wall mass at the level of T5, associated with the destruction of the fifth costovertebral junction, the right T5 pedicle, and erosion of the right T5 transverse process (Fig. 2). Given the clinical presentation and radiographic evidence, differential diagnoses included ABC, telangiectatic osteosarcoma, or metastatic disease from an unknown primary lesion. Further body imaging did not reveal lesions suspicious for cancer.
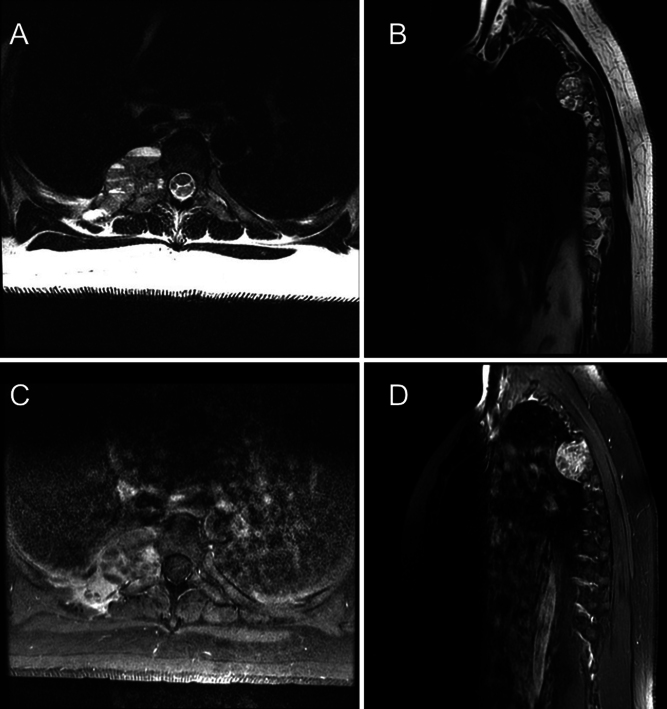
FIG. 1.
Preoperative imaging. A: Axial precontrast T2-weighted MRI at the level of T5 demonstrating 4-cm isointense lesions abutting vertebrae and encroaching on the neural foramen. B: Sagittal precontrast T2-weighted MRI of the 4-cm lesion at the T5 level abutting the pleura. C: Axial postcontrast T1-weighted MRI at the level of T5 demonstrating an avidly enhancing 4-cm lesion. D: Sagittal postcontrast T1-weighted MRI demonstrating an avidly enhancing 4-cm lesion abutting the pleura.
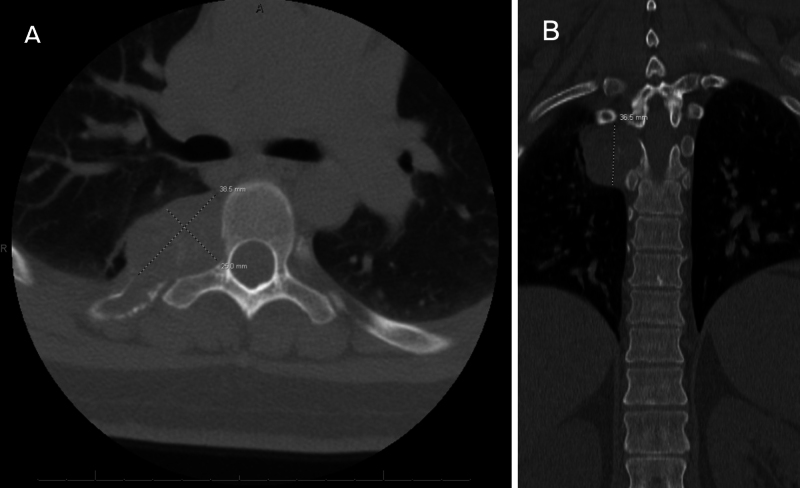
FIG. 2.
