Abstract
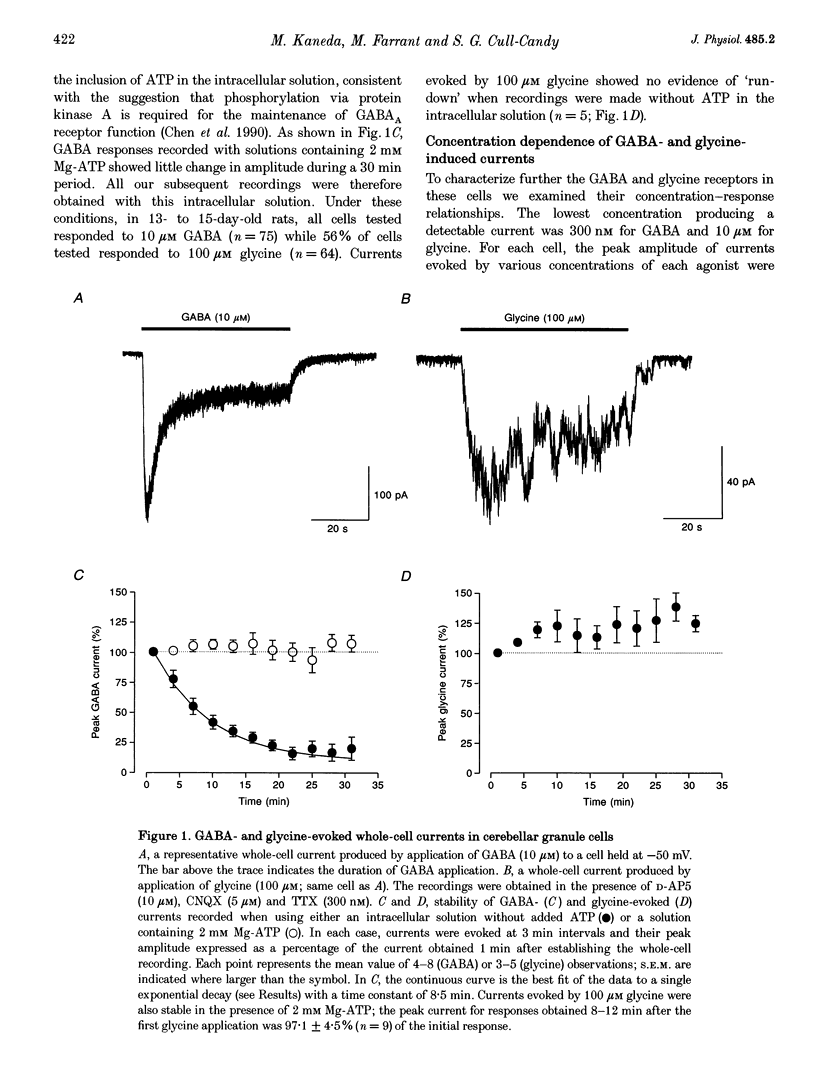
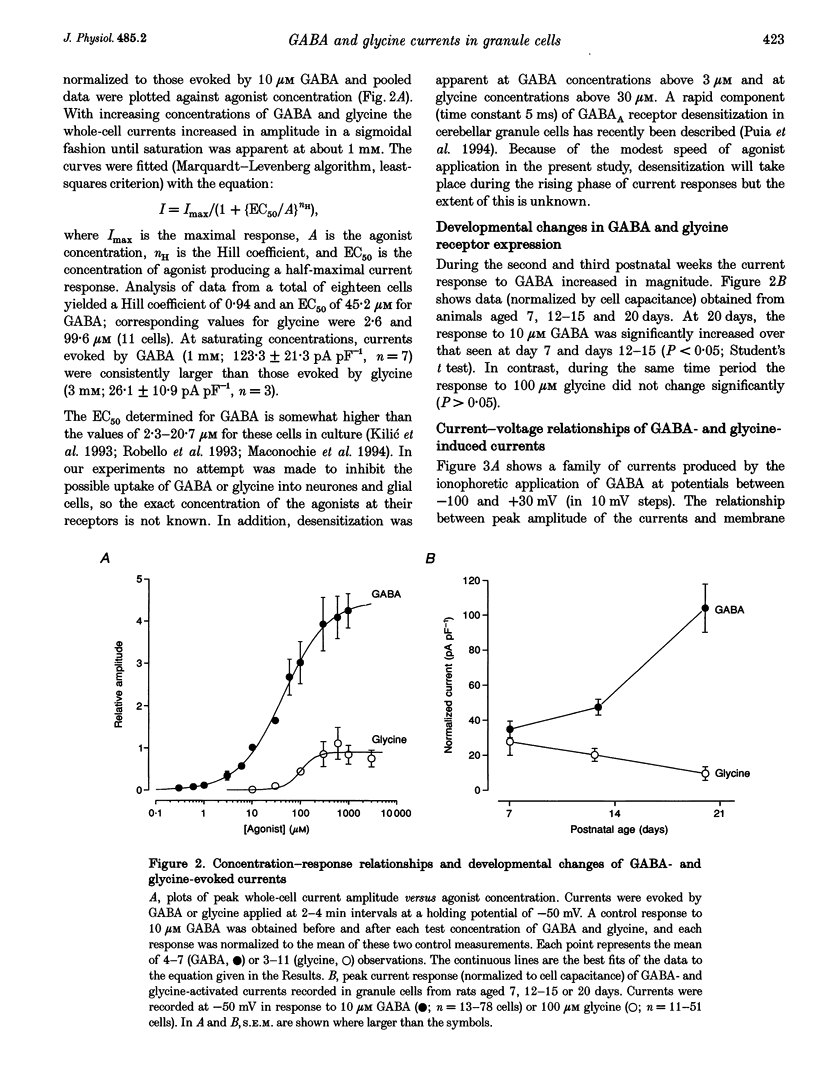
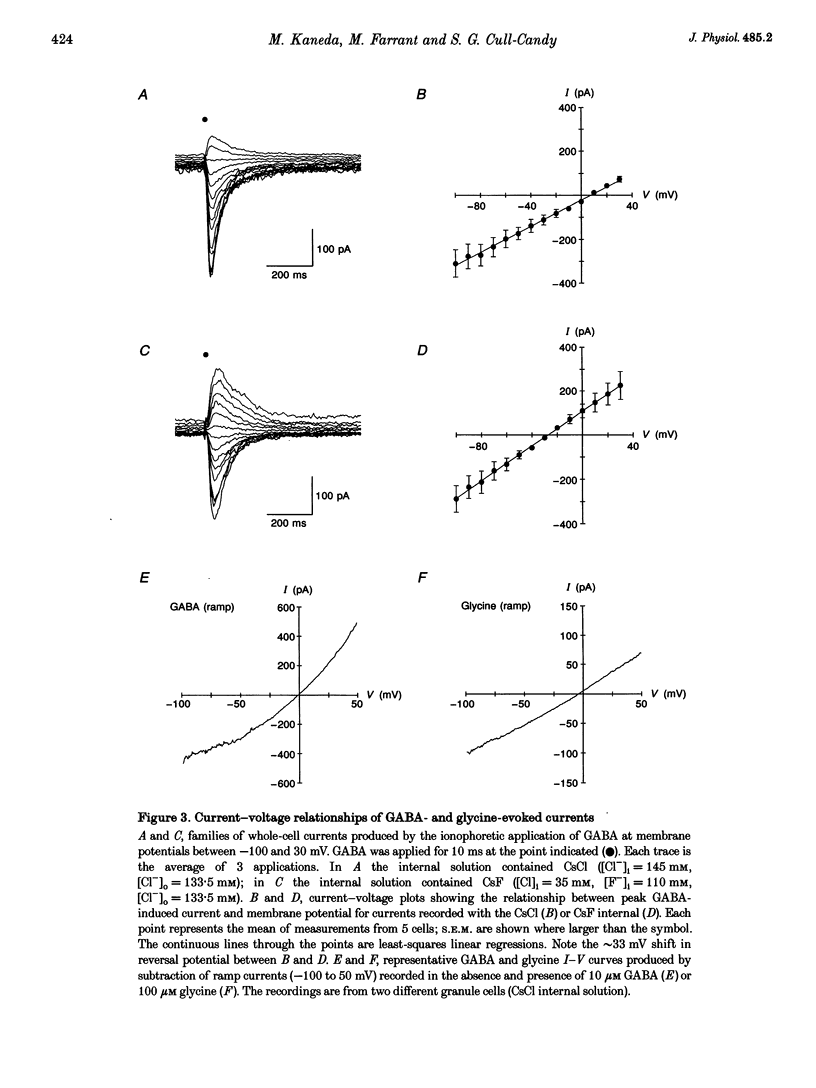
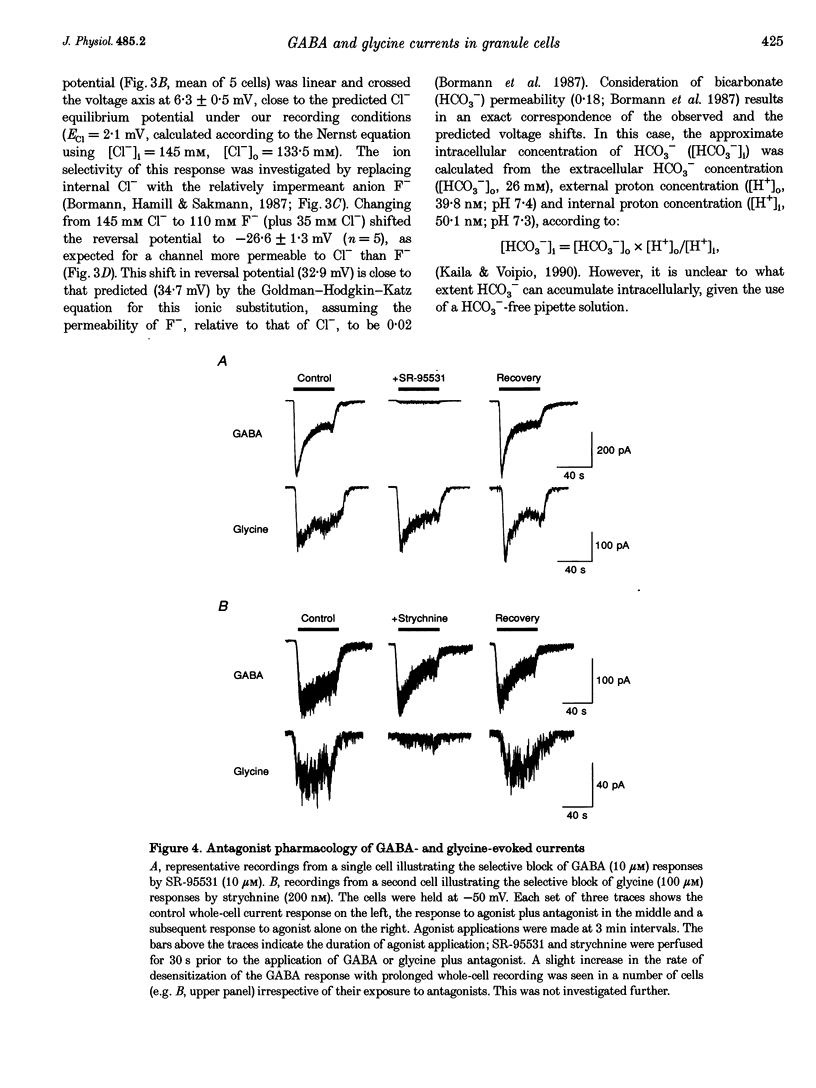
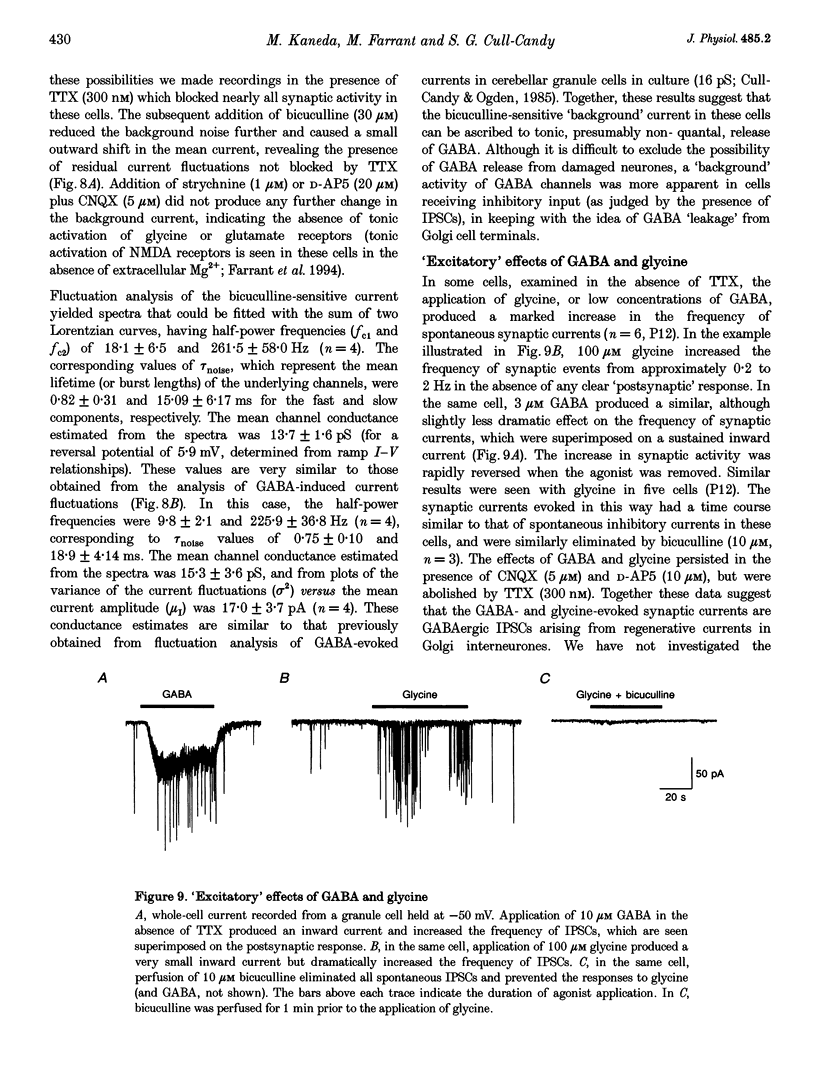
1. Patch-clamp methods have been used to characterize GABA-and glycine-activated channels and spontaneous synaptic currents in granule cells in thin cerebellar slices from 7- to 20-day-old rats. 2. All granule cells responded to 10 microM GABA, while approximately 60% responded to 100 microM glycine. With repeated against application, whole-cell responses to GABA, but not those to glycine, declined over a period of minutes unless the pipette solution contained Mg-ATP. 3. Whole-cell concentration-response curves gave EC50 values at 45.2 and 99.6 microM and Hill slopes of 0.94 and 2.6 for GABA and glycine, respectively. At saturating concentrations, currents evoked by GABA were fivefold larger than those evoked by glycine. 4. Whole-cell current-voltage (I-V) relationships of GABA- and glycine-activated currents reversed close to the predicted Cl- equilibrium potential. Partial replacement of intracellular Cl- with F- shifted the GABA reversal potential to a more negative value. 'Instantaneous' I-V relationships produced by ionophoretic application of GABA were linear, while 'steady-state' I-V relationships produced by ramp changes in potential showed outward rectification. For glycine, 'steady-state' I-V plots were linear. 5. Responses to GABA were blocked by the GABAA receptor antagonists bicuculline (15 microM), SR-95531 (10 microM) and picrotoxinin (100 microM) while responses to glycine were selectively blocked by strychnine (200 nM), indicating the presence of two separate receptor types. 6. In outside-out membrane patches, GABA opened channels with conductances of 16 and 28 pS. The proportion of openings to each of the conductances varied between patches, possibly indicating the activation of two distinct channel types. Glycine-activated single-channel currents had conductances of 32, 55 and 104 pS. Single-channel I-V relationships were linear. 