Abstract
Solid tumors generate a prothrombotic environment capable of platelet activation. Recent findings indicate that the activated platelets are crucial regulators of tumor vascular homeostasis in that they prevent tumor hemorrhage. Surprisingly, this effect is independent of platelets’ capacity to form thrombi and instead relies on the secretion of their granule content. Thus, targeting platelet secretory activities may represent a new approach to specifically destabilize tumor vasculature.
Tumor-Platelet Cross-Talk
Since the first description by Trousseau (1), an association between cancer and thrombosis has been recognized. Tumor cells may express tissue factor, release procoagulant microparticles, and secrete cytokines rendering endothelium prothrombotic (2). Moreover, sluggish blood flow and contorted vessels are features of the tumor microcirculation that contribute to the tumor thrombogenic environment (3). Thus, tumors are capable of activating platelets, and it has been suggested that platelets in turn promote tumor growth (4, 5).
Clinical and experimental evidence indicates that platelets play a role in the spread of cancer. Both thrombocytopenia and antiplatelet treatments reduce the number of experimental metastases (6, 7). Coating circulating cancer cells with platelets protects them from the immune response (8) and enhances their extravasation by increasing their adhesion to the vessel wall (7). Whereas interactions of platelets with circulating cancer cells are well documented, the actual contribution of platelets to solid tumor homeostasis prompts further analysis.
Platelets are a rich source of pro- and antiangiogenic factors that can be differentially released upon platelet activation (9). Thus platelet activation could regulate angiogenesis, a process essential in cancer progression. Furthermore, platelets support the integrity of angiogenic (10) and inflamed microvessels (11) through the prevention of hemorrhage at sites of angiogenesis and inflammation. Because angiogenesis and leukocyte infiltration are both involved in the development of solid tumors, it is conceivable that platelets may have an impact on tumor vessel integrity and homeostasis. Indeed, recently platelets have been shown to continuously prevent tumor hemorrhage by releasing their granules (12). In this review, we summarize these findings and discuss their therapeutic potential.
Platelets and Tumor Vascular Homeostasis
The role of platelets in solid tumors was addressed by inducing acute thrombocytopenia with a platelet-depleting antibody in mice bearing subcutaneous tumors or established lung metastasis (12). Thrombocytopenia consistently resulted in severe bleeding that was specific to the tumor site and not seen elsewhere in the animal. The tumors from thrombocytopenic mice were characterized by massive hemorrhage at the tumor-stroma interface, reduced tumor cell proliferation, and increased apoptosis in the hemorrhagic areas. The profound effect of platelet depletion on tumors, independent of the tumor location and age, underlined the severity of the vessel damage. The vascular breaks occurred very rapidly as bleeding became noticeable as early as 30 minutes after induction of thrombocytopenia. The continuous need for platelets to prevent tumor bleeding and consequent cell death reveals the crucial contribution of platelets to tumor vascular homeostasis. Thus, apart from their supportive role in tumor metastasis, platelets also have to be considered as key regulators of tumor vessel stability.
Prevention of tumor bleeding by platelets displays surprising mechanistic features. In contrast to platelets’ role in primary hemostasis, firm platelet adhesion is not required for maintaining vascular integrity in tumors. Intravital microscopy using fluorescently labeled platelets or immunostaining of platelets in tumor sections did not reveal the presence of thrombi in the tumor microcirculation (12). Previously, Manegold and colleagues reported platelet rolling but no adherence on tumor microvessels (13). The lack of adherent platelets led to the hypothesis that the mechanisms involved in the prevention of tumor hemorrhage by platelets differ from those required for platelet plug formation. This was confirmed genetically in experiments using mouse models of bleeding disorders due to defective platelet adhesion. These mice bleed profusely when injured but did not bleed at tumor sites. Thrombus formation relies on platelet adhesion through glycoprotein Ib alpha (GPIbα) to von Willebrand factor (VWF) and the activation of the integrin aIIbh3 leading to platelet aggregation. VWF−/−, GPIbα inhibitor-treated mice, and mice with defective activation of integrins (CalDAG-GEFI−/−) showed no spontaneous intratumor hemorrhage (12). Moreover, transfusion of platelets mutant in GPIbα prevented thrombocytopenia-induced tumor bleeding. Interestingly, the ability of these platelets to prevent tumor bleeding was lost when platelet degranulation was induced before infusion. Thus, prevention of tumor hemorrhage by platelets most likely relies on the positive effects of the granular components. Activated platelets also shed several plasma membrane receptors and procoagulant microparticles that could contribute to the biological activities platelets exert on tumor vessels. Degranulated platelets characterized by low levels of ADP and serotonin have been found in patients with malignant solid tumors, even in the absence of active consumption coagulopathy, showing the capacity of the tumors to induce platelet secretion (14).