Preoperative CT. A: Axial scan at the level of T5 demonstrating a lytic, destructive lesion involving the fifth costotransverse joint and pedicle. B: Coronal scan demonstrating a lytic, destructive lesion abutting the pedicle and pleura.
Procedures
Given the high suspicion for ABC, a spinal angiogram was obtained to both assess the vascular supply of the lesion and embolize the feeding vessels. The angiogram revealed supply to the lesion from the right T4, T5, and T6 intercostal arteries. Coil embolization of the 3 arteries was performed without complication (Fig. 3). Immediately after the procedure, the patient was taken to the operating room for a T4–6 posterolateral fusion with a T5 laminectomy, partial right T5 pedicle resection, and right T5 costotransversectomy for tumor resection.
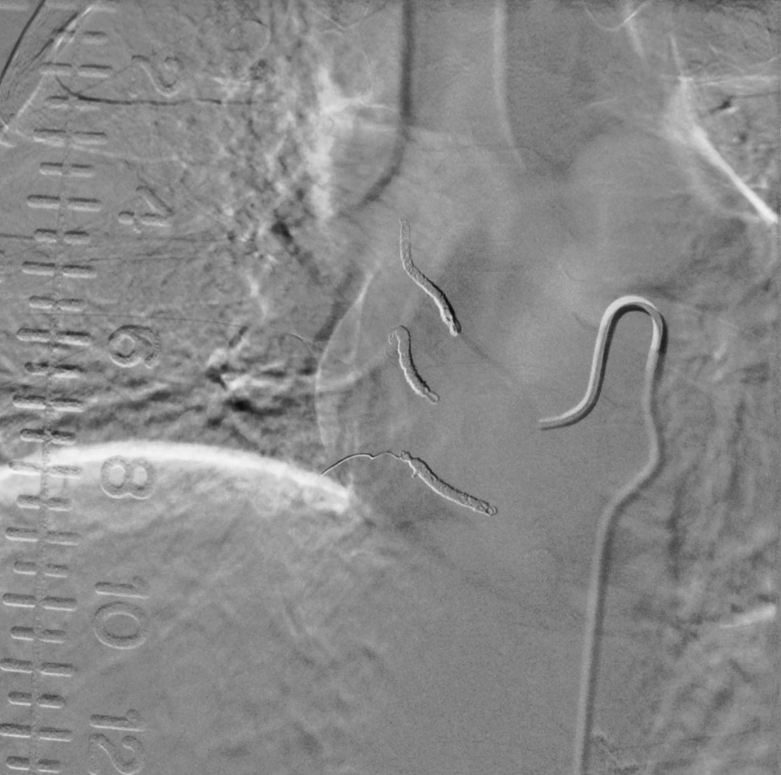
FIG. 3.
Angiogram after successful coil embolization of the right T4–6 segmental arteries.
After instrumentation and T5 laminectomy/pedicle resection with costotransversectomy, an inspection of the tumor revealed cavitations containing mixed blood products, as expected from preoperative imaging. Dissection of the tumor ensued with minimal hindrance, and the lesion was not overly bloody. Laterally, the capsule of the tumor involved the pleura of the lung, over which a plane was created to carefully separate the capsule without violating the pleura. The lesion encompassed both the right T4 and T5 nerve roots. Due to the patient’s symptoms and the degree of root involvement, both roots were tied off and transected. Intraoperatively, a near-total resection was deemed achieved, given what may have been a small rind of capsule adjacent to the pleura that was coagulated. A chest tube was inserted due to pleural involvement.
The patient had no postoperative complications and endorsed improvement from preoperative symptoms. The chest tube was removed 2 days postoperatively, and the patient was discharged without issue. Follow-up MRI with contrast showed no residual lesion, and the extent of resection was radiographically deemed GTR (Fig. 4).