7. Spontaneous synaptic currents with a rapid rise time and biexponential decay were present in more than half of the cells examined. These currents were eliminated by bicuculline (15 microM) or SR-95331 (10 microM) and were greatly reduced in frequency by tetrodotoxin (TTX; 300 nM), suggesting that they were mediated by GABA and arose from spontaneous activity in Golgi interneurones. In granule cells where this spontaneous synaptic activity was apparent, glycine and low concentrations of GABA increased the frequency of the synaptic currents.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


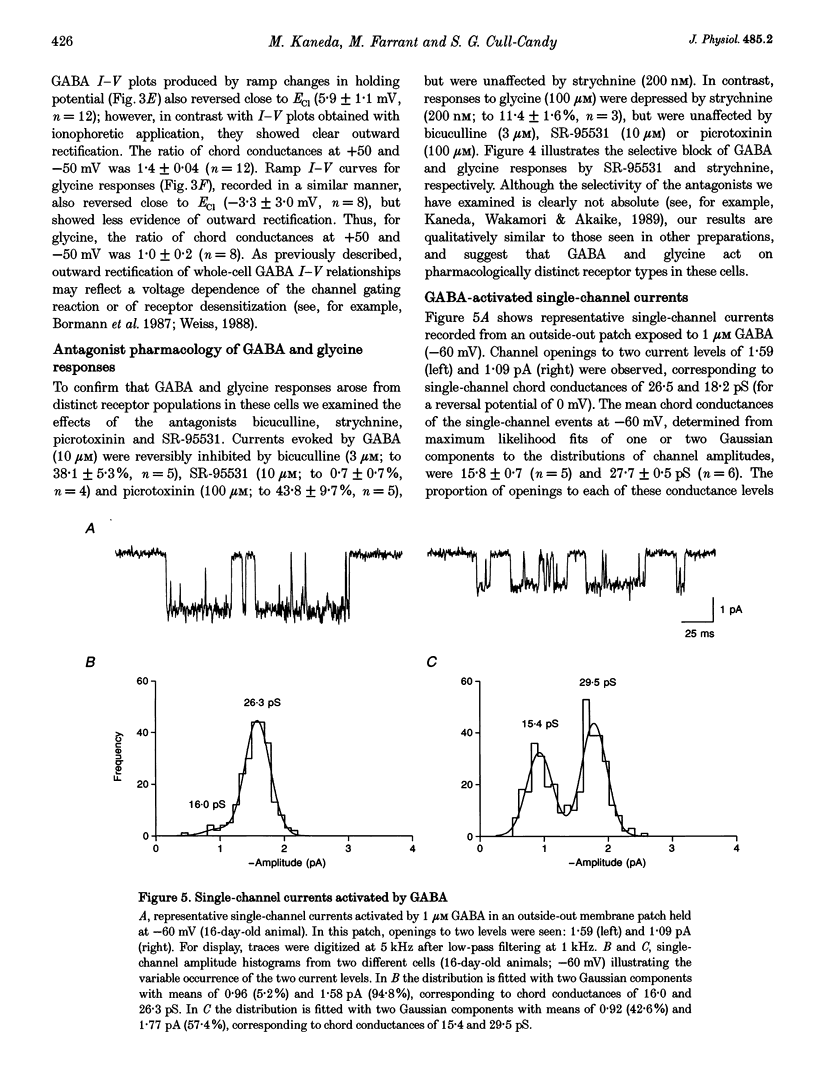
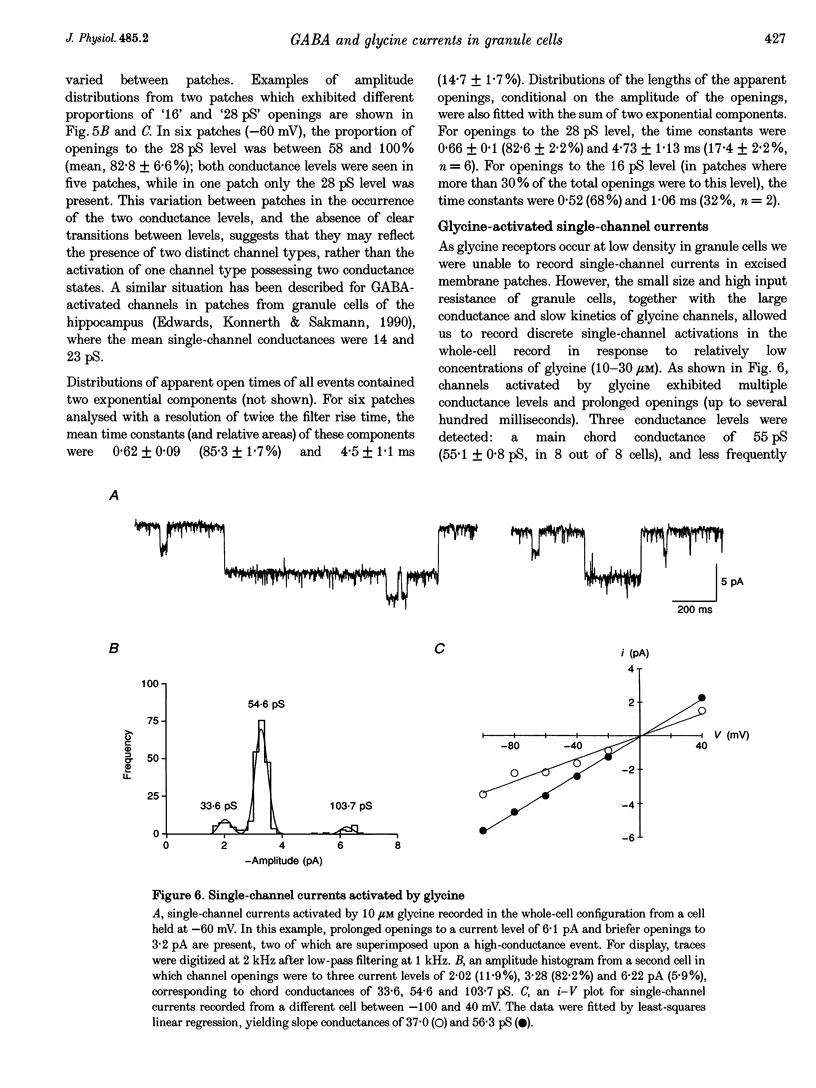
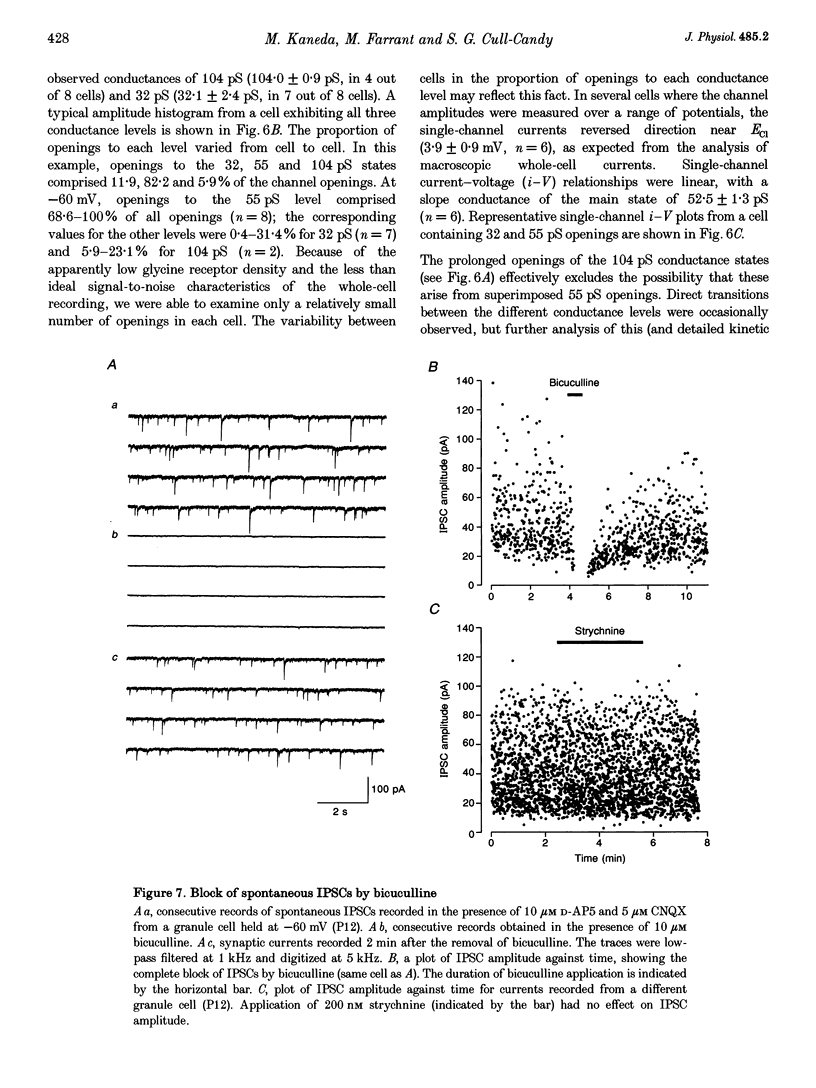
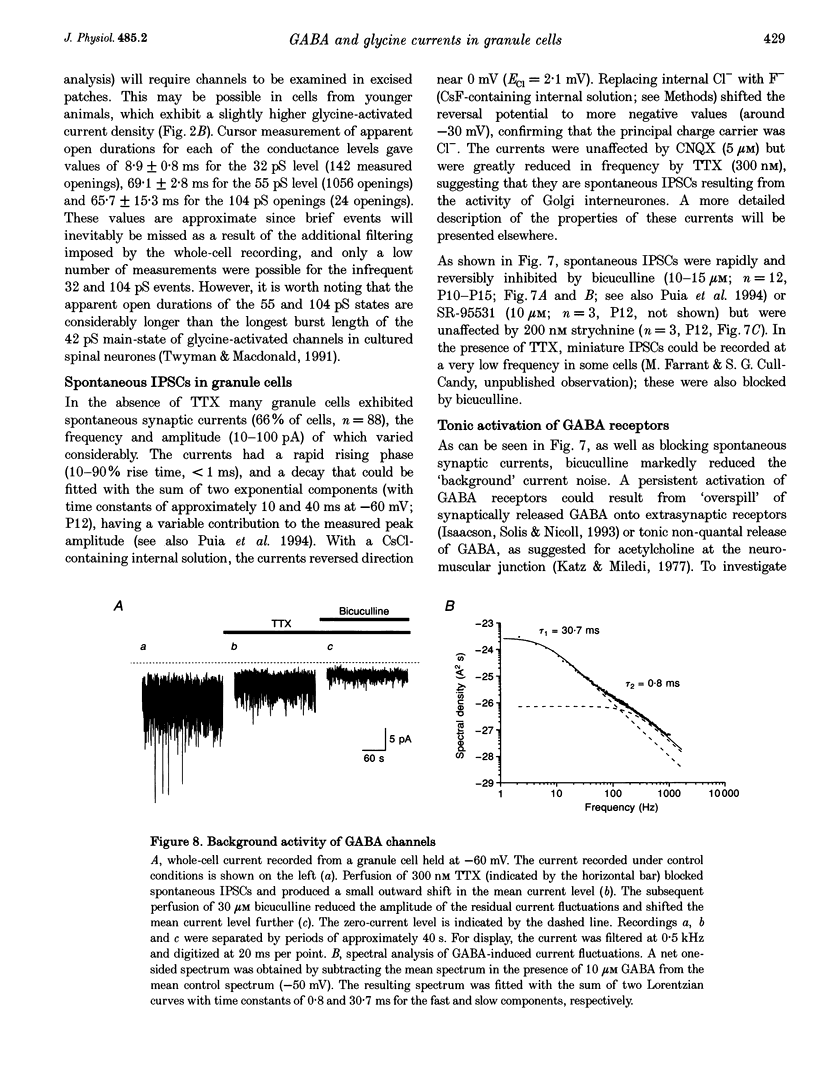





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelotti T. P., Macdonald R. L. Assembly of GABAA receptor subunits: alpha 1 beta 1 and alpha 1 beta 1 gamma 2S subunits produce unique ion channels with dissimilar single-channel properties. J Neurosci. 1993 Apr;13(4):1429–1440. doi: 10.1523/JNEUROSCI.13-04-01429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelotti T. P., Tan F., Chahine K. G., Macdonald R. L. Molecular and electrophysiological characterization of a allelic variant of the rat alpha 6 GABAA receptor subunit. Brain Res Mol Brain Res. 1992 Nov;16(1-2):173–178. doi: 10.1016/0169-328x(92)90209-t. [DOI] [PubMed] [Google Scholar]
- Araki T., Yamano M., Murakami T., Wanaka A., Betz H., Tohyama M. Localization of glycine receptors in the rat central nervous system: an immunocytochemical analysis using monoclonal antibody. Neuroscience. 1988 May;25(2):613–624. doi: 10.1016/0306-4522(88)90263-1. [DOI] [PubMed] [Google Scholar]
- Baude A., Sequier J. M., McKernan R. M., Olivier K. R., Somogyi P. Differential subcellular distribution of the alpha 6 subunit versus the alpha 1 and beta 2/3 subunits of the GABAA/benzodiazepine receptor complex in granule cells of the cerebellar cortex. Neuroscience. 1992 Dec;51(4):739–748. doi: 10.1016/0306-4522(92)90513-2. [DOI] [PubMed] [Google Scholar]
- Beattie C. E., Siegel R. E. Developmental cues modulate GABAA receptor subunit mRNA expression in cultured cerebellar granule neurons. J Neurosci. 1993 Apr;13(4):1784–1792. doi: 10.1523/JNEUROSCI.13-04-01784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y., Cherubini E., Corradetti R., Gaiarsa J. L. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989 Sep;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisti S., Iosif G., Marchesi G. F., Strata P. Pharmacological properties of inhibitions in the cerebellar cortex. Exp Brain Res. 1971;14(1):24–37. doi: 10.1007/BF00234908. [DOI] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bormann J., Rundström N., Betz H., Langosch D. Residues within transmembrane segment M2 determine chloride conductance of glycine receptor homo- and hetero-oligomers. EMBO J. 1993 Oct;12(10):3729–3737. doi: 10.1002/j.1460-2075.1993.tb06050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovolin P., Santi M. R., Puia G., Costa E., Grayson D. Expression patterns of gamma-aminobutyric acid type A receptor subunit mRNAs in primary cultures of granule neurons and astrocytes from neonatal rat cerebella. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9344–9348. doi: 10.1073/pnas.89.19.9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q. X., Stelzer A., Kay A. R., Wong R. K. GABAA receptor function is regulated by phosphorylation in acutely dissociated guinea-pig hippocampal neurones. J Physiol. 1990 Jan;420:207–221. doi: 10.1113/jphysiol.1990.sp017908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Tseng H. Y., Hockberger P. E. Depolarization- and transmitter-induced changes in intracellular Ca2+ of rat cerebellar granule cells in explant cultures. J Neurosci. 1987 May;7(5):1384–1400. doi: 10.1523/JNEUROSCI.07-05-01384.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Ogden D. C. Ion channels activated by L-glutamate and GABA in cultured cerebellar neurons of the rat. Proc R Soc Lond B Biol Sci. 1985 May 22;224(1236):367–373. doi: 10.1098/rspb.1985.0038. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G., Usowicz M. M. Whole-cell current noise produced by excitatory and inhibitory amino acids in large cerebellar neurones of the rat. J Physiol. 1989 Aug;415:533–553. doi: 10.1113/jphysiol.1989.sp017735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. A., Konnerth A., Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol. 1990 Nov;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M., Cull-Candy S. G. Excitatory amino acid receptor-channels in Purkinje cells in thin cerebellar slices. Proc Biol Sci. 1991 Jun 22;244(1311):179–184. doi: 10.1098/rspb.1991.0067. [DOI] [PubMed] [Google Scholar]
- Farrant M., Feldmeyer D., Takahashi T., Cull-Candy S. G. NMDA-receptor channel diversity in the developing cerebellum. Nature. 1994 Mar 24;368(6469):335–339. doi: 10.1038/368335a0. [DOI] [PubMed] [Google Scholar]
