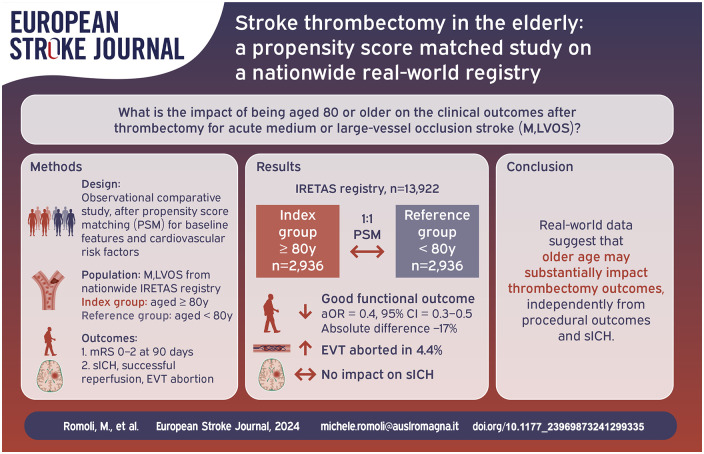
Abstract
Introduction:
Data on safety and efficacy of endovascular thrombectomy (EVT) for acute ischemic stroke in older patients are limited and controversial, and people aged 80 or older were under-represented in randomized trials. Our aim was to assess EVT effect for ischemic stroke patients aged ⩾80 at a nationwide level.
Patients and methods:
The cohort included stroke patients undergoing EVT from the Italian Registry of Endovascular Treatment in Acute Stroke (IRETAS). Patients were a priori divided into younger and older groups (<80 vs ⩾80). Primary outcome was good functional outcome (modified Rankin scale, mRS, 0–2 at 90 days). Secondary outcomes were symptomatic intracranial hemorrhage (sICH), successful reperfusion, EVT abortion. Propensity score matching (PSM) was performed between age groups for baseline features, functional status, stroke severity and neuroradiological features. Logistic regression was implemented to test the weight of age group on the predefined outcomes.
Results:
Overall, 5872 individuals (1:1 matching, n = 2936 aged ⩾80 vs n = 2936 < 80) were matched from 13,922 records. In ⩾80 group 34.1% had good functional outcome, vs 51.2% in <80 group (absolute difference = −17.1%, p < 0.001), with a 4.4% excess in EVT abortion. Age ⩾80 was a negative independent predictor of good functional outcome (aOR = 0.4, 95% CI = 0.3–0.5), but had no impact on sICH.
Discussion and conclusion:
Age ⩾80 years represents a consistent predictor of worse functional outcome, independently from successful reperfusion and sICH. Cost-effectiveness studies are needed for tailored and implement sustainable care, and research should focus on strategies to improve functional outcome in older age patient groups.
Keywords: Thrombectomy, ischemic stroke, elderly, older population, aging
Graphical abstract.
Introduction
The mean age of people suffering a stroke has gradually increased over time, in line with the global aging of the population in high and middle income countries. 1 People aged 80 or older represent up to 15% of all acute ischemic stroke cases in western world,2,3 a percentage likely to further increase. Revascularization treatment in acute ischemic stroke relies on intravenous thrombolysis (IVT) and endovascular thrombectomy (EVT), which have been shown to be effective in specific time and tissue-based windows. 4 Despite guidelines not including age-dependent restrictions for EVT, solid data supporting consistent efficacy and safety of EVT in oldest old are still lacking. 5 Randomized controlled trials on EVT had age limits for enrollment, ending up in uncertainty on the treatment effect among the oldest old groups.5,6 The HERMES (Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials) collaboration, pooling data from the seven major trials on EVT in acute ischemic stroke, reported a 24% rate of good functional outcome (defined as modified Rankin scale, mRS, 0–2 at 90 days) in people older than 85 years. 5 However data were based on only 77 patients – 4.4% of all those enrolled in the trials (n = 77/1764) –, with even larger estimates compared to younger age groups suggesting a selection bias. 5 Real world-setting studies reported significantly higher mortality and lower rates of good functional outcome in octogenarians compared to younger age groups,3,7 suggesting that expectations differ between common practice and trials.
In real world setting over patients aged ⩾80 are receiving EVT, but considering the general frailty of the elderly and the more difficult evaluation of the pre-stroke mRS, residual disability in this age group despite effective recanalization may be hypothesized. Moreover, EVT abortions due to difficult anatomy may further reduce the treatment effect.