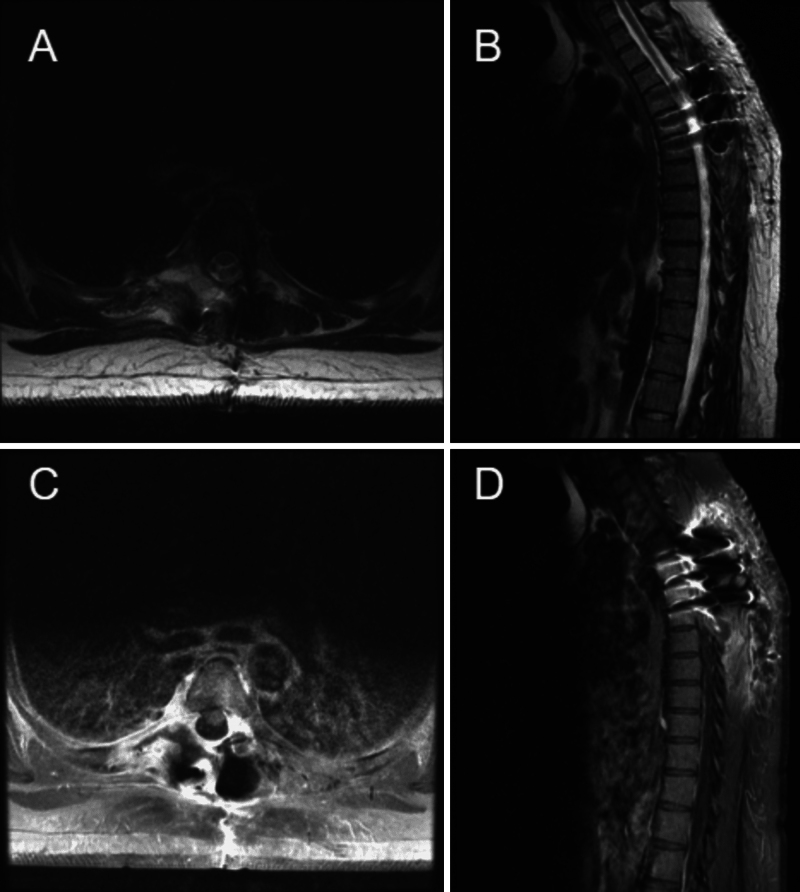
FIG. 4.
One-month postoperative MRI. A: Axial T2-weighted image at the level of T5, without obvious residual of the previous fluid-filled cystic lesion. B: Sagittal T2-weighted image showing postsurgical changes and resolution of the previous fluid-filled cystic lesion abutting the pleura. C: Axial postcontrast T1-weighted image at the level of T5 showing no enhancing remnant of the previous lesion near the vertebra. D: Sagittal postcontrast T1-weighted image without enhancing remnant of the previous lesion on the pleura.
Histology
Histological analysis of 2 specimens, both collected from the mass where it abutted the pleura between the fourth and fifth ribs, revealed an aggregate of tan-red tissue fragments and possible tan-white bone (Fig. 5A) and an aggregate of tan-pink portions of soft tissue (Fig. 5B). Sections from specimen A showed a bland, mitotically inactive spindle cell-matrix and frequent giant cells, consistent with the diagnosis of ABC (Fig. 5A). In addition, other areas in the sections displayed osteoid and woven bone, more characteristically seen in osteoblastoma, suggesting the possibility of osteoblastoma with a secondary ABC. Specimen B displayed a similar morphology (Fig. 5B).10–12 Both lesions were characterized as low grade and locally aggressive but unlikely to metastasize.
FIG. 5.