- Gyenes M., Farrant M., Farb D. H. "Run-down" of gamma-aminobutyric acidA receptor function during whole-cell recording: a possible role for phosphorylation. Mol Pharmacol. 1988 Dec;34(6):719–723. [PubMed] [Google Scholar]
- Huck S., Lux H. D. Patch-clamp study of ion channels activated by GABA and glycine in cultured cerebellar neurons of the mouse. Neurosci Lett. 1987 Aug 18;79(1-2):103–107. doi: 10.1016/0304-3940(87)90679-3. [DOI] [PubMed] [Google Scholar]
- Isaacson J. S., Solís J. M., Nicoll R. A. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993 Feb;10(2):165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- Ito S., Cherubini E. Strychnine-sensitive glycine responses of neonatal rat hippocampal neurones. J Physiol. 1991;440:67–83. doi: 10.1113/jphysiol.1991.sp018696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda M., Nakamura H., Akaike N. Mechanical and enzymatic isolation of mammalian CNS neurons. Neurosci Res. 1988 Apr;5(4):299–315. doi: 10.1016/0168-0102(88)90032-6. [DOI] [PubMed] [Google Scholar]
- Kaneda M., Wakamori M., Akaike N. GABA-induced chloride current in rat isolated Purkinje cells. Am J Physiol. 1989 Jun;256(6 Pt 1):C1153–C1159. doi: 10.1152/ajpcell.1989.256.6.C1153. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Transmitter leakage from motor nerve endings. Proc R Soc Lond B Biol Sci. 1977 Feb 11;196(1122):59–72. doi: 10.1098/rspb.1977.0029. [DOI] [PubMed] [Google Scholar]
- Khan Z. U., Gutierrez A., De Blas A. L. The subunit composition of a GABAA/benzodiazepine receptor from rat cerebellum. J Neurochem. 1994 Jul;63(1):371–374. doi: 10.1046/j.1471-4159.1994.63010371.x. [DOI] [PubMed] [Google Scholar]
- Kilić G., Moran O., Cherubini E. Currents activated by GABA and their modulation by Zn2+ in cerebellar granule cells in culture. Eur J Neurosci. 1993 Jan 1;5(1):65–72. doi: 10.1111/j.1460-9568.1993.tb00206.x. [DOI] [PubMed] [Google Scholar]
