Abstract
In this article, we provide an overview of our panel presentation at the American Epilepsy Society meeting in December2023. Our presentation reviewed functional mapping methods for epilepsy surgery including well-established and newer methods, focusing mostly on language and memory. Dr Leigh Sepeta (Chair) and Dr Jana Jones (Chair) organized the presentation, which included 5 presenters. Dr Christopher Benjamin discussed the history and current and future mapping practices using functional magnetic resonance imaging; Ms. Freya Prentice reviewed functional mapping of language and memory in pediatric epilepsy; Dr Marla Hamberger compared pros and cons of functional mapping between subdural electrodes and stereoelectroencephalography (SEEG); Dr Donald J. Bearden presented a brief how-to guide on cognitive mapping using SEEG; and Dr Alena Stasenko discussed the complexities of functional mapping of bilingual patients. We have included references for more detailed information on the content of our presentation.
Keywords: fMRI, stereoelectroencephalography, language mapping, memory mapping, epilepsy surgery
Introduction
Functional mapping provides critical information on lateralization and localization of eloquent cortex supporting cognition, which is used to guide epilepsy surgery for patients with drug-resistant epilepsy.1–3 Neuropsychological testing served this purpose prior to the advent of the Wada test and neuroimaging, but is an indirect measure of brain function. 4 Though the Wada test is more useful than neuropsychological testing for language lateralization, functional magnetic resonance imaging (fMRI) has become the preferred method for language mapping due to its ability to noninvasively lateralize and localize eloquent cortex. 5 fMRI is also increasingly being used for memory lateralization. 6 Subdural electrodes (SDE) and stereoelectroencephalography (SEEG) provide an opportunity for functional mapping directly within cortex, each method having pros and cons.7,8 Our review summarizes the current state of cognitive mapping using fMRI, SDE, and SEEG, focusing primarily on language and memory, with an eye toward the future.
Clinical Language fMRI in Epilepsy: Current Practices and Opportunities
Neurosurgery may not be offered if hemispheric language lateralization is unknown because most epilepsy programs consider damage to language an unacceptable risk of surgery. Historically, the language-critical hemisphere was identified with the Wada protocol. 5 The Wada remains an invaluable tool in select cases, but it has largely been replaced by functional magnetic resonance imaging for language mapping (fMRI). 2
Highly standardized language fMRI performed by a skilled team lateralizes language and can predict postsurgical language decline as well as most typical Wada protocols.9,10 In rare cases where the Wada and language fMRI provide contradictory language lateralization, good-quality fMRI may predict the risk of language decline more accurately than Wada testing. 11 A remaining challenge is fMRI's promise to locate areas of critical language cortex which, if resected, would result in functional language impairment (ie, language “localization”). 12 Some initial findings are impressive and promising. 13
A fundamental and typically unvoiced assumption is that language fMRI is indeed “highly standardized and performed by a skilled team.” In practice, essentially all protocol aspects as well as the skills of those completing fMRI vary by fMRI program.14,15 In this context, different professions have issued unique clinical guidelines in efforts to standardize care,16,17 sometimes in domains outside their expertise. 18 This dramatic variation in protocols and skills is very unlikely to impact language lateralization, but extremely likely to impact language localization. 14 If the fMRI approach used follows a standardized, validated paradigm, it is typically able to locate islands of language-critical cortex in surgical planning. Whether fMRI has the ability to reliably identify specific margins to guide resection, remains an open question.