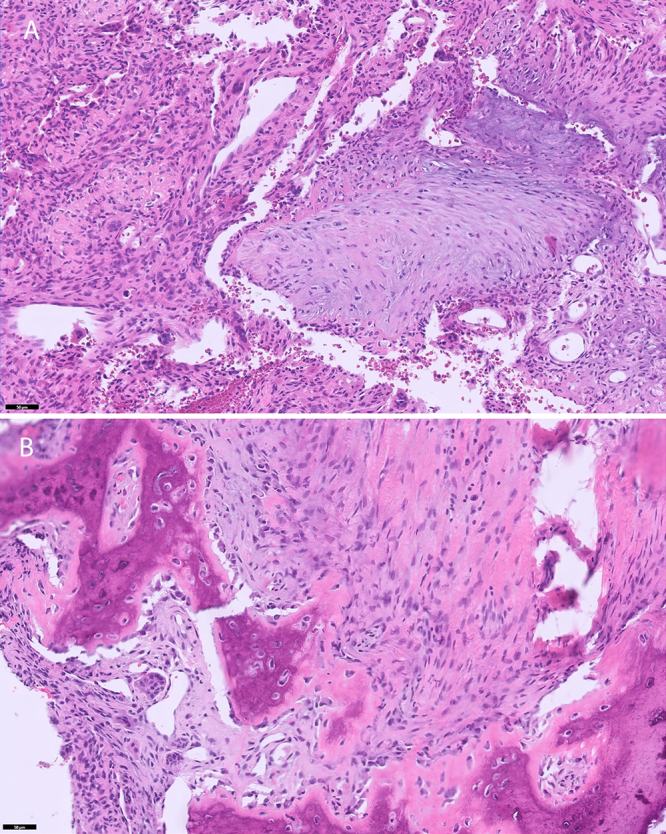
Specimens collected from the mass where it abutted the pleura between the fourth and fifth ribs. A: Cystic spaces, some containing residual blood and separated by fibrous septa containing occasional osteoclast-like giant cells, are characteristic of an ABC. Basophilic reticulated chondroid-like foci, another common feature, appear in the center of the image. There is no atypia. B: In some areas, trabeculated osteoid and woven bone surrounded by activated osteoblasts predominate. Mitotic activity is minimal, and atypia is absent. Hematoxylin and eosin. Bar = 50 μm.
Informed Consent
The necessary informed consent was obtained in this study.
Discussion
Observations
The above case details an ABC, secondary to osteoblastoma, in a 35-year-old female who was symptomatic from the lesion and required a multidisciplinary approach for resection. There is no standardized treatment consensus within the literature for ABC treatments, but based on the reviews currently published, complete resection is recommended to limit recurrence.2, 5, 6, 12 Embolization of the tumor allows better vascular control, as these lesions can require multiple units of blood transfusions intraoperatively.6, 12, 13 In this case, preoperative embolization optimized the resection, which allowed for a GTR. The surgical plan, involving a right T5 laminectomy, pedicle resection with costotransversectomy, and T4–6 posterolateral fusion, provided adequate working corridors for both resection and spine stabilization.
The treatment of ABCs depends on the location and size. Because of the locally aggressive nature and high recurrence rate, maximal safe resection is the primary goal of treatment.2, 5, 6, 12, 14 Neoadjuvant therapy with selective arterial embolization is often included to cut off blood supply to the lesion, minimizing intraoperative blood loss.5 En bloc excision has been shown to have the lowest recurrence rates when it is possible to perform it safely.15, 16 However, when neurological function is at risk from en bloc resection, less aggressive curettage with adjuvant therapies is most common.5, 12 Some of these adjuvant therapies include liquid nitrogen, phenol, argon beam coagulation, and calcitonin-methylprednisolone, which have been used in different instances with varying effects.17–22 Radiation therapy as an adjunct to surgery has also been reported to improve outcomes in these ABC patients.23
In this case, en bloc resection was attempted without violation of the pleura or excessive destabilization of the spine. The involved neurological structures were only the right T4 and T5 nerve roots, with minimal involvement of the T5 vertebral body. Resection of the lesion would not compromise the anterior column of the thoracic spine, which would require a corpectomy, and stabilization for the involved body resection could be achieved with a posterolateral fusion. Because of strong radiographic and clinical evidence that the lesion was an ABC, this strategy was favored over just a biopsy since the former conferred the least risk of recurrence, even if the lesion was any of those from the differential diagnosis. Given the acuity of the patient’s symptoms, the decision to proceed with surgery, rather than just radiation, was made. During surgery, the lesion was not removed en bloc but rather in a piecemeal fashion without too much bleeding. The portions of the tumor capsule adherent to the pleura were able to be dissected without violating the pleura, except for one small portion of the capsule that was adherent to the pleura and subsequently coagulated. The decision to not resect that portion of the pleura was made to avoid a pneumothorax; however, there was no obvious infiltration of the lesion within the pleura that would make a wide pleural resection favorable otherwise. After a multidisciplinary meeting with our institution’s oncologists, postoperative radiation was deferred in the setting of GTR based on postoperative images and the recency of surgery. Serial imaging and close monitoring were planned despite immediate postoperative images suggesting GTR since a small portion was left on the pleura despite being heavily coagulated. Adjuvant chemotherapies were also held off and planned for utilization in the event of recurrence. In patients with ABCs that are unresectable due to invasion of eloquent nervous system structures, denosumab has shown promise as both a neoadjuvant adjunct to allow for resection and a second-line treatment option when used alone.24–27 However, denosumab’s efficacy as an adjuvant therapy has not been fully proven.