How Do Platelets Stabilize Tumor Vessels?
The platelet-derived factors involved in the prevention of tumor hemorrhage have not been identified. Additionally, the cell type that platelets influence most is not known and several types of cells could be affected. The soluble factors released by platelets may regulate the endothelial stability of the angiogenic tumor vessels and/or prevent vascular damages induced by the tumor cells or inflammatory cells associated with tumors.
Platelets have long been known to enhance endothelial barrier function and help preserve endothelium during organ reperfusion (15-17). Kitchens and Weis (18) reported thinning of endothelium in microvessels within 6 hours of platelet depletion in rabbits and an increase in vessel permeability. But platelet depletion was not sufficient to induce significant hemorrhage such as was observed in tumor vessels of the thrombocytopenic mice (12). Thus, tumor vessels have a greater need for the mediators released by platelets than the general microcirculation of the animal.
Several classes of molecules released by platelets were shown to reduce vascular permeability. The best known are angiopoietin-1 (ang-1) (refs. 12, 19), serotonin (5-HT) (ref. 20), and sphingosin-1-P (S1P) (ref. 21). Absence of the platelet-released factors was proposed to cause extravasation of red blood cells from postcapillary venules in thrombocytopenic patients (19, 22). In contrast to normal vessel wall, tumors abundantly express vascular endothelial growth factor (VEGF), also known as vascular permeability factor. As the name implies, it has a potent ability to increase vascular permeability. Therefore, in the tumors, platelets may have to counteract the effects of VEGF. Indeed, thrombocytopenia dramatically impairs the balance between pro- and antipermeability factors in tumor-bearing mice, in particular depleting blood of angiopoietin-1 and serotonin (12). Of note however, extravasation of red blood cells does not occur only through the endothelium but also through the basement membrane, which implies degradation or rupture of the basal lamina surrounding vasculature. It is likely that the massive extravasation of red blood cells observed in thrombocytopenic tumors involves factors capable of damaging basement membranes and/or abnormalities of the basement membrane in tumor vessels. Profound structural abnormalities of basement membrane in tumors, including broad extensions away from the vessel wall, were noted (23).
In addition to the beneficial effects platelets have on resting endothelium, platelets could diminish injuries produced by inflammation. Areas of inflammation are also unusually susceptible to hemorrhage in thrombocytopenia, both in the skin and in internal organs (11). Solid tumors show signs of chronic inflammation. These include the presence of infiltrating leukocytes, the expression of cytokines such as tumor necrosis factor-α (TNF-α) or interleukin-1, chemokines, and active tissue remodeling with fibrin deposition, all potentially affecting vascular integrity (24). In addition, leukocytes secrete injurious products such as matrix metalloproteases (MMPs), serine proteases, and reactive oxygen species (ROS). Platelets could modulate the activity of leukocytes and also neutralize some of their harmful products through release of serpins, tissue inhibitors of metalloproteinases (TIMPs), and ROS scavengers from their granules (25-27). Therefore, the need for platelets to prevent tumor hemorrhage may involve not only their positive effects on endothelium (18) and role in angiogenesis (10) but also their ability to tighten endothelial junctions (15, 16, 19, 21) and to prevent potential vascular injuries by tumor-infiltrating leukocytes (Fig. 1) (ref. 11).
Figure 1.