This highlights a separate challenge for the field: establishing a successful interdisciplinary model for clinical fMRI. Mapping language cortex with language fMRI is a cognitive assessment if we accept the brain and mind are linked. Changing only the cognitive strategy a patient uses to solve an fMRI task—while all else is held constant—changes the results. 19 The same is true for other forms of cognitive assessment, including neuropsychological testing, direct cortical stimulation mapping, and the Wada protocol. This is why neuropsychologists typically complete a 5-year doctorate to learn how to accurately measure cognition, and why hospitals credential cognitive assessment. Vitally, fMRI is also equally a radiological exam, a complex image analysis, and a clinical assessment for planning neurosurgery. Expertise in all these areas in both program design and the day-to-day imaging of patients is required to ensure reliable, accurate data and patient care. 16 Such collaboration is likely to involve experts from at least 2 complementary professions, such as a neuropsychologist and radiologist, or an epileptologist and fMRI researcher. 15
Functional Mapping of Memory Circuits in Pediatric Epilepsy
Verbal memory deficits have been identified after left temporal lobe resection (TLR) in adults, with evidence for similar, although less prevalent deficits in children. Children who undergo a left TLR including the mesial structures are at greater risk of decline in verbal memory, 20 compared to those with lateral resections only. This is likely due to the critical role of the hippocampus in delayed recall of episodic information. 21 However, the lateralization of these mesial memory circuits may mediate this relationship, with those who are left dominant for verbal memory and language being most likely to decline after a left mesial TLR. 20
Given widespread use of language fMRI, language lateralization has been used as a proxy for lateralization of verbal memory functions, and in turn, to predict postoperative verbal memory. Adult studies have shown that left lateralization on several different language tasks is predictive of greater declines in delayed verbal memory.22–24 This likely reflects the co-localization of language and verbal memory functions in adults. However, such co-localization may occur over development, as lateralization of frontotemporal language regions and the mesial temporal lobe are correlated in adults but not children. 25 This sheds doubt on whether language fMRI can serve as a reliable predictor of postoperative verbal memory in children.
Consequently, there is a need for fMRI paradigms that specifically map memory functions in the mesial temporal lobe. Left hippocampal activation on word encoding paradigms has been associated with a greater risk of postoperative verbal memory decline in adults.23,26 Overall, memory fMRI has proven to be a stronger predictor than language fMRI of postoperative verbal memory in adults. 24 A recent study using an autobiographical memory paradigm in children showed that overlap between mesial activation and resection was associated with a decline in delayed word-pair recall, 6 suggesting memory fMRI is feasible and clinically relevant in children.
Although these initial findings suggest that memory fMRI may be predictive of postoperative verbal memory, limitations still exist in the memory paradigms used. Most paradigms rely on memory encoding, and measure later retrieval outside of the scanner. Recent work suggests that including both encoding and recall-based retrieval inside the scanner may produce more consistent and robust hippocampal activation.27,28 Such ongoing memory fMRI research serves to expand our understanding of the developmental trajectory of mesial memory circuits and improve the prediction of postoperative verbal memory in both children and adults.
Farewell Subdural… Hello SEEG Language Mapping: Pros, Cons, and Unknowns
The inherent challenge in epilepsy surgery is to remove a sufficient volume of epileptogenic tissue to eliminate seizures, while minimizing the amount of brain tissue removed to reduce risk of functional decline. SDE recording has been the preferred method for intracranial mapping in North America for decades, with very few epilepsy surgery programs using stereotactically placed EEG electrodes (SEEG) due to its associated risks. However, with the recent development of a robotic arm, SEEG has become faster and more accurate, considerably reducing risk of hemorrhage, and enabling electrode trajectories that were not possible previously. 29 Multiple studies have shown that SEEG is now safer, better tolerated, and in some cases, associated with better seizure outcome than SDE, rendering it the current preferred method for intracranial recording.7,8
Electrocortical stimulation mapping (ESM) is the gold standard for identification of essential language cortex. Both SDE and SEEG use ESM to create a temporary lesion in the area stimulated, enabling characterization of the deficit that would result from removal of the area.1,30,31 Important differences between SDE and SEEG include the location and density of areas tested and stimulation parameters. 32 SDE provides dense coverage of the cortical surface without access to deeper structures, whereas SEEG provides sparse coverage of lateral cortex while allowing access to deeper targets. 33
It remains unclear whether language mapping via SEEG reduces the risk of postoperative language decline to the same extent as SDE. Multiple observational studies examining postoperative language outcome via SDE (or intraoperative cortical) mapping have found that encroachment upon or removal of language-positive tissue results in decline.34–38 The limited available research on SEEG language mapping suggests that language outcomes following SEEG mapping are comparable to those with SDE39,40; however, more studies are needed. Nevertheless, SEEG's reduced safety risk, increased tolerability, and access to sulci and deeper brain structures compared to SDE have made it the preferred intracranial seizure mapping tool in North America—despite its low electrode density that leaves many brain regions untested.