Because of the predicted vascularity of the tumor, embolization of the right T4, T5, and T6 intercostal arteries was accomplished before surgery, allowing for a safer attempt at resection and limiting intraoperative blood loss. Resection corridors established in this case have been seldom described in the treatment of ABCs.28, 29 This was indicated due to the location of the lesion and compensatory fixation achieved by posterolateral fusion from T4 to T6. This approach allowed resection of the lesion. In this patient, the right T4 and T5 nerve roots were sacrificed, and the patient did not experience significant hypoesthesia in those dermatomes postoperatively.
Reactive lesions, or secondary ABCs, such as in this patient based on histology, are associated with worse progression-free survival and overall survival rates when compared to primary ABCs.8 In a 220-patient systematic review, patients with primary ABCs more frequently underwent curettage with bone grafting and laminectomy. In contrast, patients with secondary ABCs were more likely to undergo corpectomy and spinal fixation.8 In the same study, significantly higher rates of ABC recurrence and death were noted in patients with reactive lesions.8 However, it is difficult to determine to what extent the differences in outcomes were influenced by the etiology of ABC or the treatment applied. In patients with secondary ABCs, the underlying tumor can often be diagnosed via characteristic imaging findings.30 However, in equivocal cases, a biopsy may be necessary before treatment to make an accurate diagnosis. After a brief review of the available literature, no investigations of treatment choice on outcomes were found stratified by the type of inciting lesion. This information would prove useful in future treatment decisions and is a direction for future investigation. Continual postoperative management of this patient will involve close interval imaging to follow the diagnosis of osteoblastoma with plans for radiation or surgery should there be a recurrence in the future.
Lessons
The combination of preoperative endovascular intervention and the chosen surgical approach for this secondary ABC in the thoracic spine has not been described to date for GTR. This case is an important addition to the literature on ABCs by further establishing the possible range of cyst placement, the heterogeneity of origin, and the need for specialized approaches. While vertebral ABCs are rare, awareness of their local aggression and frequent recurrence demands accurate diagnosis and maximum total resection. Additionally, as more information is learned about the genetics and treatment responses of different types of ABCs, their etiology will become increasingly important in planning treatment. Finally, treatments and approaches must be tailored to each patient, as these cysts can present in unexpected locations. For these reasons, this unique case is instructive in diagnosing, understanding, and managing ABCs.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Price, D’Souza, Hayworth, Kan, Lall. Acquisition of data: Price, D’Souza, Hayworth, Campbell, Kan, Lall. Analysis and interpretation of data: Price, Bartman, Hayworth, Campbell, Kan. Drafting the article: Price, Fredricks, Bartman, D’Souza, Kan, Lall. Critically revising the article: Price, Fredricks, Bartman, D’Souza, Kan, Lall. Reviewed submitted version of manuscript: Price, Fredricks, Bartman, Hayworth, Campbell, Kan, Lall. Approved the final version of the manuscript on behalf of all authors: Price. Administrative/technical/material support: Kan. Study supervision: Kan.
