Abstract
BACKGROUND
The leading method of identifying critical functional regions during brain tumor resection is direct electrical stimulation (DES). In awake craniotomy patients, DES employs electric current to induce functional responses or task inhibition. In contrast, thermography uses infrared imaging to detect regions of increased blood flow from patient tasks, inferring the location of functional activity similarly to blood oxygen level–dependent (BOLD) functional magnetic resonance imaging (fMRI). DES seldom produces no detectable response, but the case herein is an example featuring the subsequent use of thermography.
OBSERVATIONS
The authors present the case of a 40-year-old male in whom awake craniotomy DES for high-grade glioma re-resection produced no detectable response at the upper levels of tolerated current amplitude. Following inconclusive DES, infrared thermography was performed with a lip-pursing task, and face motor activation was thermally detected in regions corroborated by both preoperative BOLD fMRI and literature on BOLD fMRI face motor mapping.
LESSONS
The lack of a detectable DES response was attributed to significant peritumoral edema, as evidenced by preoperative fluid-attenuated inversion recovery MRI. Findings indicate that infrared thermography overcomes the limitations of DES in an extensive edema setting and that thermography offers a useful complement to standard cortical mapping protocols for resection planning.
Keywords: thermography, glioma resection, direct electrical stimulation, functional mapping, awake craniotomy
ABBREVIATIONS: BOLD = blood oxygen level–dependent, CT = computed tomography, DES = direct electrical stimulation, FLAIR = fluid-attenuated inversion recovery, fMRI = functional magnetic resonance imaging, MRI = magnetic resonance imaging, nTMS = navigational transcranial magnetic stimulation, PCA = principal component analysis, TRF = thermodynamic response function.
Functional mapping is indispensable to the preservation of patient daily life functions following glioma resection.1, 2 Resection planning is a careful balance of two objectives: maximizing the extent of resection, which improves survival outcomes, and minimizing functional damage, which worsens quality of life and survival outcomes.3, 4 An ensemble of preoperative and intraoperative mapping modalities informs surgical planning to this end. The current structural imaging standard for visualizing gliomas is T2/fluid-attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI) or T1-weighted gadolinium contrast imaging.5, 6 Preoperative functional imaging, such as blood oxygen level–dependent (BOLD) functional magnetic resonance imaging (fMRI), detects oxygenated hemoglobin following task-induced activation to infer regions of neuronal activity.7–9 The primary limitations of BOLD fMRI are brain shift, leading to a loss of spatial correspondence between presurgical imaging and functional anatomy, and the modality’s inability to specifically indicate whether a given region is critical for a given function. While helpful, preoperative BOLD fMRI alone is inadequate for resection guidance and requires confirmation by intraoperative methods.10, 11
The gold standard for awake intraoperative brain mapping is direct electrical stimulation (DES).12–14 Having been practiced for approximately a century, DES has a demonstrable history of reducing postoperative neurological deficits.15, 16 DES is advantageous in its ability to either elicit detectable functional responses or temporarily produce reversible dysfunction in discrete cortical regions.17 Electrical current, delivered to the cortical surface via a monopolar or bipolar probe, identifies functional regions by positive or negative responses. Positive responses, such as subjective sensations or physical movement, are involuntarily elicited by stimulation, while the disruption of ongoing task-directed behavior constitutes a negative response. The resulting maps of function inform resection planning effectively: there is significant evidence associating DES with more extensive resection and fewer permanent neurological deficits.18 Albeit practical, reliable, and established, DES has some limitations. Intraoperative seizures due to DES are the leading cause of aborted awake craniotomy operations,19 occurring at rates between 2.5% and 54% at various European surgical centers.20 In rare cases in which the awake craniotomy continues despite these seizures, intractable DES portends delayed mapping and difficult interpretation of intraoperative task responses. The exact electrophysiological mechanism by which DES produces responses remains poorly understood.21 As a result, there is no standardized amperage considered generally adequate for the disruption of cortical function, which is a problem exacerbated by the variability of ideal current thresholds between cortical regions of the same subject.22 DES only surveys a single site at a time, which is relatively inefficient.23 Research has also indicated poor specificity and an inability to distinguish essential activated cortex from downstream activated cortex.24 Correspondingly, the network character of language activation complicates the reliability of DES, as DES can only detect language cortex by the transient disruption of language task performance in a 1-cm2 site at a time.17, 25 These limitations have invited substantial interest in noninvasive mapping modalities. Among the encouraging preoperative examples is navigational transcranial magnetic stimulation (nTMS), which employs MRI-guided magnetic pulse targeting to excite or inhibit cortical activity, not unlike a noninvasive analog of DES. nTMS has similarly improved survival outcomes by informing craniotomy extent reduction and aggressive supratotal glioma resection.26 However, as a preoperative modality, its application can be affected by brain shift, as with BOLD fMRI. In light of the disadvantages of both preoperative mapping and DES, we introduce infrared thermography.