We aimed to investigate the efficacy and safety of EVT in people aged 80 or older in real-world setting, also highlighting rates of EVT failure and EVT-related complications. using data from the large Italian Registry of Endovascular Treatment in Acute Stroke (IRETAS). The results of this study are oriented to define the impact of age in the context of precision and sustainable medicine, with the aim of estimating rate of success despite older age.
Methods
Cohort
This prospective study included data collected in the IRETAS, a nationwide multicenter, observational centralized registry running since 2011, with predefined collection tool and monitoring consortium. 8 The data collection tool, available items, and longitudinal follow-up details were previously described. 8 All acute ischemic stroke patients receiving EVT between January 2015 and December 2021 were considered for eligibility in this study. All participating centers included consecutive cases of EVT for acute ischemic stroke, independently from final outcome. An a priori stage was set for eligibility, with exclusion of cases with data lacking on age (n = 17) and endovascular procedure outcome (n = 4, STROBE checklist in Figure 1). The resulting cohort was split according to an a-priori defined age threshold of 80 years. Clinical, demographic, imaging and laboratory variables were included in the analysis. Symptomatic intracranial hemorrhage (sICH) was adjudicated locally by the treating team, and was defined as any intracranial hemorrhage associated with four point increase in 24 h NIHSS or leading to death, according to ECASS II definition. 8 EVT abortion and procedural adverse events were also registered.
Figure 1.
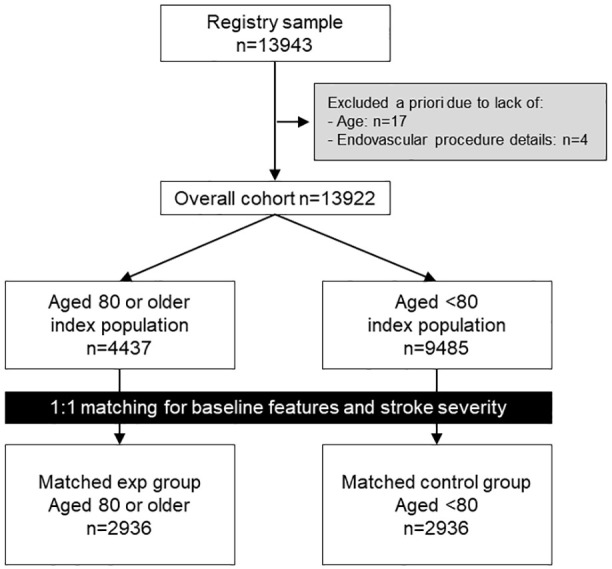
Flowchart for cohort selection and matching.
Outcomes
Primary outcome was good functional outcome, defined as mRS 0–2 at 90-day follow-up. Secondary outcome were sICH, mortality, spontaneous recanalization (defined as complete vessel patency at the moment of first diagnostic stage of EVT) and procedural outcomes, including successful reperfusion (defined as TICI 2b–3), rates of EVT abortion (defined as failed positioning or inability to reach occlusion site), SAH or vessel perforation, iatrogenic dissection, distal embolization and local hematoma requiring surgical approach or local invasive intervention.
Statistical analysis
We report continuous variables as means and standard deviations, and binary or ordinal variables as count and percentage. Parametric and non-parametric tests were used as appropriate depending on distribution of variables, to identify potential differences in the distribution of cardiovascular risk factors and outcomes across different age groups. Bonferroni adjustment was used for multiple comparisons; p-value was used to report statistical differences in factor distribution in the unmatched cohort. To account for inter-group differences in baseline features, matching was implemented using a multivariable logistic regression model with baseline characteristics as covariates, including age, sex, previous stroke/TIA, atrial fibrillation, diabetes, hypertension, coronary artery disease, heart failure, dyslipidemia, baseline mRS, thrombolysis treatment, stroke severity, ASPECT score, large vessel occlusion, reperfusion timing, known/unknown onset and blood pressure measurement at baseline. The corresponding propensity score of the grouping variable (age) was then calculated for each patient, and 1:1 Mahalanobis matching was implemented to match patients aged ⩾80 to younger pairs within predefined thresholds of the logit of the propensity score, ensuring exact matching for stroke severity, ASPECT score, gender, atrial fibrillation (AF), hypertension, dyslipidemia and independent functional status at baseline. To determine whether the approach achieved balance in confounders, standardized mean differences were estimated for all covariates, with an estimate of 0.15 or lower considered acceptable.