Correspondence
Anthony Price: The University of Texas Medical Branch at Galveston, TX. antprice@utmb.edu.
References
- 1.Aneurysmal bone cysts. In: StatPearls. Internet. StatPearls Publishing; 2024 Jan-. Accessed May 2, 2024. https://www.ncbi.nlm.nih.gov/books/NBK546654/ [Google Scholar]
- 2.Restrepo R, Zahrah D, Pelaez L, Temple HT, Murakami JW. Update on aneurysmal bone cyst: pathophysiology, histology, imaging and treatment. Pediatr Radiol. 2022;52(9):1601-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.B. Czerniak. Dorfman and Czerniak’s Bone Tumors. 2nd ed. Elsevier; 2015. [Google Scholar]
- 4.Leithner A, Windhager R, Lang S, Haas OA, Kainberger F, Kotz R. Aneurysmal bone cyst. A population based epidemiologic study and literature review. Clin Orthop Relat Res. 1999;(363):176-179. [PubMed] [Google Scholar]
- 5.Park HY, Yang SK, Sheppard WL, et al. Current management of aneurysmal bone cysts. Curr Rev Musculoskelet Med. 2016;9(4):435-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cottalorda J, Bourelle S. Modern concepts of primary aneurysmal bone cyst. Arch Orthop Trauma Surg. 2007;127(2):105-114. [DOI] [PubMed] [Google Scholar]
- 7.Wang T, Jia Q, Gao X, et al. Secondary aneurysmal bone cyst of the spine: clinicopathological features, surgical modalities and outcomes. Clin Neurol Neurosurg. 2020;188:105595. [DOI] [PubMed] [Google Scholar]
- 8.Palmisciano P, Hunter M, Lokesh N, et al. Aneurysmal bone cyst of the spine in adult patients: a systematic review and comparison of primary vs secondary lesions. J Clin Neurosci. 2022;100:15-22. [DOI] [PubMed] [Google Scholar]
- 9.Wu M, Xu K, Xie Y, et al. Diagnostic and management options of osteoblastoma in the spine. Med Sci Monit. 2019;25:1362-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucas DR. Osteoblastoma. Arch Pathol Lab Med. 2010;134(10):1460-1466. [DOI] [PubMed] [Google Scholar]
- 11.Alexiev BA. Osteoblastoma, NOS. PathologyOutlines.com. Accessed October 18, 2024. https://www.pathologyoutlines.com/topic/boneosteoblastoma.html [Google Scholar]
- 12.Nasri E, Reith JD. Aneurysmal bone cyst: a review. J Pathol Transl Med. 2023;57(2):81-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amendola L, Simonetti L, Simoes CE, Bandiera S, De Iure F, Boriani S. Aneurysmal bone cyst of the mobile spine: the therapeutic role of embolization. Eur Spine J. 2013;22(3):533-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kransdorf MJ, Sweet DE. Aneurysmal bone cyst: concept, controversy, clinical presentation, and imaging. AJR Am J Roentgenol. 1995;164(3):573-580. [DOI] [PubMed] [Google Scholar]
- 15.Mascard E, Gomez-Brouchet A, Lambot K. Bone cysts: unicameral and aneurysmal bone cyst. Orthop Traumatol Surg Res. 2015;101(1suppl):S119-S127. [DOI] [PubMed] [Google Scholar]
- 16.Mankin HJ, Hornicek FJ, Ortiz-Cruz E, Villafuerte J, Gebhardt MC. Aneurysmal bone cyst: a review of 150 patients. J Clin Oncol. 2005;23(27):6756-6762. [DOI] [PubMed] [Google Scholar]