Infrared thermography is a growing methodology for noninvasive intraoperative imaging and has demonstrated encouraging results for functional mapping.27, 28 Machine learning has also been used with infrared thermographic recordings to detect tumor margins with accuracy and specificity above 90%.29 Functional thermography is a practical technology for the surgical team. The apparatus setup takes approximately 2 minutes, primarily comprising positioning of the thermal camera. Then, thermography affords a full view of the craniotomy over time and from a distance. This presents a benefit in efficiency over site-by-site mapping methods while presenting little risk of contaminating the sterile field. Thermography allows intraoperative functional mapping via detection of the thermodynamic response function (TRF), which is the temperature impulse response of activated functional cortex. Recent work has quantitatively characterized the TRF in the context of thermographic sensorimotor mapping for awake craniotomy glioma patients.30 Thermography offers a promising avenue of intraoperative functional mapping that shares its foundational principle with BOLD fMRI.
A problem of interest is whether functional thermography provides utility comparable to that of DES and how it performs in conditions in which DES is ineffective or unsuitable, as in the rare scenario when DES fails to map expected positive sites. The 2011 case report by El Khashab et al. on inconclusive DES in an epileptogenic focus resection is one such example.31 Here, we present a case in which DES produced no detectable functional response at tolerated current amplitudes in a patient undergoing awake craniotomy. We performed intraoperative thermography while administering a face motor task. Principal component analysis (PCA) was used to create a high-resolution functional map from the spatial extent of the characteristic motor TRF. These maps agreed with expected face motor regions based on neuroanatomy and preoperative fMRI, demonstrating the utility of functional thermography as a propitious complement to DES mapping. We further discuss the correspondence of activation maps from preoperative fMRI and thermography.
Illustrative Case
Presentation
A 40-year-old male presented with a history of IDH-wildtype, MGMT unmethylated high-grade glioma. He had undergone a prior craniotomy for gross-total resection, followed by radiotherapy with concomitant then adjuvant temozolomide. Surveillance MRI demonstrated progressive enhancement in the right frontoparietal operculum with elevated perfusion, indicating tumor progression. Concurrent with these radiographic changes, the patient exhibited worsening seizures. After discussing treatment options in detail with the patient, the decision was made to proceed with resection of the presumably recurring glioma, with the goals of cytoreduction and improvement in seizure control. Intraoperative motor mapping and electrocorticography were planned with the objective of maximizing safe resection.
Preoperative Imaging
Preoperative multimodal MRI was performed with a Siemens Magnetom Prisma 3T. On gadolinium contrast MRI slices, the glioma presented as an enhanced heterogeneous mass with a hypointense necrotic core in the right frontoparietal operculum, with an irregular shape and ill-defined borders (Fig. 1). BOLD fMRI was recorded with a task comprising 20 seconds of continuous lip-pursing followed by 20 seconds of rest. Bilaterally symmetric activation of the tongue was observed on axial fMRI slices, but lip activation only occurred contralesionally (Fig. 2). One week after resection, postoperative computed tomography (CT) imaging was performed using a Siemens Somatom X.cite in a routine noncontrast axial scan.
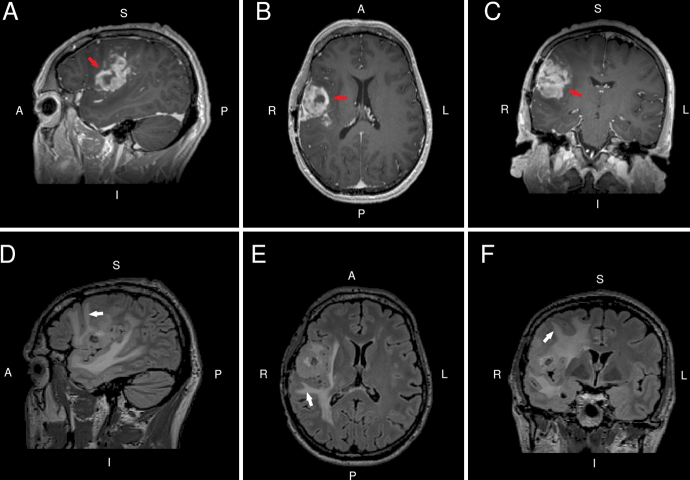
FIG. 1.