Cognitive Mapping Using SEEG
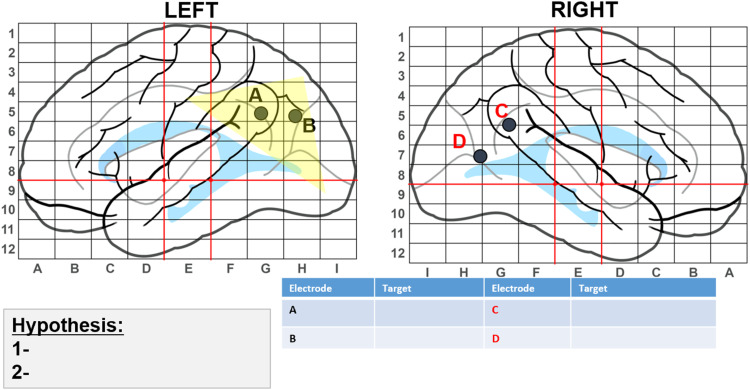
The way in which cognitive mapping with SEEG is performed varies by institution. At Children's Healthcare of Atlanta (CHOA), SEEG cognitive mapping begins with creation of a hypothesis-driven map of patients’ brains that identifies areas believed to be the epileptogenic zone (EZ) based on previously gathered neuroimaging, EEG, and neuropsychological data, which guides electrode placement. Figure 1 is an example of the map we use to depict electrode placement. The lettered dots represent electrodes and the suspected EZ is highlighted in yellow.
Figure 1.
Hypothesis-driven map to guide electrode placement.
After electrode implantation, our neurosurgeon creates a table indicating electrode trajectories (Table 1). The neuropsychologist uses this information to create a test battery that includes tasks tapping into putative functions along each electrode trajectory (Table 2). For example, Table 2 corresponds with trajectories shown in Table 1. Table 3 provides additional information on tasks we often use for SEEG cognitive mapping at CHOA.
Table 1.
Postimplantation Electrode Trajectories.
| Millimeters | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Electrode | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Left hemisphere | ||||||||||
| A | Cuneus | Cuneus | Cuneus | Cuneus | WM | WM | Middle occipital gyrus | Middle occipital gyrus | Middle occipital gyrus | Middle occipital gyrus |
| B | Lingual gyrus | Lingual gyrus | WM | WM | WM | Inferior occipital gyrus | Inferior occipital gyrus | Inferior occipital gyrus | Inferior occipital gyrus | |
| C | Temporal pole | Temporal pole | Temporal pole | Temporal pole | Temporal pole | Temporal pole | ||||
| Right hemisphere | ||||||||||
| D | HPC | HPC | HPC | Collateral sulcus | Collateral sulcus | WM | WM | WM | WM | WM |
| E | Fusiform | Fusiform | Fusiform | Fusiform | Fusiform | Inferior temporal gyrus | Inferior temporal gyrus | Inferior temporal gyrus | Inferior temporal gyrus | Inferior temporal gyrus |
| F | Heterotopic insula | Heterotopic insula | Posterior long gyrus | CSF | CSF | Parietal operculum | Parietal operculum | Parietal operculum | Parietal operculum | Parietal operculum |
Abbreviations: CSF, cerebrospinal fluid; HPC, hippocampus; WM, white matter.
Table 2.
Cognitive Task List Corresponding to Table 1.
| Electrode | Electrode path/brain region | Cognitive task |
|---|---|---|
| Left hemisphere | ||
| A | Cuneus → Middle occipital gyrus | Visual pattern matching |
| B | Lingual gyrus → Inferior occipital gyrus | Reading |
| C | Temporal pole | Picture naming |
| Right hemisphere | ||
| D | Hippocampus → Collateral sulcus | Picture naming/reading |
| E | Fusiform → Inferior temporal gyrus | Facial recognition, language comprehension |
| F | Heterotopia → Parietal operculum | Tactile discrimination |
Table 3.