- Laurie D. J., Seeburg P. H., Wisden W. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. II. Olfactory bulb and cerebellum. J Neurosci. 1992 Mar;12(3):1063–1076. doi: 10.1523/JNEUROSCI.12-03-01063.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie D. J., Wisden W., Seeburg P. H. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992 Nov;12(11):4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I., Marty A., Johnson J. W., Ascher P., Gähwiler B. H. Patch-clamp recording of amino acid-activated responses in "organotypic" slice cultures. Proc Natl Acad Sci U S A. 1988 May;85(9):3221–3225. doi: 10.1073/pnas.85.9.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüddens H., Pritchett D. B., Köhler M., Killisch I., Keinänen K., Monyer H., Sprengel R., Seeburg P. H. Cerebellar GABAA receptor selective for a behavioural alcohol antagonist. Nature. 1990 Aug 16;346(6285):648–651. doi: 10.1038/346648a0. [DOI] [PubMed] [Google Scholar]
- Macdonald R. L., Rogers C. J., Twyman R. E. Kinetic properties of the GABAA receptor main conductance state of mouse spinal cord neurones in culture. J Physiol. 1989 Mar;410:479–499. doi: 10.1113/jphysiol.1989.sp017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maconochie D. J., Zempel J. M., Steinbach J. H. How quickly can GABAA receptors open? Neuron. 1994 Jan;12(1):61–71. doi: 10.1016/0896-6273(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Malosio M. L., Marquèze-Pouey B., Kuhse J., Betz H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J. 1991 Sep;10(9):2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry D. K., Hablitz J. J. Activation of subconductance states by gamma-aminobutyric acid and its analogs in chick cerebral neurons. Pflugers Arch. 1990 Jun;416(4):454–461. doi: 10.1007/BF00370754. [DOI] [PubMed] [Google Scholar]
- Morales E., Tapia R. Neurotransmitters of the cerebellar glomeruli: uptake and release of labeled gamma-aminobutyric acid, glycine, serotonin and choline in a purified glomerulus fraction and in granular layer slices. Brain Res. 1987 Sep 8;420(1):11–21. doi: 10.1016/0006-8993(87)90234-4. [DOI] [PubMed] [Google Scholar]
- Mori-Okamoto J., Tatsuno J. Development of sensitivity to GABA and glycine in cultured cerebellar neurons. Brain Res. 1985 Jun;352(2):249–258. doi: 10.1016/0165-3806(85)90112-9. [DOI] [PubMed] [Google Scholar]
- Newland C. F., Colquhoun D., Cull-Candy S. G. Single channels activated by high concentrations of GABA in superior cervical ganglion neurones of the rat. J Physiol. 1991 Jan;432:203–233. doi: 10.1113/jphysiol.1991.sp018382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen O. P., Storm-Mathisen J., Somogyi P. Colocalization of glycine-like and GABA-like immunoreactivities in Golgi cell terminals in the rat cerebellum: a postembedding light and electron microscopic study. Brain Res. 1988 May 31;450(1-2):342–353. doi: 10.1016/0006-8993(88)91573-9. [DOI] [PubMed] [Google Scholar]