Preoperative sagittal (A), axial (B), and coronal (C) contrast T1-weighted MRI slices indicate a heterogeneously enhancing mass (red arrows) in the right frontoparietal operculum. Corresponding preoperative FLAIR MRI slices (D−F) indicate peritumoral edema extending toward the cortical surface (white arrows).
FIG. 2.
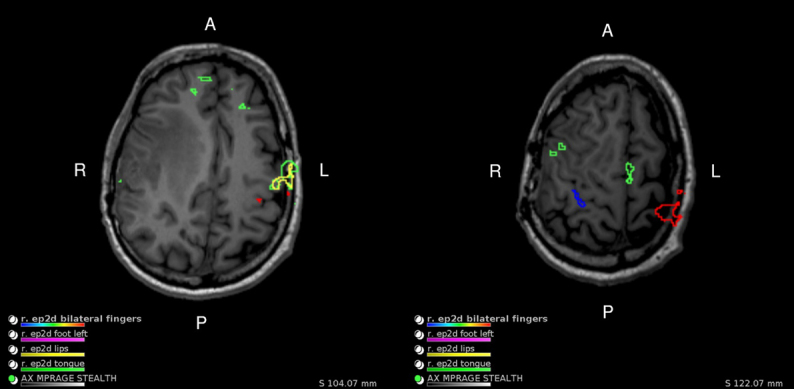
Preoperative axial BOLD fMRI slices reveal bilaterally symmetric tongue activation from a lip-pursing task (green), while lip activation (yellow) is only observed on the contralesional (left) side.
Intraoperative DES Mapping
Following the opening of the dura, DES mapping was performed using standard low-frequency parameters (bipolar probe, 60 Hz, 1-msecond pulse duration) and a current amplitude of 1–3 mA (6 mA peak-to-peak), which was not increased above 3 mA due to the presence of afterdischarges and concerns about seizures on concurrent electrocorticography. No positive motor or sensory sites were noted during DES over the exposed cortical region, which was surveyed with approximately 1-cm spacing. Digital color images of the craniotomy were then captured. The craniotomy was of mild elliptical shape and had a long axis spanning 6 cm in the anteroposterior dimension.
Intraoperative Thermography
Intraoperative thermography immediately followed DES mapping and was performed with a FLIR T1020sc thermal camera with 1024 × 768–pixel resolution, 30-Hz frame rate, and sub-20–mK noise equivalent temperature difference.. Our intraoperative imaging system has been described previously.30 A tripod and horizontal arm secure the camera approximately 20 cm from the brain surface. The preferred configuration comprises a working distance of 20–30 cm and optical orthogonality to the craniotomy apex. Data are streamed from the camera to a computer workstation for storage and offline processing. The patient participated in a face motor mapping task, which consisted of lip-pursing every other 20 seconds for 5 minutes. Start and rest cues were delivered by a series of auditory beeps from the workstation speaker. Thermographic recording had minimal effect on the standard surgical workflow, such that no changes occurred to the craniotomy, anesthesia protocol, DES procedure, or resection boundaries.
Thermography Analysis and Map Generation
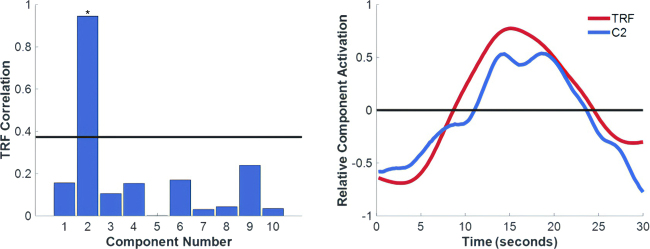
Our methods for the preprocessing and analysis of thermal data have been described previously.30 Data first underwent motion correction through linear registration, followed by nonlinear registration of all dataset frames to the data baseline (first video frame).32 Air current attenuation was performed using an exponential weighted filter.30 Data were z-score–normalized by frame and by pixel to correct for global baseline changes across space and time. PCA was performed to decompose the data into candidate functional maps and corresponding time series. The functional map was selected by correlation of all component time series with the known TRF, which describes a peak in temperature approximately 15 seconds following stimulus delivery (Fig. 3).30 Postprocessing duration is on the order of minutes. Some variation is introduced by analysis parameters, hardware specifications, and the length of recordings. Our analysis outputs high-resolution two-dimensional activation maps that can be displayed on the monitor of the mobile clinical workstation.
