Abstract
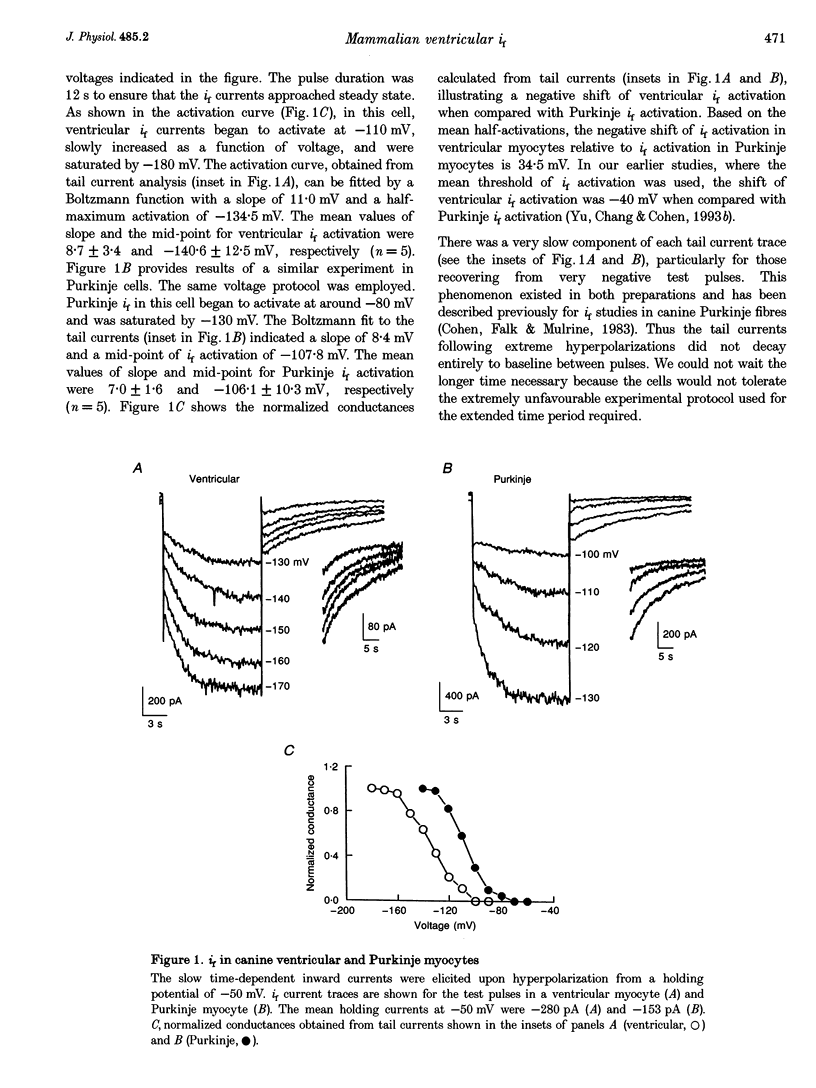
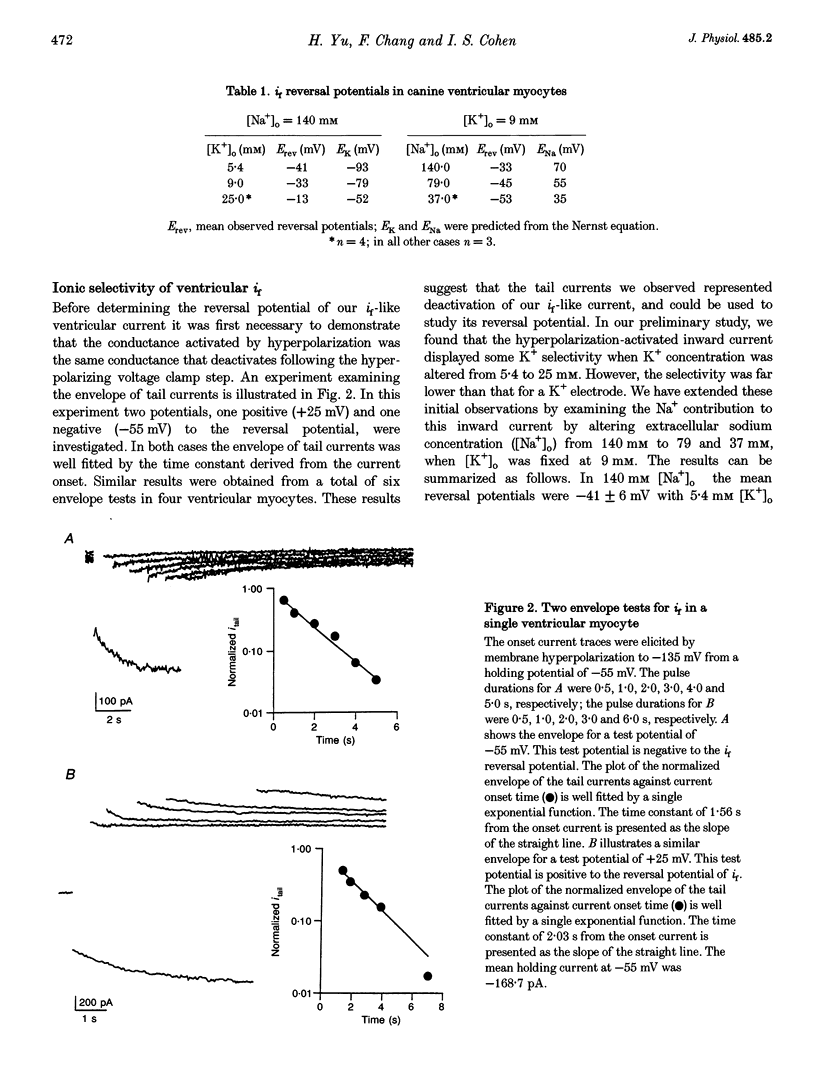
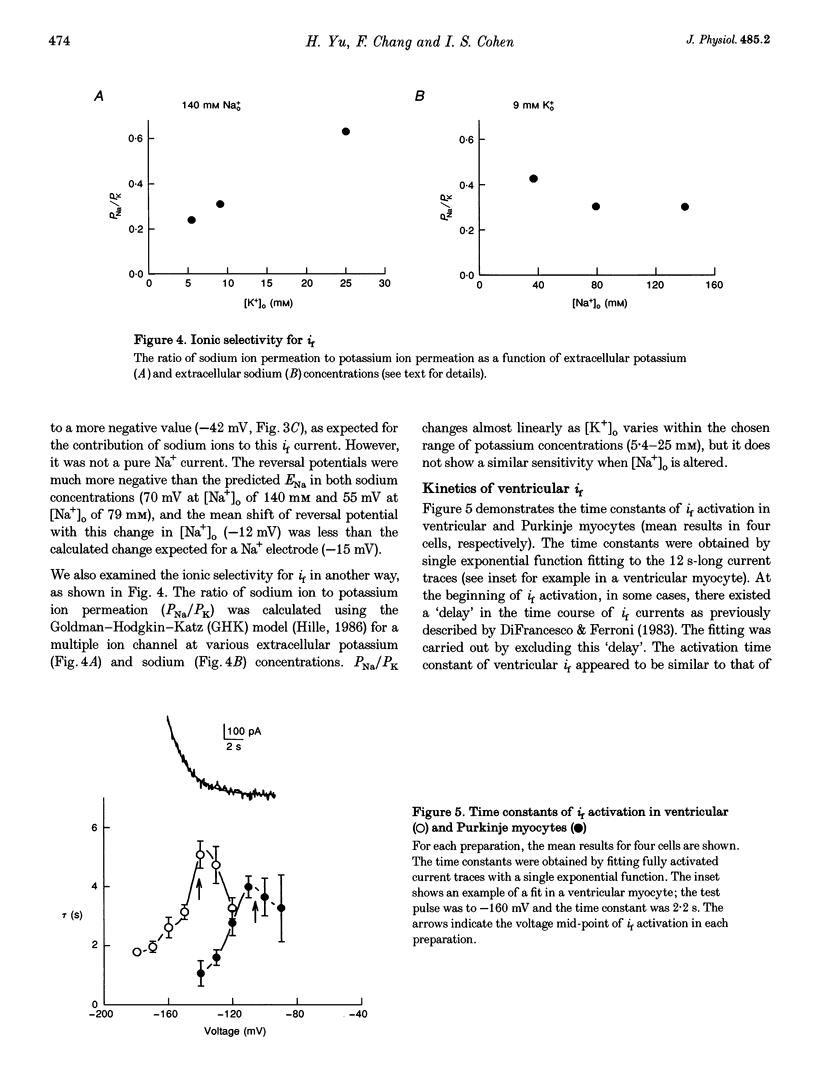