- Pribilla I., Takagi T., Langosch D., Bormann J., Betz H. The atypical M2 segment of the beta subunit confers picrotoxinin resistance to inhibitory glycine receptor channels. EMBO J. 1992 Dec;11(12):4305–4311. doi: 10.1002/j.1460-2075.1992.tb05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puia G., Costa E., Vicini S. Functional diversity of GABA-activated Cl- currents in Purkinje versus granule neurons in rat cerebellar slices. Neuron. 1994 Jan;12(1):117–126. doi: 10.1016/0896-6273(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Reichling D. B., Kyrozis A., Wang J., MacDermott A. B. Mechanisms of GABA and glycine depolarization-induced calcium transients in rat dorsal horn neurons. J Physiol. 1994 May 1;476(3):411–421. doi: 10.1113/jphysiol.1994.sp020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robello M., Amico C., Cupello A. Regulation of GABAA receptor in cerebellar granule cells in culture: differential involvement of kinase activities. Neuroscience. 1993 Mar;53(1):131–138. doi: 10.1016/0306-4522(93)90291-m. [DOI] [PubMed] [Google Scholar]
- Sieghart W., Eichinger A., Richards J. G., Möhler H. Photoaffinity labeling of benzodiazepine receptor proteins with the partial inverse agonist [3H]Ro 15-4513: a biochemical and autoradiographic study. J Neurochem. 1987 Jan;48(1):46–52. doi: 10.1111/j.1471-4159.1987.tb13125.x. [DOI] [PubMed] [Google Scholar]
- Silver R. A., Traynelis S. F., Cull-Candy S. G. Rapid-time-course miniature and evoked excitatory currents at cerebellar synapses in situ. Nature. 1992 Jan 9;355(6356):163–166. doi: 10.1038/355163a0. [DOI] [PubMed] [Google Scholar]
- Smart T. G. A novel modulatory binding site for zinc on the GABAA receptor complex in cultured rat neurones. J Physiol. 1992 Feb;447:587–625. doi: 10.1113/jphysiol.1992.sp019020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P., Takagi H., Richards J. G., Mohler H. Subcellular localization of benzodiazepine/GABAA receptors in the cerebellum of rat, cat, and monkey using monoclonal antibodies. J Neurosci. 1989 Jun;9(6):2197–2209. doi: 10.1523/JNEUROSCI.09-06-02197.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y. M., Huang L. Y. Modulation of glycine receptor chloride channels by cAMP-dependent protein kinase in spinal trigeminal neurons. Nature. 1990 Nov 15;348(6298):242–245. doi: 10.1038/348242a0. [DOI] [PubMed] [Google Scholar]