FIG. 3.
Left: Bar graph showing the correlation of each component with the TRF, which is used to infer regions of neuronal activity. The horizontal black line represents the upper end of a 95% bootstrapped (n = 1 × 106) confidence interval of the means of the correlation values. Component 2 is denoted with an asterisk to indicate it surpasses this level. Right: A time course plot of the relative activity in component 2 (C2) is shown to closely match the TRF.
Postoperative Virtual Craniotomy Visualization
Multimodal volume registration between preoperative FLAIR MRI and postoperative CT imaging was achieved by using the cranium as a reference structure. FreeSurfer software automated segmentation of the cerebral cortex from the preoperative T1 MRI volume, while the cranium was segmented from CT imaging by primitive intensity thresholding. Both structures were registered to the same virtual coordinate space using the affine three-dimensional multimodal registration function native to MATLAB. The bone flap was virtually removed by manually selecting a closed loop tracing the perimeter of the second resection craniotomy in the CT reconstruction. Lastly, the activation mapping derived from PCA was manually registered to the virtual craniotomy to confirm the neuroanatomical context of active regions.
Informed Consent
The necessary informed consent was obtained in this study.
Discussion
Observations
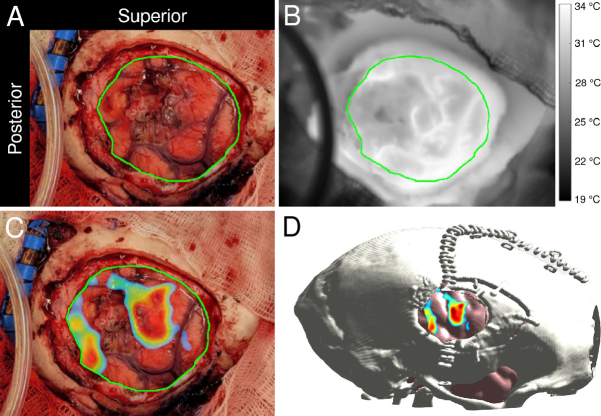
The thermographic activation map identified two distinct sites of markedly intense activity on the craniotomy (Fig. 4C). The larger, elliptical-appearing site had dimensions of approximately 2 × 1.5 cm, was located near the superior edge, and extended in the inferior direction to span approximately one-third of the anterior half of the craniotomy. The smaller site was a thin strip of approximately 2 × 0.5 cm, abutting the dura at the posterior inferior corner of the exposed cortex. The tumor was patently visible on digital color images of the craniotomy, and infrared-to-color registration situated the tumor between the two activation sites.
FIG. 4.
A visible-light image (A) and thermogram (B) were obtained via digital and infrared cameras, respectively. The major diameter of the craniotomy was approximately 6 cm. The color image was manually registered to the thermal image and then used as a reference to draw the cortical surface boundary shown in green in both images. PCA yielded a face-motor activation map superimposed onto a visible-light image of the craniotomy (C). The thermal functional map was manually registered onto a postoperatively rendered virtual craniotomy to provide more intuitive anatomical context (D).
The thermal activation map corroborated the location of the tumor by indicating a lack of functional activity between the sites. Furthermore, superimposing the thermal activation map on a virtual reconstruction of the craniotomy correctly localized the major activation site to the precentral gyrus above the sylvian fissure (Fig. 4D). The smaller activation site was further identified in the postcentral gyrus, the expected location of the sensory cortex. Functional thermography successfully detected face motor activity despite a lack of conclusive responses from DES.