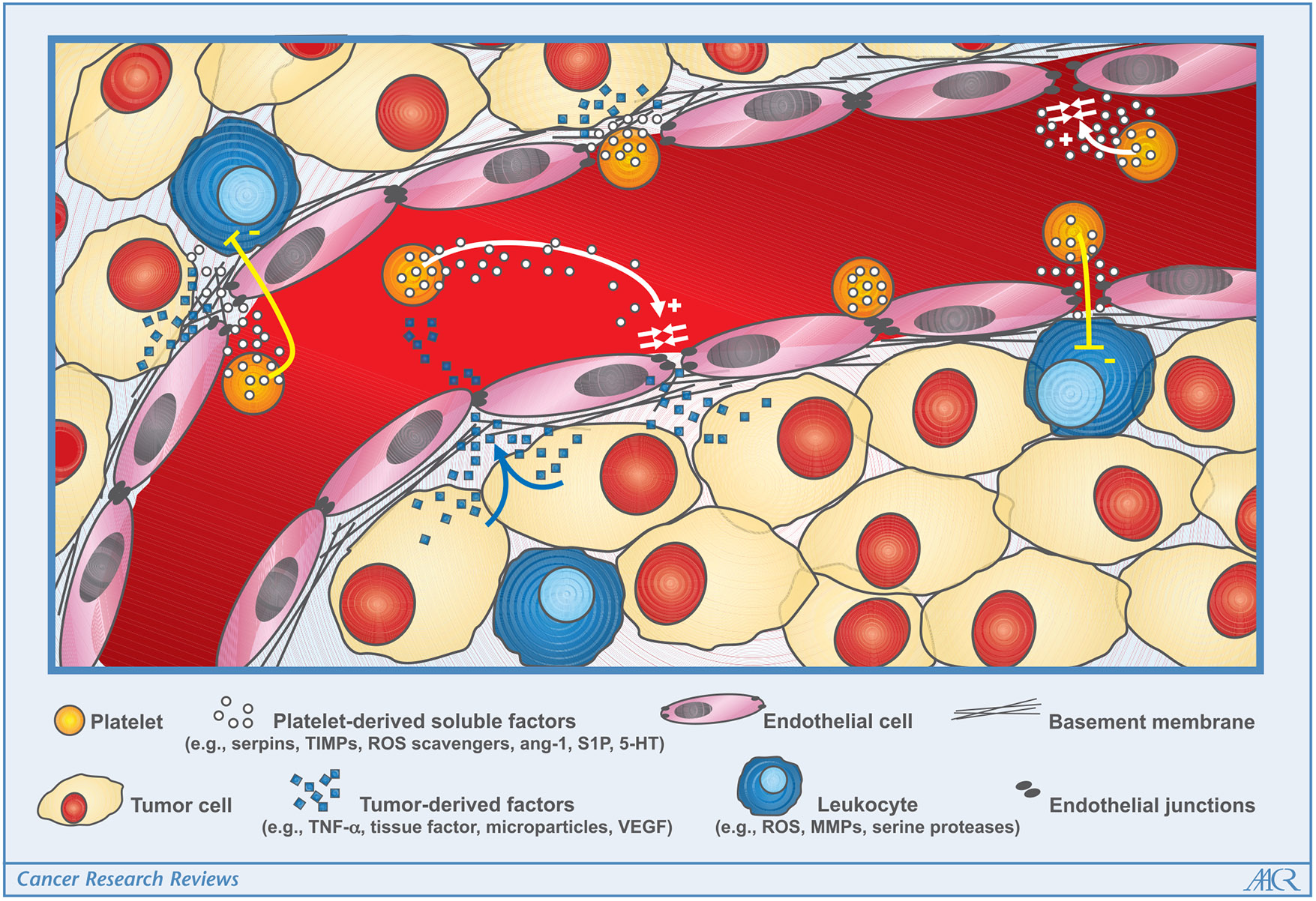
Schematic of platelet-tumor interactions relevant for maintenance of tumor vascular integrity. Tumors provide a thrombogenic proinflammatory environment that promotes coagulation and endothelial cell activation. The prothrombotic environment stimulates local degranulation of platelets. The release of vascular protective and stabilizing factors from platelets helps tighten endothelial junctions and/or inhibits injurious stimuli produced by tumor cells and tumor-infiltrating leukocytes.
The fact that genetic inhibition of platelet adhesion does not affect their capacity to stabilize tumor vessels raises questions about how platelets deliver active compounds to tumor vessels. As previously hypothesized by Folkman and colleagues (5), platelet-tumor interactions might be facilitated by impaired blood flow and localized granular release by the procoagulant environment of the tumor. Platelets have two different types of granules, dense granules where serotonin is located and alpha granules containing protein components including angiogenic factors, growth factors, and immunomodulatory cytokines. The alpha granules further differ in their composition and can be differentially secreted by platelets depending on stimulus (9, 28) as is the case for endothelial storage granules (29). Thus, there could be a very fine regulation of expression of angiogenic, inflammatory, and vascular stability modulators by granular secretion within the tumor.