- Stelzer A., Kay A. R., Wong R. K. GABAA-receptor function in hippocampal cells is maintained by phosphorylation factors. Science. 1988 Jul 15;241(4863):339–341. doi: 10.1126/science.2455347. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Momiyama A., Hirai K., Hishinuma F., Akagi H. Functional correlation of fetal and adult forms of glycine receptors with developmental changes in inhibitory synaptic receptor channels. Neuron. 1992 Dec;9(6):1155–1161. doi: 10.1016/0896-6273(92)90073-m. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Momiyama A. Single-channel currents underlying glycinergic inhibitory postsynaptic responses in spinal neurons. Neuron. 1991 Dec;7(6):965–969. doi: 10.1016/0896-6273(91)90341-v. [DOI] [PubMed] [Google Scholar]
- Thompson C. L., Stephenson F. A. GABAA receptor subtypes expressed in cerebellar granule cells: a developmental study. J Neurochem. 1994 May;62(5):2037–2044. doi: 10.1046/j.1471-4159.1994.62052037.x. [DOI] [PubMed] [Google Scholar]
- Triller A., Cluzeaud F., Korn H. gamma-Aminobutyric acid-containing terminals can be apposed to glycine receptors at central synapses. J Cell Biol. 1987 Apr;104(4):947–956. doi: 10.1083/jcb.104.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman R. E., Macdonald R. L. Kinetic properties of the glycine receptor main- and sub-conductance states of mouse spinal cord neurones in culture. J Physiol. 1991 Apr;435:303–331. doi: 10.1113/jphysiol.1991.sp018512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaello M. L., Ruiz-Gómez A., Lerma J., Mayor F., Jr Modulation of inhibitory glycine receptors by phosphorylation by protein kinase C and cAMP-dependent protein kinase. J Biol Chem. 1994 Jan 21;269(3):2002–2008. [PubMed] [Google Scholar]
- Verdoorn T. A., Draguhn A., Ymer S., Seeburg P. H., Sakmann B. Functional properties of recombinant rat GABAA receptors depend upon subunit composition. Neuron. 1990 Jun;4(6):919–928. doi: 10.1016/0896-6273(90)90145-6. [DOI] [PubMed] [Google Scholar]