Example of Tasks and Test Materials Used for SEEG Mapping.
| Task list | Example of materials used |
|---|---|
| Face recognition | Face photos of close family members and friends sent by parent |
| Visual pattern recognition | Hooper, TVPS-3, JOLO |
| Picture naming | DAS-2 Naming Vocabulary |
| Tests of apraxia | “Show me how you…” (eg, brush your teeth) |
| Comprehension | Auditory naming/comprehension questions |
| List learning/recall | 3-5 words read to patient during stimulation followed by immediate recall after stimulation ends |
| Reading | Patient's preferred reading brought from home; passages from book |
| Grasping | 3 objects easily grasped/released (eg, block) |
| Pointing | Point to objects in room/numbers on board |
| Tactile object recognition | Bayley-3 blue bag with disc, peg, block, or other objects |
Next, the neuropsychologist conducts baseline testing at bedside, removing test items that patients respond to incorrectly to ensure that patient errors during mapping are caused by ESM and not lack of familiarity. We also prepare patients/parents regarding what to expect during ESM. With extremely young, nonverbal, or low functioning patients, we gather information about how they communicate to prepare the mapping team. An interpreter is scheduled to join, if needed. After baseline testing, we conduct SEEG cognitive mapping without electrocortical stimulation (“passive mapping” or “high frequency mapping”) and share findings with the surgery team. Passive mapping is less labor intensive and allows more extensive testing of cognitive functions than ESM and will not trigger a seizure. 3 Passive mapping and ESM can be used in a complementary fashion to shorten the duration of stimulation mapping. 41
Following passive mapping, and once sufficient habitual seizure data have been collected via SEEG, ESM is conducted. Team members involved in ESM include the epileptologist, neuropsychologist, and nursing. Neuropsychology conducts testing while reporting patients’ behavioral changes/interruptions in function during ESM.
The neuropsychologist is ideally suited to play an important role in cognitive mapping using SEEG based on our extensive knowledge and training in neuroanatomy, cognitive function, and brain-behavior relationships. In addition, prior to mapping we have typically established a relationship with the patient and family during the neuropsychological evaluation process, which often involves spending a few hours with patients. This time with patients also provides important information regarding their functional level and ability to manage stress. Further, because neuropsychologists are trained in clinical psychology, we are skilled at aiding children and parents in managing anxiety associated with these stressful procedures. Going forward, standardization of SEEG cognitive mapping, including personnel, materials, and procedures across institutions will be important to conduct large-scale studies examining its validity as a tool to prevent cognitive decline associated with epilepsy surgery.
Language Mapping in the Bilingual Brain
Despite a rise in bi-/multilingualism in the United States, limited research has addressed language mapping practices for this population. This is especially problematic because many of these individuals are in racially/ethnically marginalized groups already at increased risk for healthcare disparities. Bilingual patients considered for neurosurgery are vulnerable to improper characterization of their language cortex due to ambiguous neural organization of language(s) compared to their monolingual counterparts. Specifically, languages may reside within distinct brain regions,42–44 leaving them susceptible to postoperative decline if only one language is mapped. 45
Functional MRI is a valuable tool for understanding language lateralization patterns in the bilingual brain. Several studies have demonstrated that bilingual patients tend to have bilateral, widespread language lateralization,46,47 especially for the second language. 48 This may be due to neuroplasticity associated with learning multiple languages early in life and/or extra processing demands required in bilingual language processing. 49 Increased right hemisphere activation in bilingual patients has been associated with later learning of a second language (ie, after age 6), 48 with a left hemisphere seizure focus, 47 and with a character (vs alphabet) writing system (eg, Mandarin). 50 Additional research is needed to understand how linguistic factors (proficiency, age of acquisition) and seizure variables (age of onset) interact to affect language representation. Another unanswered question is whether right hemisphere activation is essential for language function or adjunctive (eg, compensatory) in bilingual patients.
A recent review of ESM studies found that the most common pattern was a combination of completely distinct and overlapping cortical sites (ie, areas shared across languages) in both anterior and posterior language regions within the same patient. 44 Due to significant heterogeneity across ESM studies, we currently lack a model to predict which brain regions are critical for one language versus another. However, research has shown that language proficiency, age at acquisition of a second language, amount of exposure, and linguistic similarity between languages spoken influence whether multiple languages in an individual have shared or separate brain representation. 44 These factors can be used to guide the clinician's approach to mapping (eg, the decision to map one or multiple languages).