Preoperative BOLD fMRI located tongue activation in the vicinity of lip-pursing activation regions found by intraoperative functional thermography. Functional imaging literature strongly indicates the localization of lip and face motor activity to the precentral gyrus and bilateral symmetry of general face motor activity.33, 34 Our preoperative BOLD fMRI did show lip motor activation in the expected region of the contralesional (left) frontoparietal lobe, but did not show typical bilateral activation symmetry (Fig. 2). Work by Lüdemann et al. reported a reduction in the fractional blood volume of peritumoral edematous regions compared to that of the contralesional regions.35 As significant peritumoral edema was evident on preoperative FLAIR MRI (Fig. 1), we attribute the asymmetric functional activation to suppression of the BOLD signal by a combination of edema and mass effect. Functional reorganization of the lip motor cortex, common to the face motor cortex, is also plausible. However, thermography in our patient detected lip motor activation in the typical regions, so functional reorganization was ruled out. Similarly, El Khashab et al. reported that hand motor activation was detected in expected regions by postoperative BOLD fMRI after the inability of DES to detect the motor cortex in their case of epileptogenic focal resection.31 While the cause of inconclusive DES was unidentified in the report by El Khashab et al., consideration of the case details, preoperative imaging, and pertinent biochemistry implicates edema as a likely culprit in ours.
DES failed to elicit a reliable motor response in our patient at stimulation parameters within the typical range. Afterdischarges were observed in electrodes adjacent to the stimulation site at the upper range of delivered amperage (3 mA), indicating that electrical current was indeed being delivered but not sufficiently to elicit expected functional responses. Note that the maximum delivered current of 3 mA was relatively low; hence, the current could have been increased, but there were concerns about inducing seizures or the poor reliability and localization of patient responses. A comprehensive list of possible explanations for the lack of positive DES sites would include the effects of scar tissue and edema on electrical transmission, insufficient current amplitude to invoke an overt sensory or motor response, and the inability of the patient to perceive subtler sensorimotor responses. Peritumoral edema is quite common, occurring in 24% of patients with malignant glioma in a diffusion tensor imaging study,36 and DES is successful in functional region detection for the vast majority of cases. However, cerebral edematous tissue has lower measured electrical impedance than nonedematous white matter because of increased ionized water content.37 In our case, in which preoperative FLAIR MRI showed a substantial spatial extent of peritumoral edema and extension toward the cortical surface, the electrical impedance of edematous tissue presents an involved problem.
The electrical impedance experienced by deep brain stimulation probes, for example, fluctuates as a function of myriad variables, including plasma osmolality, glial and immunological response stabilization, and the frequency of delivered current.38, 39 The presence of surgical osmotic diuretics can further effect complicated changes in edematous tissue impedance; hyperosmolar cerebral edema was observed in concussed rats following mannitol infusion.40 Furthermore, mannitol-induced hyperosmolality reduced the intracellularly recorded amplitude of evoked field potentials and suppressed excitatory postsynaptic potentials in rat neocortical slices.41 Thus, mannitol and similar osmotic diuretics may decrease the neuronal excitability of peritumoral edematous tissue by increasing tissue capacitance. The combination of lower impedance in edematous tissue and diuretically induced hyperosmolality would redirect and attenuate DES excitation of functional cortical tissues. In sum, a greater current amplitude may be required to provoke detectable functional responses. Recalling El Khashab and colleagues’ case with epileptic focal resection, postictal cerebral edema may have caused the inconclusiveness of DES in the initial surgery.31 Cerebral edema is a known consequence of epileptic seizures,42, 43 and El Khashab and colleagues’ patient was brought directly from the epilepsy monitoring unit to the operating room. Our results and preoperative imaging imply that, unlike DES, thermography is not compromised by the effects of edema on electrical tissue impedance.