After age-related matching in the main groups (⩾80 vs <80 group), a binary logistic regression with backward stepwise elimination was modeled. All variables that reached statistical significance in the univariate analysis for the primary outcome were included to test for independent predictors. Age group was imputed a priori in all models. Two models were developed, one including only baseline variables (model 1) and one including also EVT procedural data (model 2) limiting collinearity, for each stratum. C-statistic is reported to provide the model accuracy in outcome prediction. Last observation carried forward was implemented in case of missing data on primary outcome >5%. 9 Sensitivity analysis included a restricted cohort excluding all cases of EVT abortion, and inverse probability of treatment weighting (IPTW) as an application of propensity scores to retain the whole cohort as compared to PSM procedure. Variables used for weighting and adjustment are shown in Supplemental Material. R v3.3.1 (R-project) was used for all analysis (packages: ipw, MatchIt, and in-house developed packages by MR), with a two-sided p-value <0.05 considered statistically significant. Data can be made available on Zenodo platform (https://zenodo.org/records/6907296).
Results
Overall, 13,922 unmatched participants (4437 in ⩾80 group vs 9485 in <80 group) were included from the original cohort, with significant differences in the distribution of cardiovascular risk factors (Table 1). After 1:1 matching, 5872 individuals were included, 2936 for each group (⩾80 group mean age = 84.6 vs 66.6 years in <80 group, Table 1). Cardiovascular risk factors, stroke severity and neuroradiological features were well-matched between groups (Table 1).
Table 1.
Demographic, clinical and imaging features of people aged 80 or older versus younger people in unmatched and matched cohort.
| Feature | Before matching |
After matching |
||||
|---|---|---|---|---|---|---|
| 80 or older (n = 4437) | <80 (n = 9485) | p-Value | 80 or older (n = 2936) | <80 (n = 2936) | SMD | |
| Age | 84.7 ± 3.7 | 65.4 ± 11.9 | ns | 84.6 ± 3.7 | 66.6 ± 11.7 | NA b |
| Sex (female) | 2834 (63.9%) | 4208 (44.4%) | <0.001 | 1765 (60.1%) | 1923 (65.5%) | 0.1 |
| Previous stroke/TIA a | 199 (4.8%) | 422 (4.9%) | ns | 142 (4.8%) | 137 (4.7%) | 0.02 |
| Atrial fibrillation | 1923 (43.3%) | 2157 (22.7%) | <0.001 | 1244 (42.4%) | 1210 (41.2%) | 0.02 |
| Diabetes | 734 (16.5%) | 1424 (15.0%) | ns | 486 (16.6%) | 493 (16.8%) | 0.02 |
| Hypertension | 3148 (70.9%) | 5135 (54.1%) | <0.001 | 2152 (73.3%) | 2053 (69.9%) | 0.08 |
| Coronary artery disease a | 506 (12.3%) | 846 (9.9%) | <0.001 | 374 (12.7%) | 262 (8.9%) | 0.1 |
| Heart failure a | 344 (8.4%) | 540 (6.3%) | <0.001 | 221 (5.5%) | 163 (5.6%) | 0.07 |
| Dyslipidemia | 1008 (22.7%) | 2176 (22.9%) | ns | 707 (24.1%) | 604 (20.6%) | 0.08 |
| mRS 0–2 baseline | 3615 (81.5%) | 8088 (85.3%) | <0.001 | 2798 (95.3%) | 2823 (96.2%) | −0.02 |
| mRS 0–1 | 3123 (70.4%) | 7642 (80.6%) | <0.001 | 2455 (83.6%) | 2671 (91.0%) | NA b |
| Thrombolysis | 1993 (44.9%) | 4357 (45.9%) | ns | 1378 (48.6%) | 1364 (47.8%) | 0.03 |
| NIHSS | 17 (15–24) | 15 (10–20) | <0.001 | 17 (12–20) | 17 (12–20) | 0.01 |
| ASPECTS | 10 (9–10) | 10 (9–10) | ns | 10 (9–10) | 10 (9–10) | −0.03 |
| Large vessel occlusion | 3414 (76.9%) | 7165 (75.5%) | 0.07 | 2498 (85.1%) | 2570 (87.5%) | −0.1 |
| Onset to EVT | 283 ± 190 | 283 ± 208 | ns | 273 ± 201 | 281 ± 179 | −0.06 |
| Systolic BP (mmHg) | 150.6 ± 24.9 | 145.4 ± 23.8 | <0.001 | 150.1 ± 24 | 145.6 ± 23.8 | 0.09 |
| Diastolic BP (mmHg) | 81.2 ± 13.3 | 81 ± 13 | ns | 81.2 ± 13.1 | 80.9 ± 12.7 | 0.04 |
| Wake-up onset | 478 (10.8%) | 1067 (11.2%) | ns | 338 (11.5%) | 307 (10.5%) | 0.07 |
AF: atrial fibrillation; CAD: coronary artery disease; DBP: diastolic blood pressure; LVO: large vessel occlusion, including internal carotid artery, middle cerebral artery M1 and proximal M2 segment, anterior cerebral artery A1 segment; mRS: modified Rankin scale; SBP: systolic blood pressure.
