Abstract
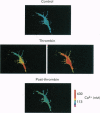
1. Glial cells are known to play a role in regulating the microenvironment of the nervous system. While earlier considerations of glial function assumed a passive, static physiology for these cells, this is not likely to be the case. In this study, we begin to examine how the physiology of Müller glial cells changes in response to molecules in the microenvironment. 2. Perforated-path recordings and intracellular calcium measurements were performed on human retinal Müller cells in vitro. 3. Analysis of whole-cell currents revealed that the human Müller glial cells have an inwardly rectifying K+ current (IK(IR) which is active near the resting membrane potential. This IK(IR) is significantly inhibited when the Müller cell is exposed to thrombin, a molecule that is likely to enter the retina with a breakdown of the blood-retinal barrier and may be endogenous to the nervous system. 4. A variety of experiments point to a role for Ca2+ as a second messenger mediating the inhibitory effect of thrombin on the IK(IR) of Müller cells. Specifically, thrombin evokes an increase in intracellular [Ca2+] in the Müller cells; the Ca2+ chelator BAPTA blocks the effects of thrombin on both the inhibition of IK(IR) and the rise in intracellular [Ca2+]; exposure to ionomycin, a calcium ionophore, induces a reduction in the IK(IR) of Müller cells. 5. A thrombin- induced inhibition in the IK(IR) of Müller cells is likely to have significant functional consequences for the retina since these ion channels are involved in K+ homeostasis. 6. Our experiments support the idea that the physiology of Müller glial cells is dynamic and can be markedly affected by molecules in the microenvironment.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Barakeh J., Laskey R., Van Breemen C. Ion channels and regulation of intracellular calcium in vascular endothelial cells. FASEB J. 1989 Oct;3(12):2389–2400. doi: 10.1096/fasebj.3.12.2477294. [DOI] [PubMed] [Google Scholar]
- Aotaki-Keen A. E., Harvey A. K., de Juan E., Hjelmeland L. M. Primary culture of human retinal glia. Invest Ophthalmol Vis Sci. 1991 May;32(6):1733–1738. [PubMed] [Google Scholar]
- Barbour B., Brew H., Attwell D. Electrogenic glutamate uptake in glial cells is activated by intracellular potassium. Nature. 1988 Sep 29;335(6189):433–435. doi: 10.1038/335433a0. [DOI] [PubMed] [Google Scholar]
- Barres B. A., Chun L. L., Corey D. P. Ion channels in vertebrate glia. Annu Rev Neurosci. 1990;13:441–474. doi: 10.1146/annurev.ne.13.030190.002301. [DOI] [PubMed] [Google Scholar]
- Benveniste E. N. Inflammatory cytokines within the central nervous system: sources, function, and mechanism of action. Am J Physiol. 1992 Jul;263(1 Pt 1):C1–16. doi: 10.1152/ajpcell.1992.263.1.C1. [DOI] [PubMed] [Google Scholar]
- Brass L. F. Homologous desensitization of HEL cell thrombin receptors. Distinguishable roles for proteolysis and phosphorylation. J Biol Chem. 1992 Mar 25;267(9):6044–6050. [PubMed] [Google Scholar]
- Brew H., Gray P. T., Mobbs P., Attwell D. Endfeet of retinal glial cells have higher densities of ion channels that mediate K+ buffering. Nature. 1986 Dec 4;324(6096):466–468. doi: 10.1038/324466a0. [DOI] [PubMed] [Google Scholar]
- Chan C. C., Rozenszajn L. A., Nussenblatt R. B., Muellenberg-Coulombre C., Hsu S. M., Palestine A. G., Lando Z., BenEzra D. Monoclonal antibodies to Müller's cells of the retina. Invest Ophthalmol Vis Sci. 1984 Sep;25(9):1007–1012. [PubMed] [Google Scholar]
- Chao T. I., Henke A., Reichelt W., Eberhardt W., Reinhardt-Maelicke S., Reichenbach A. Three distinct types of voltage-dependent K+ channels are expressed by Müller (glial) cells of the rabbit retina. Pflugers Arch. 1994 Jan;426(1-2):51–60. doi: 10.1007/BF00374670. [DOI] [PubMed] [Google Scholar]
- Crook R. B., Lui G. M., Polansky J. R. Thrombin stimulates inositol phosphate formation, intracellular calcium fluxes and DNA synthesis in cultured fetal human non-pigmented ciliary epithelial cells. Exp Eye Res. 1992 Dec;55(6):785–795. doi: 10.1016/0014-4835(92)90005-d. [DOI] [PubMed] [Google Scholar]