- 17.Peeters SP, Van der Geest ICM, de Rooy JWJ, Veth RPH, Schreuder HWB. Aneurysmal bone cyst: the role of cryosurgery as local adjuvant treatment. J Surg Oncol. 2009;100(8):719-724. [DOI] [PubMed] [Google Scholar]
- 18.Bitzan P, Windhager R, Lang S, Richling B, Kotz R. Incidence of recurrence of aneurysmal bone cysts following surgical treatment and adjuvant therapy with phenol. Z Orthop Ihre Grenzgeb. 1995;133(5):422-428. [PubMed] [Google Scholar]
- 19.Schreuder HW, Veth RP, Pruszczynski M, Lemmens JA, Koops HS, Molenaar WM. Aneurysmal bone cysts treated by curettage, cryotherapy and bone grafting. J Bone Joint Surg Br. 1997;79(1):20-25. [DOI] [PubMed] [Google Scholar]
- 20.Feigenberg SJ, Marcus RB, Zlotecki RA, Scarborough MT, Berrey BH, Enneking WF. Megavoltage radiotherapy for aneurysmal bone cysts. Int J Radiat Oncol Biol Phys. 2001;49(5):1243-1247. [DOI] [PubMed] [Google Scholar]
- 21.Cummings JE, Smith RA, Heck RK. Argon beam coagulation as adjuvant treatment after curettage of aneurysmal bone cysts: a preliminary study. Clin Orthop Relat Res. 2010;468(1):231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliveira MBDR, Meohas W, Silva RR, de Carvalho GS, Mello FCQ, Paschoal MEM. Percutaneous treatment of aneurysmal bone cyst with calcitonin and methylprednisolone. Acta Ortop Bras. 2018;26(5):314-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu S, Hitchcock KE, Mendenhall WM. Radiation therapy for aneurysmal bone cysts. Am J Clin Oncol. 2017;40(6):621-624. [DOI] [PubMed] [Google Scholar]
- 24.Kurucu N, Akyuz C, Ergen FB, et al. Denosumab treatment in aneurysmal bone cyst: evaluation of nine cases. Pediatr Blood Cancer. 2018;65(4). [DOI] [PubMed] [Google Scholar]
- 25.Lange T, Stehling C, Fröhlich B, et al. Denosumab: a potential new and innovative treatment option for aneurysmal bone cysts. Eur Spine J. 2013;22(6):1417-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skubitz KM, Peltola JC, Santos ER, Cheng EY. Response of aneurysmal bone cyst to denosumab. Spine (Phila PA 1976). 2015;40(22):E1201-E1204. [DOI] [PubMed] [Google Scholar]
- 27.Hung YP, Bredella MA, Lobmaier IVK, Lozano-Calderón SA, Rosenberg AE, Nielsen GP. Aneurysmal bone cyst and osteoblastoma after neoadjuvant denosumab: histologic spectrum and potential diagnostic pitfalls. APMIS. 2022;130(4):206-214. [DOI] [PubMed] [Google Scholar]
- 28.Lubelski D, Abdullah KG, Mroz TE, et al. Lateral extracavitary vs costotransversectomy approaches to the thoracic spine: reflections on lessons learned. Neurosurgery. 2012;71(6):1096-1102. [DOI] [PubMed] [Google Scholar]
- 29.Chandra SP, Ramdurg SR, Kurwale N, et al. Extended costotransversectomy to achieve circumferential fusion for pathologies causing thoracic instability. Spine J. 2014;14(9):2094-2101. [DOI] [PubMed] [Google Scholar]
- 30.Sasaki H, Nagano S, Shimada H, et al. Diagnosing and discriminating between primary and secondary aneurysmal bone cysts. Oncol Lett. 2017;13(4):2290-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]