Data available for 12,700 patients in unmatched cohort.
mRS 0–2 used for matching.
In ⩾80 group 34.1% reached good functional outcome, versus 51.2% in <80 group (−17.1% absolute difference, p < 0.0; Table 2). Mortality was significantly higher in the ⩾80 group (27% vs 15.6%, p < 0.001), as was EVT abortion (4.4% increase in ⩾80 group, Table 2). sICH, successful reperfusion and procedural outcomes were similar across groups (Table 2). The trend against good functional outcome in ⩾80 group also emerged from additional analysis excluding EVT abortion cases, and also considering mRS 0–2 or unchanged compared to baseline as good functional outcome (Supplemental Table 1 and 2).
Table 2.
Outcome distribution across age groups.
| Outcome | 80 or older (n = 2936) (%) | <80 (n = 2936) (%) | p-Value |
|---|---|---|---|
| mRS 0–2 | 1000 (34.1) | 1503 (51.2) | <0.001 |
| Death | 792 (27) | 457 (15.6) | <0.001 |
| sICH | 201 (7.2) | 203 (7.3) | 0.95 |
| Successful reperfusion | 2120 (74.5) | 2178 (76.4) | 0.1 |
| Procedural outcomes | |||
| EVT attempt abortion | 345 (11.8) | 216 (7.4) | <0.001 |
| SAH or vessel perforation | 80 (2.7) | 89 (3) | 0.44 |
| Iatrogenic dissection | 34 (1.2) | 52 (1.8) | 0.05 |
| Distal embolization | 201 (6.9) | 206 (7) | 0.79 |
| Local hematoma | 31 (1.1) | 25 (0.9) | 0.42 |
EVT: endovascular thrombectomy; mRS: modified Rankin Scale; SAH: subarachnoid hemorrhage; sICH: symptomatic intracranial hemorrhage; Successful reperfusion: TICI score 2b–3.
Backward conditional logistic regression confirmed the negative impact of age on good functional outcome, with age ⩾80 emerging as the most detrimental predictor (adjusted ORmodel1 = 0.40, 95% CI = 0.40–0.50, Table 3; c-statisticmodel1 = 0.81, 95% CI = 0.80–0.83). When including EVT procedural outcomes to the predictive model, age ⩾80 still represented a factor negatively impacting prognosis beyond sICH (aOR = 0.40, 95% CI = 0.40–0.50, Table 3; c-statisticmodel2 = 0.85, 95% CI = 0.82–0.87). Such findings were confirmed also in sensitivity analysis with good functional outcome defined as mRS 0–2 or recovery to baseline functional status (Supplemental Table 2). No impact of age ⩾80 emerged on sICH rate, which was significantly influenced solely by factors related to stroke severity (Supplemental Table 3).
Table 3.
Independent predictors of good functional outcome.
| Factor | mRS 0–2 (n =2503) | mRS > 2 (n =3369) | p-Value | Prediction modeling with baseline features only | Prediction model including procedural data | ||||
|---|---|---|---|---|---|---|---|---|---|
| Adjusted OR (95%CI) | p-Value | Elimination step | Adjusted OR (95%CI) | p-Value | Elimination step | ||||
| Age > 80 | 1000 (40%) | 1936 (57.5%) | <0.001 | 0.4 (0.4–0.5) | <0.001 | 0.4 (0.4–0.5) | <0.001 | ||
| Sex (female) | 1609 (64.3%) | 2079 (61.7%) | 0.04 | 1.0 (0.8–1.2) | / | 1 | 1.0 (0.8–1.2) | / | 1 |
| Previous stroke or TIA | 119 (4.8%) | 160 (4.7%) | 0.99 | ||||||
| AF | 929 (37.1%) | 1525 (45.3%) | <0.001 | 0.8 (0.7–1) | / | 5 | 0.7 (0.6–0.9) | 0.003 | |
| Diabetes | 290 (11.6%) | 689 (20.5%) | <0.001 | 0.6 (0.5–0.8) | <0.001 | 0.6 (0.4–0.8) | <0.001 | ||
| Hypertension | 1733 (69.2%) | 2472 (73.4%) | 0.01 | 0.7 (0.6–0.9) | 0.003 | 0.8 (0.6–1) | 0.035 | ||
| CAD | 205 (8.2%) | 431 (12.8%) | <0.001 | 0.6 (0.4–0.8) | <0.001 | 0.6 (0.4–0.9) | 0.004 | ||
| Heart failure | 125 (5%) | 259 (7.7%) | <0.001 | 1.2 (0.8–1.7) | / | 3 | 1.1 (0.7–1.7) | / | 2 |
| Dyslipidemia | 541 (21.6%) | 770 (22.9%) | 0.25 | ||||||
| Thrombolysis | 1262 (51.8%) | 1480 (45.5%) | <0.001 | 1.3 (1.1–1.5) | <0.001 | 1.2 (1–1.5) | / | 5 | |