Neurosurgeons and patients stand to gain substantially from multimodal DES with thermography for awake craniotomy. Thermography offers potential as an intraoperative noninvasive complement to DES when high-resolution composite-function activation maps are desired or when DES or fMRI is difficult to interpret. In cases in which seizure minimization is imperative or DES is anticipated to be ineffective, intraoperative thermography may work especially well with preoperative nTMS in a multimodal battery of noninvasive mapping methods. The active mapping of nTMS is similar to DES in its ability to differentiate stimulatory and inhibitory regions while inducing seizures at a subpercentage rate far lower than that of DES.44 Meanwhile, thermography avoids intraoperative electromagnetic manipulation of brain tissue entirely. However, for this reason, we caution that thermography and DES are not strictly equivalent in the intraoperative context. Thermography detects blood flow in a region associated with the task whether the relevant function is stimulatory, inhibitory, downstream, or any combination thereof. In contrast, DES relies heavily on detecting either motor responses or inhibition of activity in one site at a time. DES further depends on the constitutive electromagnetic properties of cortical tissue, increasing the probability of seizures when severe edema demands greater current. Hence, a study accounting for survival outcomes, functional outcomes, and failure modes would be required to determine if thermography is comparable to or a viable replacement for DES. Overall, the correspondence between thermography results, the literature, and preoperative imaging in this case is promising. Our findings invite a deeper, longitudinal investigation of cases in which thermography proves to be a more reliable mapping modality.
Lessons
Our case is a unique example in which functional thermography detected face motor activation in the precentral gyrus despite inconclusive DES. Thermographically detected activation regions agreed with both preoperative BOLD fMRI and the fMRI literature, which indicates the success of thermography in a severe edema setting. Thermography stands to reinforce DES findings by detecting composite-function activation across the entire craniotomy. Furthermore, thermography relies on cortical heating patterns as opposed to the conductive properties of cortical tissue, so it was not substantially affected by the peritumoral edema observed in our patient. More extensive study is required to assess whether, and in which contexts, thermography is more reliable than DES. We emphasize the availability of functional thermography for multimodal resection guidance and its utility when complications arise in traditional functional mapping.
Acknowledgments
We thank Dr. Mohit Saxena for critical information on clinical protocols for fMRI activation mapping. We also thank Professor Luis Lujan for insightful discussions on the electrical properties of pathological cortical tissues and pertinent literature recommendations.
This work was supported by a grant from the National Institute of Neurological Disorders and Stroke (no. R01NS116190).
Disclosures
Dr. Parrish reported a patent pending for infrared thermography for intraoperative functional mapping and a patent pending for automated detection and tracking of cortical blood vessel temperature during neurosurgery, both to Northwestern University.
Author Contributions
Conception and design: Tran, Parrish, Tate, Iorga. Acquisition of data: Schneider, Cho, Tate. Analysis and interpretation of data: Tran, Tate, Iorga. Drafting the article: Tran, Iorga. Critically revising the article: Tran, Parrish, Tate, Iorga. Reviewed submitted version of manuscript: Tran, Schneider, Parrish, Iorga. Approved the final version of the manuscript on behalf of all authors: Tran. Administrative/technical/material support: Tran. Study supervision: Parrish, Iorga.
Correspondence
Phillip Tran: Northwestern University, Chicago, IL. phillip.tran@northwestern.edu.
References
- 1.Sagar S, Rick J, Chandra A, Yagnik G, Aghi MK. Functional brain mapping: overview of techniques and their application to neurosurgery. Neurosurg Rev. 2019;42(3):639-647. [DOI] [PubMed] [Google Scholar]
- 2.Saito T, Muragaki Y, Tamura M, et al. Awake craniotomy with transcortical motor evoked potential monitoring for resection of gliomas within or close to motor-related areas: validation of utility for predicting motor function. J Neurosurg. 2022;136(4):1052-1061. [DOI] [PubMed] [Google Scholar]