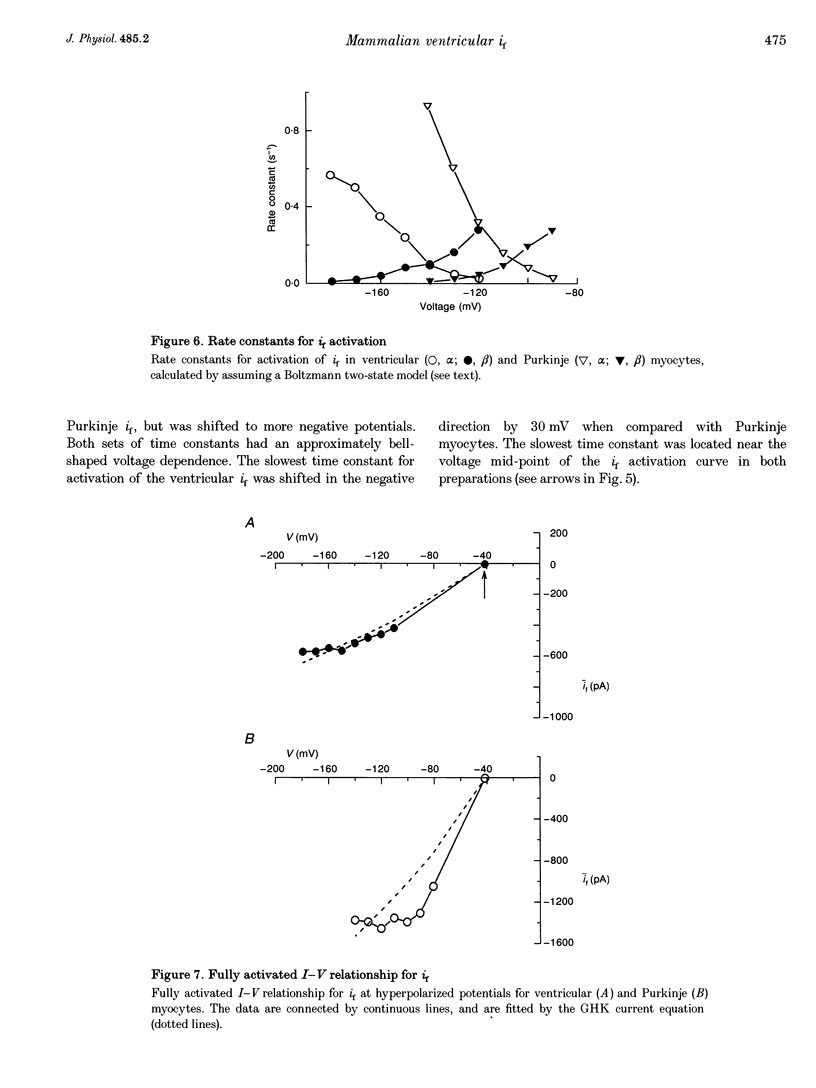
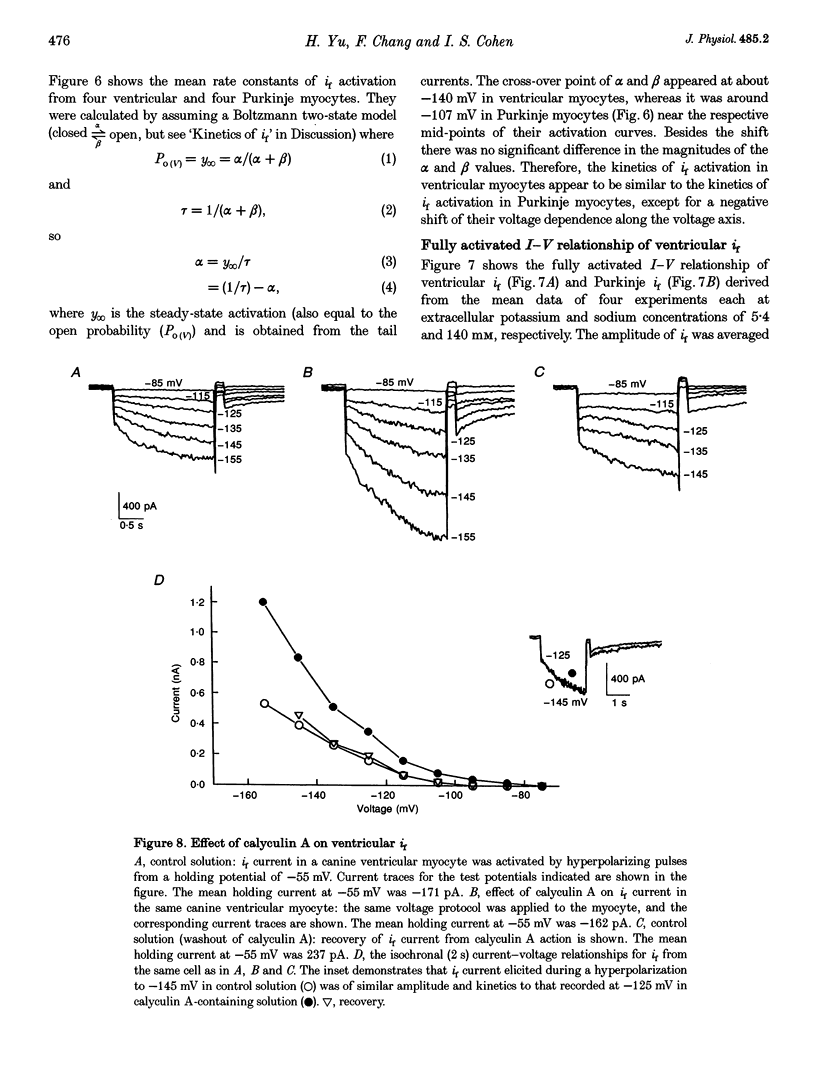
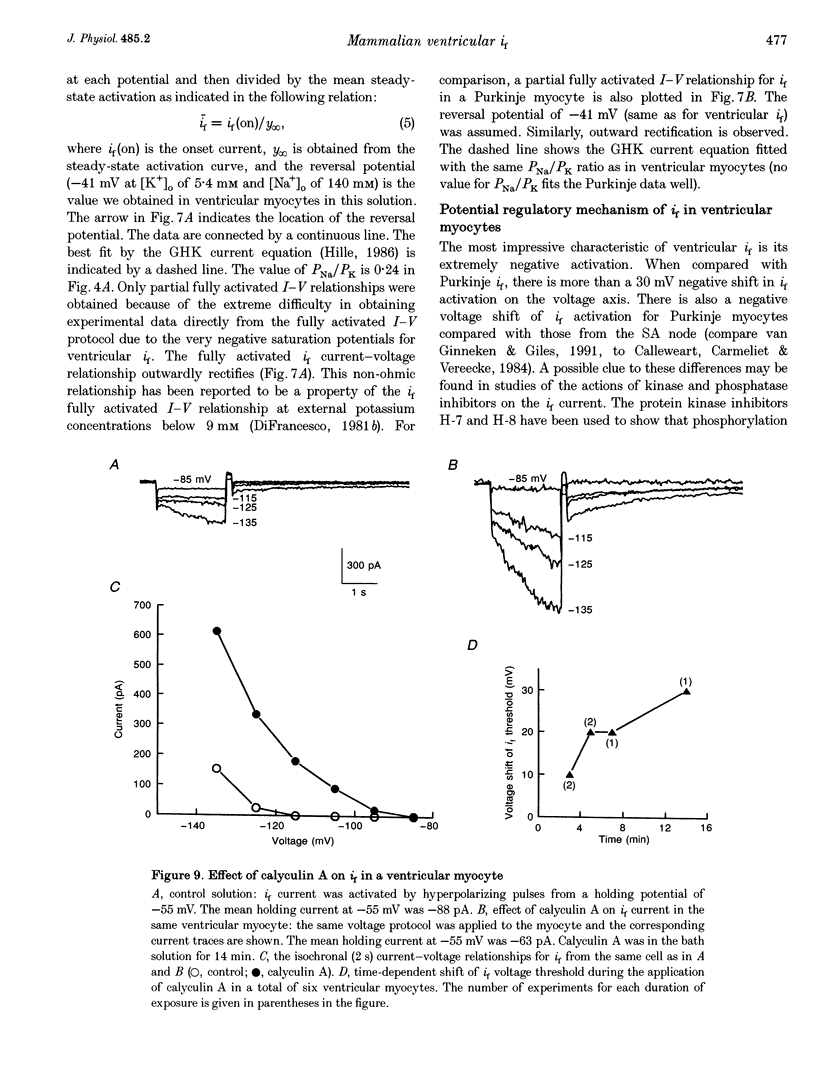