| mRS 0–2 baseline | 2467 (98.6%) | 3154 (93.6%) | <0.001 | 4.1 (2.3–7.5) | <0.001 | 3.9 (2.1–7.3) | <0.001 | ||
| NIHSS | 14 (9–18) | 18 (15–22) | <0.001 | 0.9 (0.9–0.9) | <0.001 | 0.9 (0.9–0.9) | <0.001 | ||
| ASPECTS | 10 (9–10) | 10 (8–10) | <0.001 | 1.0 (0.9–1.1) | / | 2 | 1.0 (0.9–1) | / | 3 |
| Large vessel occlusion | 2102 (84.6%) | 2966 (88.5%) | 0.001 | 0.6 (0.5–0.8) | <0.001 | 0.6 (0.5–0.8) | <0.001 | ||
| Onset to EVT | 283 ± 196 | 267 ± 195 | 0.09 | ||||||
| SBP | 146.6 ± 22.7 | 149 ± 25 | 0.01 | 0.99 (0.99–1) | / | 4 | 0.99 (0.99–1) | / | 4 |
| DBP | 80.8 ± 12 | 81.2 ± 13.7 | 0.23 | ||||||
| Wake-up onset | 259 (10.3%) | 386 (11.5%) | 0.179 | ||||||
| Procedural outcomes | |||||||||
| sICH | 35 (1.5%) | 369 (11.7%) | <0.001 | 0.1 (0–0.1) | <0.001 | ||||
| Successful reperfusion | 2160 (88.6%) | 2138 (65.5%) | <0.001 | 5.0 (3.9–6.4) | <0.001 | ||||
| EVT attempt abortion | 201 (8.2%) | 359 (10.8%) | 0.01 | ||||||
| SAH or perforation | 31 (1.2%) | 138 (4.1%) | <0.001 | ||||||
| Dissection | 31 (1.2%) | 55 (1.6%) | 0.21 | ||||||
| Distal embolization | 134 (5.4%) | 273 (8.1%) | <0.001 | ||||||
| Local hematoma | 15 (0.6%) | 41 (1.2%) | 0.02 | ||||||
AF: atrial fibrillation; CAD: coronary artery disease; DBP: diastolic blood pressure; mRS: modified Rankin scale; SBP: systolic blood pressure.
Sensitivity analysis retaining the whole cohort through IPW confirmed a negative trend for all older age strata, although mitigating the larger difference found thorugh PSM. Age ⩾80 had a significant negative impact on the primary outcome (adjusted ORmodel1 = 0.88, 95%CI = 0.85–0.91; Supplemental Table 4). All older age strata confirmed a substantial negative impact on good functional outcome (adjusted ORmodel1 for age ⩾ 85 = 0.89, 95% CI = 0.86–0.92; adjusted ORmodel1 for age ⩾ 90 = 0.9, 95% CI = 0.85–0.96; adjusted ORmodel1 for age ⩾ 95 = 0.83, 95%CI = 0.7–0.98; Supplemental Table 5). Older age groups maintained an independent negative impact on functional outcome also in models including EVT procedural data, therefore independently from sICH and successful reperfusion (Supplemental Table 6).
Discussion
The impact of age on the outcomes of EVT, particularly in older people, is still poorly defined. With an aging population, the mean age of people suffering a stroke has gradually increased over time, 1 leading to perform EVT also outside the boundaries of exploration of RCT. 10 Rates of good functional outcome were, however, reported to be critically low, around 10%–39% in preliminary reports.10–12
Our results, emerging from a propensity score matched study on a real-world setting large national registry of EVT, highlight that age ⩾80 has a clear detrimental effect on outcome. Being 80 or older at the moment of EVT reduces the chances of a good functional outcome by around 50% regardless from the degree of reperfusion or the occurrence of sICH. Indeed, rates of sICH seems to not significantly differ between aged population and younger pairs, excluding that the effect could be driven solely by bleeding complications. The negative impact of age on functional status after EVT was confirmed with sensitivity analysis based on IPW, where older age was associated to lower odds of achieving a good functional outcome, even after adjusting for sICH and successful reperfusion. As a consistent proportion of patients aged ⩾80 can achieve a good functional status, efforts should be put toward the identification of the best possible strategies to foster recovery, a more ambitious challenge in older age groups.