Another challenge when mapping language in bilingual patients is establishing a linguistically and culturally appropriate set of mapping stimuli for all techniques. Ideally, items are matched in difficulty and familiarity across languages. Otherwise, it is difficult to determine whether, for example, naming errors during ESM are due to these factors rather than to the stimulation. This can lead to erroneous conclusions about language representation and could explain some of the heterogeneity observed in bilingual mapping studies. Therefore, extensive premapping sessions and piloting of stimuli are critical for obtaining valid data. 49
Taken together, emerging evidence suggests that bilingualism can affect the neural organization of languages. Thus, bilingual status should be carefully considered in language mapping methods (eg, selection of stimuli) and in conceptualization and interpretation of results to improve clinical care of this growing demographic group.
Conclusion
Functional mapping technology and methodology are advancing rapidly. However, there are many questions left unanswered, including: How useful is fMRI at reliably identifying eloquent cortex in the developing brains of children? Should fMRI be used to localize language function to guide surgical tissue destruction? How can we adequately identify critical language areas with SEEG considering its sparse density and limited cortical coverage? How do we standardize SEEG cognitive mapping across institutions in order to study its validity as a tool to prevent cognitive decline associated with epilepsy surgery? How can we improve our ability to predict risk for postsurgical cognitive decline in bilingual patients, many of whom are already vulnerable to major disparities in healthcare?
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NIH R01 NS 35140 (Hamberger); MAYDAY Fund (Bearden); K23 NINDS NS093152 (Sepeta); NIH 1F32NS119285 and T32 5T32MH019934-30 (Stasenko).
ORCID iDs: Donald Jay Bearden https://orcid.org/0000-0003-1306-5615
Jana J. Jones https://orcid.org/0000-0003-1723-0163
References
- 1.Hamberger MJ. Cortical language mapping in epilepsy: a critical review. Neuropsychol Rev. 2007;17:477–489. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin CF. Cognitive biomarkers in the clinic: lessons from presurgical fMRI. J Clin Neurophysiol. 2022;39(2):121–128. [DOI] [PubMed] [Google Scholar]
- 3.Bearden DJ, Ehrenberg A, Selawski Ret al. Four-Way Wada: SEEG-based mapping with electrical stimulation, high frequency activity, and phase amplitude coupling to complement traditional Wada and functional MRI prior to epilepsy surgery. Epilepsy Res. 2023;192:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loring DW. History of neuropsychology through epilepsy eyes. Arch Clin Neuropsychol. 2010;25(4):259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boas WE. Juhn A. Wada and the sodium amytal test the first (and last?) 50 years. J Hist Neurosci. 1999;8(3):286–292. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein HE, Poliakov A, Shaw DWet al. Precision medicine in pediatric temporal epilepsy surgery: optimization of outcomes through functional MRI memory tasks and tailored surgeries. J Neurosurg Pediatr. 2022;30(3):272–283. [DOI] [PubMed] [Google Scholar]
- 7.Jehi L, Morita-Sherman M, Love TEet al. Comparative effectiveness of stereotactic electroencephalography versus subdural grids in epilepsy surgery. Annals Neurol. 2021;90(6):927–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tandon N, Tong BA, Friedman ERet al. Analysis of morbidity and outcomes associated with use of subdural grids vs stereoelectroencephalography in patients with intractable epilepsy. JAMA Neurol. 2019;76(6):672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janecek JK, Swanson SJ, Sabsevitz DSet al. et al. Language lateralization by f MRI and Wada testing in 229 patients with epilepsy: rates and predictors of discordance. Epilepsia. 2013;54(2):314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabsevitz DS, Swanson SJ, Hammeke TAet al. Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology. 2003;60(11):1788–1792. [DOI] [PubMed] [Google Scholar]