In parallel to studies reporting cancer-associated platelet activation, enrichment in intraplatelet angiogenic factors has also been described in cancer patients. Platelet lysate from breast cancer patients was noted to contain higher VEGF and angiopoietin-1 levels than from healthy subjects (30). Thus, it could be that solid tumors not only stimulate platelet granule secretion, but also load platelets with proangiogenic factors that support tumor progression.
Therapeutic Implications for the Role of Platelets in Maintenance of Tumor Vessel Integrity
One important aspect of recent findings about platelet-tumor interactions is that platelet-mediated prevention of tumor vessel damage does not require platelet plug formation. The mechanistic dichotomy of platelet-dependent primary hemostasis and maintenance of tumor vessel integrity may allow specific pharmacological targeting of the two processes. Inhibitors of the platelet-derived soluble factors responsible for protection of tumor vessels may destabilize the tumor vasculature without affecting primary hemostasis and increasing the risk of bleeding outside the tumor. On the other hand, blockage of platelet activation that is involved in both integrin activation and degranulation would probably alter both primary hemostasis and tumor vessel stability.
The identification of the specific vascular protective factor(s) delivered by platelets to solid tumors or discovery of inhibitors preventing their secretion could open new therapeutic perspectives. Targeting of these molecules or their secretion could selectively destabilize tumor vasculature and, thus, affect tumor cell viability. It is conceivable that destabilization of the tumor vessels might have additional beneficial consequences, such as promoting antitumor immunity through better exposure of tumor antigens to circulating immune cells, improving the delivery of chemotherapeutic agents, even larger nanoparticles, to solid tumors. Alternatively, mimicking the stabilizing effect of platelets on tumor vessels by providing the stabilizing factors might promote normalization of the tumor vasculature and its function. Normalizing tumor vessels is a strategy that was proposed to increase the efficiency of oxygen and drug delivery to solid tumors and that was shown to have a synergistic effect with cytotoxic therapy (31).
In conclusion, interference in platelet-tumor vessel cross-talk represents an interesting and challenging approach for manipulating tumor vasculature in order to improve anticancer therapies. New insights could be provided by the identification of the beneficial factors that platelets deliver to the tumor vasculature and of the cell type(s) platelets regulate to prevent tumor hemorrhage.
Acknowledgments
Grant support: National Heart, Lung, and Blood Institute of the National Institutes of Health grants P01 HL066105 and R37 HL041002 (to D.D. Wagner).
We thank Dr. Joseph Italiano for helpful discussions and reading of the manuscript and Lesley Cowan for help with manuscript preparation.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Trousseau A. Phlegmatia alba dolens, vol. 3. Paris: JB Baillere et Fils; 1865. p. 654–712. [Google Scholar]
- 2.Amin C, Mackman N, Key NS. Microparticles and cancer. Pathophysiol Haemost Thromb 2008;36:177–83. [DOI] [PubMed] [Google Scholar]
- 3.Dvorak HF, Nagy JA, Berse B, et al. Vascular permeability factor, fibrin, and the pathogenesis of tumor stroma formation. Ann N Y Acad Sci 1992;667:101–11. [DOI] [PubMed] [Google Scholar]
- 4.Nash GF, Turner LF, Scully MF, Kakkar AK. Platelets and cancer. Lancet Oncol 2002;3:425–30. [DOI] [PubMed] [Google Scholar]
- 5.Pinedo HM, Verheul HM, D’Amato RJ, Folkman J. Involvement of platelets in tumour angiogenesis? Lancet 1998;352:1775–7. [DOI] [PubMed] [Google Scholar]
- 6.Gasic GJ, Gasic TB, Stewart CC. Antimetastatic effects associated with platelet reduction. Proc Natl Acad Sci U S A 1968;61:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karpatkin S, Pearlstein E, Salk PL, Yogeeswaran G. Role of platelets in tumor cell metastases. Ann N Y Acad Sci 1981;370:101–18. [DOI] [PubMed] [Google Scholar]
- 8.Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 1999;59:1295–300. [PubMed] [Google Scholar]