- Verdoorn T. A. Formation of heteromeric gamma-aminobutyric acid type A receptors containing two different alpha subunits. Mol Pharmacol. 1994 Mar;45(3):475–480. [PubMed] [Google Scholar]
- Vicini S., Wroblewski J. T., Costa E. Pharmacological modulation of GABAergic transmission in cultured cerebellar neurons. Neuropharmacology. 1986 Feb;25(2):207–211. doi: 10.1016/0028-3908(86)90043-2. [DOI] [PubMed] [Google Scholar]
- Wahl P., Elster L., Schousboe A. Identification and function of glycine receptors in cultured cerebellar granule cells. J Neurochem. 1994 Jun;62(6):2457–2463. doi: 10.1046/j.1471-4159.1994.62062457.x. [DOI] [PubMed] [Google Scholar]
- Weiss D. S., Barnes E. M., Jr, Hablitz J. J. Whole-cell and single-channel recordings of GABA-gated currents in cultured chick cerebral neurons. J Neurophysiol. 1988 Feb;59(2):495–513. doi: 10.1152/jn.1988.59.2.495. [DOI] [PubMed] [Google Scholar]
- Weiss D. S. Membrane potential modulates the activation of GABA-gated channels. J Neurophysiol. 1988 Feb;59(2):514–527. doi: 10.1152/jn.1988.59.2.514. [DOI] [PubMed] [Google Scholar]
- Wilkin G. P., Csillag A., Balázs R., Kingsbury A. E., Wilson J. E., Johnson A. L. Localization of high affinity [3H]glycine transport sites in the cerebellar cortex. Brain Res. 1981 Jul 6;216(1):11–33. doi: 10.1016/0006-8993(81)91275-0. [DOI] [PubMed] [Google Scholar]
- Wisden W., Seeburg P. H. GABAA receptor channels: from subunits to functional entities. Curr Opin Neurobiol. 1992 Jun;2(3):263–269. doi: 10.1016/0959-4388(92)90113-y. [DOI] [PubMed] [Google Scholar]
- Yuste R., Katz L. C. Control of postsynaptic Ca2+ influx in developing neocortex by excitatory and inhibitory neurotransmitters. Neuron. 1991 Mar;6(3):333–344. doi: 10.1016/0896-6273(91)90243-s. [DOI] [PubMed] [Google Scholar]
- Zarbin M. A., Wamsley J. K., Kuhar M. J. Glycine receptor: light microscopic autoradiographic localization with [3H]strychnine. J Neurosci. 1981 May;1(5):532–547. doi: 10.1523/JNEUROSCI.01-05-00532.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng T., Santi M. R., Bovolin P., Marlier L. N., Grayson D. R. Developmental expression of the alpha 6 GABAA receptor subunit mRNA occurs only after cerebellar granule cell migration. Brain Res Dev Brain Res. 1993 Sep 17;75(1):91–103. doi: 10.1016/0165-3806(93)90068-l. [DOI] [PubMed] [Google Scholar]