- 3.Rahman M, Abbatematteo J, De Leo EK, et al. The effects of new or worsened postoperative neurological deficits on survival of patients with glioblastoma. J Neurosurg. 2017;127(1):123-131. [DOI] [PubMed] [Google Scholar]
- 4.Brown TJ, Brennan MC, Li M, et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol. 2016;2(11):1460-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verburg N, de Witt Hamer PC. State-of-the-art imaging for glioma surgery. Neurosurg Rev. 2021;44(3):1331-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haddad AF, Young JS, Morshed RA, Josephson SA, Cha S, Berger MS. Pseudo-insular glioma syndrome: illustrative cases. J Neurosurg Case Lessons. 2021;2(26):CASE21481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Logothetis NK, Pfeuffer J. On the nature of the BOLD fMRI contrast mechanism. Magn Reson Imaging. 2004;22(10):1517-1531. [DOI] [PubMed] [Google Scholar]
- 8.Champod AS, Ferreira E, Amiez C, et al. Preoperative and postoperative functional magnetic resonance imaging and intraoperative assessment of mental spatial transformations in patients undergoing surgery for brain tumors. In: Brain Mapping: From Neural Basis of Cognition to Surgical Applications. Springer; 2011:167-180. [Google Scholar]
- 9.Amiez C, Kostopoulos P, Champod AS, et al. Preoperative functional magnetic resonance imaging assessment of higher-order cognitive function in patients undergoing surgery for brain tumors. J Neurosurg. 2008;108(2):258-268. [DOI] [PubMed] [Google Scholar]
- 10.Brahimaj BC, Kochanski RB, Pearce JJ, et al. Structural and functional imaging in glioma management. Neurosurgery. 2021;88(2):211-221. [DOI] [PubMed] [Google Scholar]
- 11.Bizzi A, Blasi V, Falini A, et al. Presurgical functional MR imaging of language and motor functions: validation with intraoperative electrocortical mapping. Radiology. 2008;248(2):579-589. [DOI] [PubMed] [Google Scholar]
- 12.Ottenhausen M, Krieg SM, Meyer B, Ringel F. Functional preoperative and intraoperative mapping and monitoring: increasing safety and efficacy in glioma surgery. Neurosurg Focus. 2015;38(1):E3. [DOI] [PubMed] [Google Scholar]
- 13.De Witte E, Satoer D, Colle H, Robert E, Visch-Brink E, Mariën P. Subcortical language and non-language mapping in awake brain surgery: the use of multimodal tests. Acta Neurochir. 2015;157(4):577-588. [DOI] [PubMed] [Google Scholar]
- 14.Mahon BZ, Miozzo M, Pilcher WH. Direct electrical stimulation mapping of cognitive functions in the human brain. Cogn Neuropsychol. 2019;36(3-4):97-102. [DOI] [PubMed] [Google Scholar]
- 15.D’Amico RS, Englander ZK, Canoll P, Bruce JN. Extent of resection in glioma—a review of the cutting edge. World Neurosurg. 2017;103:538-549. [DOI] [PubMed] [Google Scholar]
- 16.Paiva WS, Fonoff ET, Beer-Furlan A, et al. Evaluation of postoperative deficits following motor cortex tumor resection using small craniotomy. Surgery. 2019;5(1):e8-e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamberger MJ. Cortical language mapping in epilepsy: a critical review. Neuropsychol Rev. 2007;17(4):477-489. [DOI] [PubMed] [Google Scholar]
- 18.Gerritsen JKW, Arends L, Klimek M, Dirven CMF, Vincent AJE. Impact of intraoperative stimulation mapping on high-grade glioma surgery outcome: a meta-analysis. Acta Neurochir. 2019;161(1):99-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larkin CJ, Yerneni K, Karras CL, et al. Impact of intraoperative direct cortical stimulation dynamics on perioperative seizures and afterdischarge frequency in patients undergoing awake craniotomy. J Neurosurg. 2022;137(6):1853-1861. [DOI] [PubMed] [Google Scholar]
- 20.Spena G, Schucht P, Seidel K, et al. Brain tumors in eloquent areas: a European multicenter survey of intraoperative mapping techniques, intraoperative seizures occurrence, and antiepileptic drug prophylaxis. Neurosurg Rev. 2017;40(2):287-298. [DOI] [PubMed] [Google Scholar]
- 21.Borchers S, Himmelbach M, Logothetis N, Karnath HO. Direct electrical stimulation of human cortex—the gold standard for mapping brain functions? Nat Rev Neurosci. 2011;13(1):63-70. [DOI] [PubMed] [Google Scholar]
- 22.Corley JA, Nazari P, Rossi VJ, et al. Cortical stimulation parameters for functional mapping. Seizure. 2017;45:36-41. [DOI] [PubMed] [Google Scholar]
- 23.Trébuchon A, Chauvel P. Electrical stimulation for seizure induction and functional mapping in stereoelectroencephalography. J Clin Neurophysiol. 2016;33(6):511-521. [DOI] [PubMed] [Google Scholar]