- 11.Janecek JK, Swanson SJ, Sabsevitz DSet al. et al. Naming outcome prediction in patients with discordant Wada and fMRI language lateralization. Epilepsy Behav. 2013;27(2):399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benjamin CF, Walshaw PD, Hale Ket al. Presurgical language fMRI: mapping of six critical regions. Human Brain Map. 2017;38(8):4239–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolinski R, Austermuehle A, Wiggs Eet al. Functional MRI and direct cortical stimulation: prediction of postoperative language decline. Epilepsia. 2019;60(3):560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benjamin CF, Li AX, Blumenfeld Het al. Presurgical language fMRI: clinical practices and patient outcomes in epilepsy surgical planning. Human Brain Map. 2018;39(7):2777–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benjamin CF, Dhingra I, Li AXet al. Presurgical language fMRI: technical practices in epilepsy surgical planning. Human Brain Map. 2018;39(10):4032–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Div40. Official position of the division of clinical neuropsychology (APA division 40) on the role of neuropsychologists in clinical use of fMRI: approved by the division 40 executive committee July 28, 2004. Clin Neuropsychol. 2004;18:349–351. [DOI] [PubMed] [Google Scholar]
- 17.Szaflarski JP, Gloss D, Binder JRet al. Practice guideline summary: use of fMRI in the presurgical evaluation of patients with epilepsy: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2017;88(4):395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Black DF, Vachha B, Mian Aet al. American Society of functional neuroradiology–recommended fMRI paradigm algorithms for presurgical language assessment. Am J Neuroradiol. 2017;38(10):E65–E73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fink G, Marshall J, Weiss P, Toni I, Zilles K. Task instructions influence the cognitive strategies involved in line bisection judgements: evidence from modulated neural mechanisms revealed by fMRI. Neuropsychologia. 2002;40:119–130. [DOI] [PubMed] [Google Scholar]
- 20.Danguecan AN, Smith ML. Verbal associative memory outcomes in pediatric surgical temporal lobe epilepsy: exploring the impact of mesial structures. Epilepsy Behav. 2019;101:1–8. [DOI] [PubMed] [Google Scholar]
- 21.Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277(5324):376–380. [DOI] [PubMed] [Google Scholar]
- 22.Binder JR, Swanson SJ, Sabsevitz DS, Hammeke TA, Raghavan M, Mueller WM. A comparison of two fMRI methods for predicting verbal memory decline after left temporal lobectomy: language lateralization versus hippocampal activation asymmetry. Epilepsia. 2010;51(4):618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sidhu MK, Stretton J, Winston GPet al. et al. Memory fMRI predicts verbal memory decline after anterior temporal lobe resection. Neurology. 2015;84(15):1512–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crow AJ, Thomas A, Rao Yet al. et al. Task-based functional magnetic resonance imaging prediction of postsurgical cognitive outcomes in temporal lobe epilepsy: a systematic review, meta-analysis, and new data. Epilepsia. 2023;64(2):266–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sepeta LN, Berl MM, Wilke Met al. Age-dependent mesial temporal lobe lateralization in language fMRI. Epilepsia. 2016;57(1):122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strandberg M, Mannfolk P, Stenberg Let al. et al. A functional MRI-based model for individual memory assessment in patients eligible for anterior temporal lobe resection. The Open Neuroimag J. 2017;11:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buck S, Bastos F, Baldeweg T, Vargha-Khadem F. A functional MRI paradigm suitable for language and memory mapping in pediatric temporal lobe epilepsy. Front Neurol. 2020;10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buck S, Sidhu MK. A guide to designing a memory fMRI paradigm for pre-surgical evaluation in temporal lobe epilepsy. Front Neurol. 2020;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKhann GM. Dulling the double-edged sword of human SEEG research. Neurosurg Focus. 2020;48(4):E3. [DOI] [PubMed] [Google Scholar]
- 30.Jayakar P, Gaillard WD, Tripathi M, Libenson MH, Mathern GW, Cross JH. Task force for paediatric epilepsy surgery, commission for paediatrics, and the diagnostic commission of the International league against epilepsy. Diagnostic test utilization in evaluation for resective epilepsy surgery in children. Epilepsia. 2014;55(4):507–518. [DOI] [PubMed] [Google Scholar]