In addition, there seems to be room to support an independent reduction in good functional outcome carried by older age, independently from successful recanalization. Even after adjusting for EVT abortion cases, the distribution of good functional outcome still negatively affects patients ⩾80. Our results seem to suggest that the relationship between successful reperfusion and good functional outcome in the ⩾80 population may be less direct than in younger age groups. In a previous HERMES study a similar trend emerged, whose interpretation was however limited by several factors, including underrepresentation of older people in the original trials, selection bias, lack of data on pre-stroke functional status/disability and ASPECTS consistently favoring EVT group. 5 Our study, counting on robust matching on a large nationwide registry and sensitivity analysis through IPW, confirms that age ⩾80 represents a detrimental factor for EVT outcome, independently from the degree of reperfusion or sICH. At the same time, the detrimental effect does not seem so consistent to recommend any withdrawal of EVT to patients meeting functional status requisites for undergoing reperfusion treatments. Therefore, the estimates from our study can be of help in framing the expected benefit when proposing EVT to older age groups, as well as in informing relatives of patients on what to expect from the procedure.
On a broader scale, as EVT rates increase and procedural complications become more rare, these data may help the clinician in defining the appropriate pathway for older patients. As frail people may be over-exposed to in-hospital complications, optimal and tailored care should not stop with EVT, but extend daily to ensure that the advantage of EVT does not regress over time. Older people can still achieve a good functional outcome, therefore efforts should be directed to ensure that such treatment effect is met and preserved. This particularly applies to the implementation of rehabilitation programs, 13 with the aim of capitalizing the net benefit obtained with recanalization.
A further point raised by our study stands in the significantly higher rates of EVT abortion. An absolute 4% higher rate of attempt abortion was registered in patients aged ⩾80 compared to their pairs. This may be considered for technical development of endovascular tools and alternative vascular access sites, which may allow to reduce this percentage even further in the coming decades. On the other hand, it could stimulate stroke interventionists to face new technical challenges to also overcome these procedural difficulties in the elderly population. Reducing the rate of adverse procedural events may indeed represent a first step to contain the economic burden of care, and maximize the cost-effectiveness profile of EVT in older age groups.
Our results should be considered in light of some limitations. First, IRETAS is a nationwide registry which resembles Italian practice. As Italy is among the countries with the longest life expectancy, our results may be flawed by the good-fitness of the aging population and by local practice tending to treat people in older age groups similarly to younger pairs. At the same time, although IRETAS enrolls consecutive patients throughout the whole nation, there is still an intrinsic risk of bias, particularly regarding inclusion and ASPECTS assessment. Second, our results derive from a dedicated matching procedure, which may still leave out unmeasured confounders, including frailty and cognitive status, which will need to be integrated in future studies. Similarly, the impact of AF detection on stroke severity and potential implications for clinical practice should further be elucidated. 14 Third, as devices for EVT evolve, there may be room for thinking that abortion cases will progressively be limited, also increasing the safety of EVT in people ⩾80. Since the effect of EVT was weaker in patients aged ⩾80, strategies need to evolve regarding hyperacute but also post-acute care. It may be insufficient to guarantee EVT treatment in hyperacute stage if people are then displaced from a dedicated rehabilitation path. This can be particularly important in the older population, as these patients still benefit from rehabilitation, and improvement of mRS over time can still occur after the first 3 months, though at times limited by the exposure to an active environment. On the other side, careful consideration of frailty index and age-related conditions15,16 may allow clinicians to provide a more reasonable estimate of treatment effect to orient patient and caregivers expectations.