- 9.Italiano JE Jr., Richardson JL, Patel-Hett S, et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet a granules and differentially released. Blood 2008;111:1227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kisucka J, Butterfield CE, Duda DG, et al. Platelets and platelet adhesion support angiogenesis while preventing excessive hemorrhage. Proc Natl Acad Sci U S A 2006;103:855–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goerge T, Ho-Tin-Noe B, Carbo C, et al. Inflammation induces hemorrhage in thrombocytopenia. Blood 2008;111:4958–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho-Tin-Noe B, Goerge T, Cifuni SM, Duerschmied D, Wagner DD. Platelet granule secretion continuously prevents intratumor hemorrhage. Cancer Res 2008;68:6851–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manegold PC, Hutter J, Pahernik SA, Messmer K, Dellian M. Platelet-endothelial interaction in tumor angiogenesis and microcirculation. Blood 2003;101:1970–6. [DOI] [PubMed] [Google Scholar]
- 14.Boneu B, Bugat R, Boneu A, Eche N, Sie P, Combes PF. Exhausted platelets in patients with malignant solid tumors without evidence of active consumption coagulopathy. Eur J Cancer Clin Oncol 1984;20:899–903. [DOI] [PubMed] [Google Scholar]
- 15.Danielli JF. Capillary permeability and oedema in the perfused frog. J Physiol 1940;98:109–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gimbrone MA Jr., Aster RH, Cotran RS, Corkery J, Jandl JH, Folkman J. Preservation of vascular integrity in organs perfused in vitro with a platelet-rich medium. Nature 1969;222:33–6. [DOI] [PubMed] [Google Scholar]
- 17.D’Amore P. First annual Lamport award manuscript. Platelet-endothelial interaction and the maintenance of the microvasculature. Microvasc Res 1978;15:137–45. [DOI] [PubMed] [Google Scholar]
- 18.Kitchens CS, Weiss L. Ultrastructural changes of endothelium associated with thrombocytopenia. Blood 1975;46:567–78. [PubMed] [Google Scholar]
- 19.Li JJ, Huang YQ, Basch R, Karpatkin S. Thrombin induces the release of angiopoietin-1 from platelets. Thromb Haemost 2001;85:204–6. [PubMed] [Google Scholar]
- 20.Shepro D, Welles SL, Hechtman HB. Vasoactive agonists prevent erythrocyte extravasation in thrombocytopenic hamsters. Thromb Res 1984;35:421–30. [DOI] [PubMed] [Google Scholar]
- 21.Schaphorst KL, Chiang E, Jacobs KN, et al. Role of sphingosine-1 phosphate in the enhancement of endothelial barrier integrity by platelet-released products. Am J Physiol Lung Cell Mol Physiol 2003;285:L258–67. [DOI] [PubMed] [Google Scholar]
- 22.Nachman RL, Rafii S. Platelets, petechiae, and preservation of the vascular wall. N Engl J Med 2008;359:1261–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol 2003;163:1801–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol 2006;1:119–50. [DOI] [PubMed] [Google Scholar]
- 25.Mansilla S, Boulaftali Y, Venisse L, et al. Macrophages and platelets are the major source of protease nexin-1 in human atherosclerotic plaque. Arterioscler Thromb Vasc Biol 2008;28:1844–50. [DOI] [PubMed] [Google Scholar]
- 26.Radomski A, Jurasz P, Sanders EJ, et al. Identification, regulation and role of tissue inhibitor of metalloproteinases-4 (TIMP-4) in human platelets. Br J Pharmacol 2002;137:1330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGarrity ST, Stephenson AH, Hyers TM, Webster RO. Inhibition of neutrophil superoxide anion generation by platelet products: role of adenine nucleotides. J Leukoc Biol 1988;44:411–21. [DOI] [PubMed] [Google Scholar]
- 28.Cognasse F, Hamzeh-Cognasse H, Lafarge S, et al. Toll-like receptor 4 ligand can differentially modulate the release of cytokines by human platelets. Br J Haematol 2008;141:84–91. [DOI] [PubMed] [Google Scholar]
- 29.Cleator JH, Zhu WQ, Vaughan DE, Hamm HE. Differential regulation of endothelial exocytosis of P-selectin and von Willebrand factor by protease-activated receptors and cAMP. Blood 2006;107:2736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caine GJ, Lip GY, Blann AD. Platelet-derived VEGF, Flt-1, angiopoietin-1 and P-selectin in breast and prostate cancer: further evidence for a role of platelets in tumour angiogenesis. Ann Med 2004;36:273–7. [DOI] [PubMed] [Google Scholar]
- 31.Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res 2007;74:72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]