- 31.Mahon BZ, Miozzo M, Pilcher WH. Direct electrical stimulation mapping of cognitive functions in the human brain. Cognit Neuropsychol. 2019;36(3-4):97–102. [DOI] [PubMed] [Google Scholar]
- 32.Lesser RP, Crone NE, Webber WRS. Subdural electrodes. Clin Neurophysiol. 2010;121(9):1376–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ritaccio AL, Brunner P, Schalk G. Electrical stimulation mapping of the brain: basic principles and emerging alternatives. J Clin Neurophysiol. 2018;35(2):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ojemann GA, Dodrill CB. Predicting postoperative language and memory deficits after dominant hemisphere anterior temporal lobectomy by intraoperative stimulation mapping. In: Abstracts, American association of neurological surgeons, 50th annual meeting. 1981:76–77. [Google Scholar]
- 35.Haglund MM, Berger MS, Shamseldin M, Lettich E, Ojemann GA. Cortical localization of temporal lobe language sites in patients with gliomas. Neurosurgery. 1994;34(4):567–576. [DOI] [PubMed] [Google Scholar]
- 36.Hermann BP, Perrine K, Chelune GJet al. et al. Visual confrontation naming following left anterior temporal lobectomy: a comparison of surgical approaches. Neuropsychology. 1999;13(1):3–9. [DOI] [PubMed] [Google Scholar]
- 37.Hamberger MJ, Seidel WT, Mckhann GM, Perrine K, Goodman RR. Brain stimulation reveals critical auditory naming cortex. Brain. 2005;128(11):2742–2749. [DOI] [PubMed] [Google Scholar]
- 38.Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. New England J Med. 2008;358(1):18–27. [DOI] [PubMed] [Google Scholar]
- 39.Arya R, Frink C, Kargol Cet al. Neuropsychological outcomes after epilepsy surgery: a comparison of stereo-EEG and subdural electrodes. Eur J Neurol. 2023;30:2986–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdallah C, Brissart H, Colnat-Coulbois Set al. Stereoelectroencephalographic language mapping of the basal temporal cortex predicts postoperative naming outcome. J Neurosurg. 2021;135(5):1466–1476. [DOI] [PubMed] [Google Scholar]
- 41.Cuisenier P, Testud B, Minotti Let al. Relationship between direct cortical stimulation and induced high-frequency activity for language mapping during SEEG recording. J Neurosurg. 2020;134(4):1251–1261. [DOI] [PubMed] [Google Scholar]
- 42.Cargnelutti E, Tomasino B, Fabbro F. Language brain representation in bilinguals with different age of appropriation and proficiency of the second language: a meta-analysis of functional imaging studies. Front Human Neurosci. 2019;13:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lucas TH, McKhann GM, Ojemann GA. Functional separation of languages in the bilingual brain: a comparison of electrical stimulation language mapping in 25 bilingual patients and 117 monolingual control patients. J Neurosurg. 2004;101(3):449–457. [DOI] [PubMed] [Google Scholar]
- 44.Połczyńska MM, Bookheimer SY. Factors modifying the amount of neuroanatomical overlap between languages in bilinguals—a systematic review of neurosurgical language mapping studies. Brain Sci. 2020;10(12):983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cervenka MC, Boatman-Reich DF, Ward J, Franaszczuk PJ, Crone NE. Language mapping in multilingual patients: electrocorticography and cortical stimulation during naming. Front Human Neurosci. 2011;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Połczyńska MM, Japardi K, Bookheimer SY. Lateralizing language function with pre-operative functional magnetic resonance imaging in early proficient bilingual patients. Brain Language. 2017;170:1–11. [DOI] [PubMed] [Google Scholar]
- 47.Stasenko A, Schadler A, Kaestner Eet al. et al. Can bilingualism increase neuroplasticity of language networks in epilepsy? Epilepsy Res. 2022;182:1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Centeno M, Koepp MJ, Vollmar Cet al. Language dominance assessment in a bilingual population: validity of fMRI in the second language. Epilepsia. 2014;55:1504–1511. [DOI] [PubMed] [Google Scholar]
- 49.Stasenko A, Hays C, Wierenga CE, Gollan TH. Cognitive control regions are recruited in bilinguals’ silent reading of mixed-language paragraphs. Brain Language. 2020;204:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheung M, Chan AS, Chan Y, Lam JMK. Language lateralization of Chinese-English bilingual patients with temporal lobe epilepsy: a functional MRI study. Neuropsychology. 2006;20:589–597. [DOI] [PubMed] [Google Scholar]