Overall, our results suggest that people ⩾80 have halved odds of reaching a good functional outcome compared to their matched peers after EVT for acute ischemic stroke, independently from sICH and successful reperfusion. Further studies are needed to optimize prognostication, implement tailored care, and maximize the cost-effectiveness of EVT.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873241299335 for Stroke thrombectomy in the elderly: A propensity score matched study on a nationwide real-world registry by Michele Romoli, Ludovica Migliaccio, Valentina Saia, Giovanni Pracucci, Luigi Cirillo, Stefano Forlivesi, Daniele Romano, Ilaria Casetta, Enrico Fainardi, Fabrizio Sallustio, Nicola Limbucci, Patrizia Nencini, Valerio Da Ros, Marina Diomedi, Stefano Vallone, Guido Bigliardi, Sergio Lucio Vinci, Paolino La Spina, Mauro Bergui, Paolo Cerrato, Sandra Bracco, Rossana Tassi, Andrea Saletti, Cristiano Azzini, Maria Ruggiero, Lucio Castellan, Tiziana Benzi Markushi, Roberto Menozzi, Alessandro Pezzini, Guido Andrea Lazzarotti, Nicola Giannini, Davide Castellano, Andrea Naldi, Alessio Comai, Elisa Dall’Ora, Mauro Plebani, Manuel Cappellari, Giulia Frauenfelder, Edoardo Puglielli, Alfonsina Casalena, Nicola Burdi, Giovanni Boero, Sergio Nappini, Nicola Davide Loizzo, Nicola Cavasin, Adriana Critelli, Diego Ivaldi, Tiziana Tassinari, Francesco Biraschi, Ettore Nicolini, Sergio Zimatore, Marco Petruzzellis, Pietro Filauri, Berardino Orlandi, Ivan Gallesio, Delfina Ferrandi, Marco Pavia, Paolo Invernizzi, Pietro Amistá, Monia Russo, Adriana Paladini, Annalisa Rizzo, Michele Besana, Alessia Giossi, Marco Filizzolo, Marina Mannino, Salvatore Mangiafico, Danilo Toni and Andrea Zini in European Stroke Journal
Acknowledgments
None.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MR declares support for educational activities from CLS-Behring and PRESTIGE-AF trial. SN declares consulting fees from Medtronic, Cerenovus, Stryker, and Balt. MC declares consultancy or advisory board fees or speaker’s honoraria from Pfizer/Bristol Meyer Squibb and Daiichi Sankyo. AZ received speaker and consultation fees from Alexion, CLS-Behring, Boehringer-Ingelheim. All the other authors report no disclosures.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The publication of this article was supported by the “Ricerca Corrente.” MR is supported by Young Investigator Grants from the Italian Stroke Association (ISA-AII).
Ethical approval: IRETAS registry was approved by the Ministry of Health (2015) 8
Informed consent: Informed consent was waived due to anonymized data.
Guarantor: MR
Contributorship: MR, AZ: concept, design, writing, reviewing. MR: data analysis. All other authors: manuscript review.
ORCID iDs: Michele Romoli  https://orcid.org/0000-0001-8009-8543
https://orcid.org/0000-0001-8009-8543
Valentina Saia  https://orcid.org/0000-0001-9855-8894
https://orcid.org/0000-0001-9855-8894
Ilaria Casetta  https://orcid.org/0000-0003-4099-8875
https://orcid.org/0000-0003-4099-8875
Mauro Bergui  https://orcid.org/0000-0002-5336-695X
https://orcid.org/0000-0002-5336-695X
Rossana Tassi  https://orcid.org/0000-0002-5906-8718
https://orcid.org/0000-0002-5906-8718
Maria Ruggiero  https://orcid.org/0000-0002-3612-4289
https://orcid.org/0000-0002-3612-4289
Alessandro Pezzini  https://orcid.org/0000-0001-8629-3315
https://orcid.org/0000-0001-8629-3315
Alessio Comai  https://orcid.org/0000-0002-0566-395X
https://orcid.org/0000-0002-0566-395X
Edoardo Puglielli  https://orcid.org/0000-0002-3082-249X
https://orcid.org/0000-0002-3082-249X
Nicola Cavasin  https://orcid.org/0000-0001-6246-3543
https://orcid.org/0000-0001-6246-3543
Francesco Biraschi  https://orcid.org/0000-0001-7660-2401
https://orcid.org/0000-0001-7660-2401
Ettore Nicolini  https://orcid.org/0000-0002-8481-6327
https://orcid.org/0000-0002-8481-6327
Salvatore Mangiafico  https://orcid.org/0000-0002-5844-3117
https://orcid.org/0000-0002-5844-3117
Supplemental material: Supplemental material for this article is available online.