- Dihanich M., Kaser M., Reinhard E., Cunningham D., Monard D. Prothrombin mRNA is expressed by cells of the nervous system. Neuron. 1991 Apr;6(4):575–581. doi: 10.1016/0896-6273(91)90060-d. [DOI] [PubMed] [Google Scholar]
- Frishman L. J., Steinberg R. H. Light-evoked increases in [K+]o in proximal portion of the dark-adapted cat retina. J Neurophysiol. 1989 Jun;61(6):1233–1243. doi: 10.1152/jn.1989.61.6.1233. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gupta S. K., Diez E., Heasley L. E., Osawa S., Johnson G. L. A G protein mutant that inhibits thrombin and purinergic receptor activation of phospholipase A2. Science. 1990 Aug 10;249(4969):662–666. doi: 10.1126/science.2166341. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hung D. T., Wong Y. H., Vu T. K., Coughlin S. R. The cloned platelet thrombin receptor couples to at least two distinct effectors to stimulate phosphoinositide hydrolysis and inhibit adenylyl cyclase. J Biol Chem. 1992 Oct 15;267(29):20831–20834. [PubMed] [Google Scholar]
- Karwoski C. J., Lu H. K., Newman E. A. Spatial buffering of light-evoked potassium increases by retinal Müller (glial) cells. Science. 1989 May 5;244(4904):578–580. doi: 10.1126/science.2785716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCAS D. R., NEWHOUSE J. P. The toxic effect of sodium L-glutamate on the inner layers of the retina. AMA Arch Ophthalmol. 1957 Aug;58(2):193–201. doi: 10.1001/archopht.1957.00940010205006. [DOI] [PubMed] [Google Scholar]
- Newman E. A. Distribution of potassium conductance in mammalian Müller (glial) cells: a comparative study. J Neurosci. 1987 Aug;7(8):2423–2432. [PMC free article] [PubMed] [Google Scholar]
- Newman E. A., Frambach D. A., Odette L. L. Control of extracellular potassium levels by retinal glial cell K+ siphoning. Science. 1984 Sep 14;225(4667):1174–1175. doi: 10.1126/science.6474173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E. A. Inward-rectifying potassium channels in retinal glial (Müller) cells. J Neurosci. 1993 Aug;13(8):3333–3345. doi: 10.1523/JNEUROSCI.13-08-03333.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E. A. Membrane physiology of retinal glial (Müller) cells. J Neurosci. 1985 Aug;5(8):2225–2239. doi: 10.1523/JNEUROSCI.05-08-02225.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puro D. G., Mano T., Chan C. C., Fukuda M., Shimada H. Thrombin stimulates the proliferation of human retinal glial cells. Graefes Arch Clin Exp Ophthalmol. 1990;228(2):169–173. doi: 10.1007/BF00935728. [DOI] [PubMed] [Google Scholar]
- Puro D. G., Mano T. Modulation of calcium channels in human retinal glial cells by basic fibroblast growth factor: a possible role in retinal pathobiology. J Neurosci. 1991 Jun;11(6):1873–1880. doi: 10.1523/JNEUROSCI.11-06-01873.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991 Mar;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Reichelt W., Müller T., Pastor A., Pannicke T., Orkand P. M., Kettenmann H., Schnitzer J. Patch-clamp recording from Müller (glial) cell endfeet in the intact isolated retina and acutely isolated Müller cells of mouse and guinea-pig. Neuroscience. 1993 Dec;57(3):599–613. doi: 10.1016/0306-4522(93)90009-5. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M. Voltage-gated ion channels in Schwann cells and glia. Trends Neurosci. 1992 Sep;15(9):345–351. doi: 10.1016/0166-2236(92)90052-a. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarantis M., Attwell D. Glutamate uptake in mammalian retinal glia is voltage- and potassium-dependent. Brain Res. 1990 May 21;516(2):322–325. doi: 10.1016/0006-8993(90)90935-5. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. L-glutamate conditionally modulates the K+ current of Müller glial cells. Neuron. 1993 Jun;10(6):1141–1149. doi: 10.1016/0896-6273(93)90062-v. [DOI] [PubMed] [Google Scholar]
- Siess W. Molecular mechanisms of platelet activation. Physiol Rev. 1989 Jan;69(1):58–178. doi: 10.1152/physrev.1989.69.1.58. [DOI] [PubMed] [Google Scholar]
- Stuenkel E. L., Nordmann J. J. Intracellular calcium and vasopressin release of rat isolated neurohypophysial nerve endings. J Physiol. 1993 Aug;468:335–355. doi: 10.1113/jphysiol.1993.sp019775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]