- 24.Mandonnet E. Intraoperative electrical mapping: advances, limitations and perspectives. In: Brain Mapping: From Neural Basis of Cognition to Surgical Applications. Springer; 2011:101-108. [Google Scholar]
- 25.Zhao Z, Liu Y, Zhang J, Lu J, Wu J. Where is the speech production area? Evidence from direct cortical electrical stimulation mapping. Brain. 2021;144(7):e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Umana GE, Scalia G, Graziano F, et al. Navigated transcranial magnetic stimulation motor mapping usefulness in the surgical management of patients affected by brain tumors in eloquent areas: a systematic review and meta-analysis. Front Neurol. 2021;12:644198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorbach AM, Heiss JD, Kopylev L, Oldfield EH. Intraoperative infrared imaging of brain tumors. J Neurosurg. 2004;101(6):960-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ecker RD, Goerss SJ, Meyer FB, Cohen-Gadol AA, Britton JW, Levine JA. Vision of the future: initial experience with intraoperative real-time high-resolution dynamic infrared imaging. Technical note. J Neurosurg. 2002;97(6):1460-1471. [DOI] [PubMed] [Google Scholar]
- 29.Cardone D, Trevisi G, Perpetuini D, Filippini C, Merla A, Mangiola A. Intraoperative thermal infrared imaging in neurosurgery: machine learning approaches for advanced segmentation of tumors. Phys Eng Sci Med. 2023;46(1):325-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iorga M, Schneider N, Cho J, Tate MC, Parrish TB. A novel intraoperative mapping device detects the thermodynamic response function. Brain Sci. 2023;13(7):1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Khashab M, Liu WC, Fried A, Gabay TM, Pierce SD, Ghacibeh GA. When the direct electrical cortical stimulation failed: a case report. J Neurol Res. 2011;1(1):30-33. [Google Scholar]
- 32.Iorga M, Tate MC, Parrish TB. A robust motion correction technique for infrared thermography during awake craniotomy. Int J Comput Assist Radiol Surg. 2023;18(12):2223-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao FL, Gao PY, Sui BB, et al. Time-course of changes in activation among facial nerve injury: a functional imaging study. Medicine. 2015;94(43):e1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rottler P, Schroeder HWS, Lotze M. Outcome-dependent coactivation of lip and tongue primary somatosensory representation following hypoglossal-facial transfer after peripheral facial palsy. Hum Brain Mapp. 2014;35(2):638-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lüdemann L, Förschler A, Grieger W, Zimmer C. BOLD signal in the motor cortex shows a correlation with the blood volume of brain tumors. J Magn Reson Imaging. 2006;23(4):435-443. [DOI] [PubMed] [Google Scholar]
- 36.Ibrahim AS, Gomaa M, Sakr HM, Elzaher YA. Role of diffusion tensor imaging in characterization and preoperative planning of brain neoplasms. Egypt J Radiol Nucl Med. 2013;44(2):297-307. [Google Scholar]
- 37.Abboud T, Hahn G, Just A, et al. An insight into electrical resistivity of white matter and brain tumors. Brain Stimul. 2021;14(5):1307-1316. [DOI] [PubMed] [Google Scholar]
- 38.Lempka SF, Miocinovic S, Johnson MD, Vitek JL, McIntyre CC. In vivo impedance spectroscopy of deep brain stimulation electrodes. J Neural Eng. 2009;6(4):046001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei XF, Grill WM. Impedance characteristics of deep brain stimulation electrodes in vitroandin vivo. J Neural Eng. 2009;6(4):046008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Todd MM, Cutkomp J, Brian JE. Influence of mannitol and furosemide, alone and in combination, on brain water content after fluid percussion injury. Anesthesiology. 2006;105(6):1176-1181. [DOI] [PubMed] [Google Scholar]
- 41.Rosen AS, Andrew RD. Osmotic effects upon excitability in rat neocortical slices. Neuroscience. 1990;38(3):579-590. [DOI] [PubMed] [Google Scholar]
- 42.Lassmann H, Petsche U, Kitz K, et al. The role of brain edema in epileptic brain damage induced by systemic kaini acid injection. Neuroscience. 1984;13(3):691-704. [DOI] [PubMed] [Google Scholar]
- 43.Sammaritano M, Andermann F, Melanson D, et al. Prolonged focal cerebral edema associated with partial status epilepticus. Epilepsia. 1985;26(4):334-339. [DOI] [PubMed] [Google Scholar]
- 44.Stultz DJ, Osburn S, Burns T, Pawlowska-Wajswol S, Walton R. Transcranial magnetic stimulation (TMS) safety with respect to seizures: a literature review. Neuropsychiatr Dis Treat. 2020;16:2989-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]