References
- 1. Rothwell PM, Coull AJ, Silver LE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford vascular study). Lancet 2005; 366: 1773–1783. [DOI] [PubMed] [Google Scholar]
- 2. Sharobeam A, Cordato DJ, Manning N, et al. Functional outcomes at 90 days in octogenarians undergoing thrombectomy for acute ischemic stroke: a prospective cohort study and meta-analysis. Front Neurol 2019; 10: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meyer L, Alexandrou M, Flottmann F, et al. Endovascular treatment of very elderly patients aged ⩾90 with acute ischemic stroke. J Am Heart Assoc 2020; 9: e014447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 5. McDonough RV, Ospel JM, Campbell BCV, et al. Functional outcomes of patients ⩾85 years with acute ischemic stroke following EVT: a HERMES substudy. Stroke 2022; 53: 2220–2226. [DOI] [PubMed] [Google Scholar]
- 6. Goyal M, Almekhlafi M, Dippel DW, et al. Rapid alteplase administration improves functional outcomes in patients with stroke due to large vessel occlusions. Stroke 2019; 50: 645–651. [DOI] [PubMed] [Google Scholar]
- 7. Sussman ES, Martin B, Mlynash M, et al. Thrombectomy for acute ischemic stroke in nonagenarians compared with octogenarians. J Neurointerv Surg 2020; 12: 266–270. [DOI] [PubMed] [Google Scholar]
- 8. Mangiafico S, Pracucci G, Saia V, et al. The Italian registry of endovascular treatment in acute stroke: rationale, design and baseline features of patients. Neurol Sci 2015; 36: 985–993. [DOI] [PubMed] [Google Scholar]
- 9. Fernandez-Ferro J, Schwamm LH, Descalzo MA, et al. Missing outcome data management in acute stroke trials testing iv thrombolytics. Is there risk of bias? Eur Stroke J 2020; 5: 148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dunphy H, Garcia-Esperon C, Beom Hong J, et al. Endovascular thrombectomy for acute ischaemic stroke improves and maintains function in the very elderly: a multicentre propensity score matched analysis. Eur Stroke J 2023; 8: 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Möhlenbruch M, Pfaff J, Schönenberger S, et al. Endovascular stroke treatment of nonagenarians. Am J Neuroradiol 2017; 38: 299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khan MA, Baird GL, Miller D, et al. Endovascular treatment of acute ischemic stroke in nonagenarians compared with younger patients in a multicenter cohort. J Neurointerv Surg 2017; 9: 727–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Markle-Reid M, Fisher K, Walker KM, et al. The stroke transitional care intervention for older adults with stroke and multimorbidity: a multisite pragmatic randomized controlled trial. BMC Geriatr 2023; 23: 687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sposato LA, Field TS, Schnabel RB, et al. Towards a new classification of atrial fibrillation detected after a stroke or a transient ischaemic attack. Lancet Neurol 2024; 23: 110–122. [DOI] [PubMed] [Google Scholar]
- 15. Benali F, Singh N, Fladt J, et al. Mediation of age and thrombectomy outcome by neuroimaging markers of frailty in patients with stroke. JAMA Netw Open 2024; 7: e2349628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen H, Colasurdo M, Phipps MS, et al. The BAND score: a simple model for upfront prediction of poor outcomes despite successful stroke thrombectomy. J Stroke Cerebrovasc Dis 2024; 33: 107608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873241299335 for Stroke thrombectomy in the elderly: A propensity score matched study on a nationwide real-world registry by Michele Romoli, Ludovica Migliaccio, Valentina Saia, Giovanni Pracucci, Luigi Cirillo, Stefano Forlivesi, Daniele Romano, Ilaria Casetta, Enrico Fainardi, Fabrizio Sallustio, Nicola Limbucci, Patrizia Nencini, Valerio Da Ros, Marina Diomedi, Stefano Vallone, Guido Bigliardi, Sergio Lucio Vinci, Paolino La Spina, Mauro Bergui, Paolo Cerrato, Sandra Bracco, Rossana Tassi, Andrea Saletti, Cristiano Azzini, Maria Ruggiero, Lucio Castellan, Tiziana Benzi Markushi, Roberto Menozzi, Alessandro Pezzini, Guido Andrea Lazzarotti, Nicola Giannini, Davide Castellano, Andrea Naldi, Alessio Comai, Elisa Dall’Ora, Mauro Plebani, Manuel Cappellari, Giulia Frauenfelder, Edoardo Puglielli, Alfonsina Casalena, Nicola Burdi, Giovanni Boero, Sergio Nappini, Nicola Davide Loizzo, Nicola Cavasin, Adriana Critelli, Diego Ivaldi, Tiziana Tassinari, Francesco Biraschi, Ettore Nicolini, Sergio Zimatore, Marco Petruzzellis, Pietro Filauri, Berardino Orlandi, Ivan Gallesio, Delfina Ferrandi, Marco Pavia, Paolo Invernizzi, Pietro Amistá, Monia Russo, Adriana Paladini, Annalisa Rizzo, Michele Besana, Alessia Giossi, Marco Filizzolo, Marina Mannino, Salvatore Mangiafico, Danilo Toni and Andrea Zini in European Stroke Journal