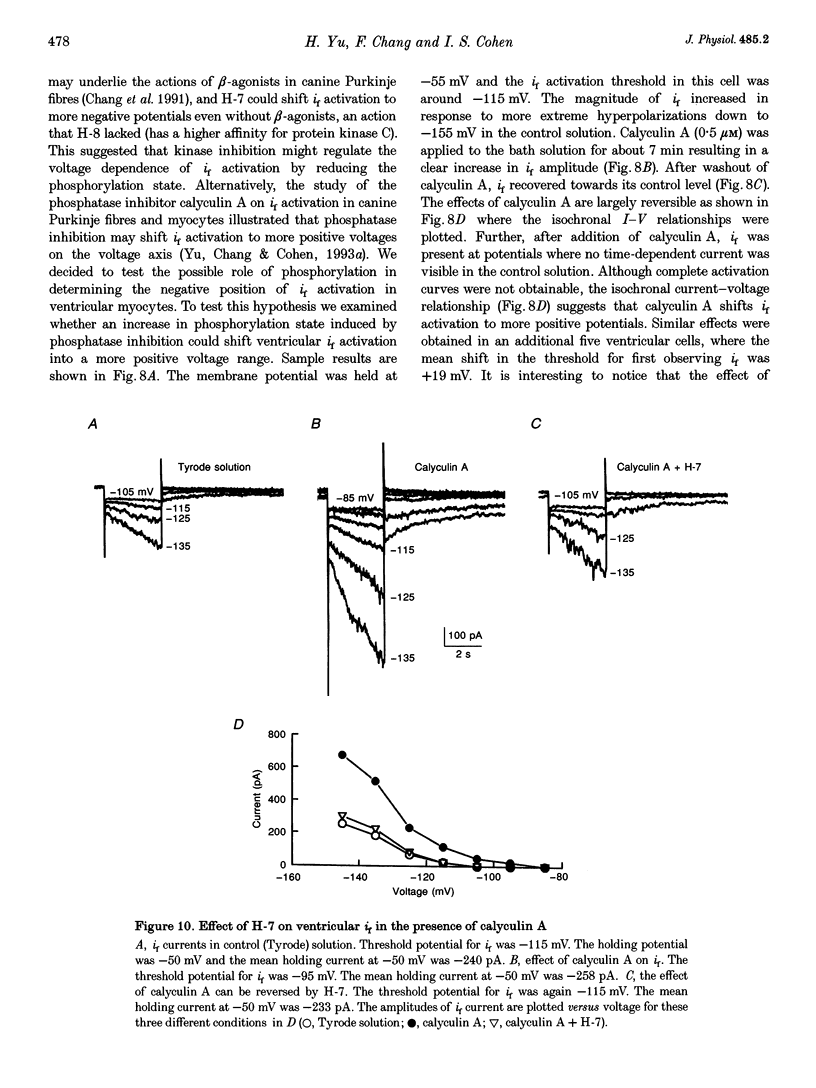
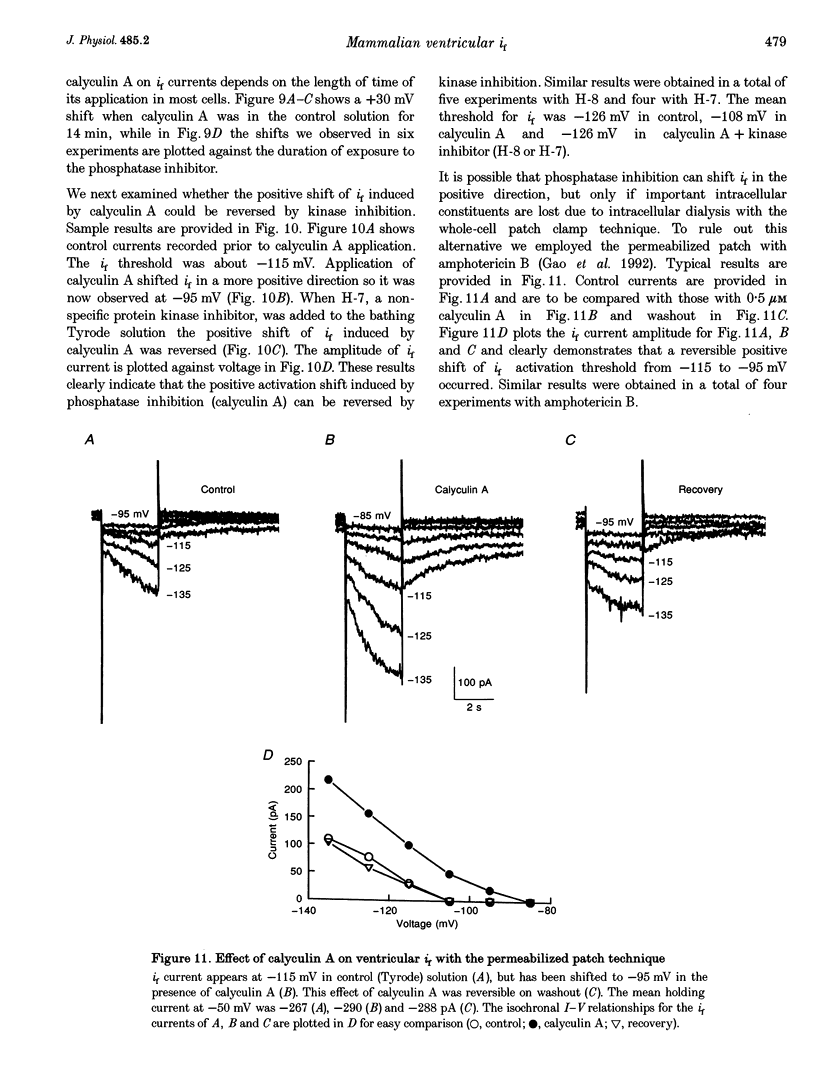
1. Single cells enzymatically isolated from canine ventricle and canine Purkinje fibres were studied with the whole-cell patch clamp technique, and the properties of the pacemaker current i(f) compared. 2. Steady-state i(f) activation occurred in canine ventricular myocytes at more negative potentials (-120 to -170 mV) than in canine Purkinje cells (-80 to -130 mV). 3. Reversal potentials were obtained in various extracellular Na+ (140, 79 or 37 mM) and K+ concentrations (25, 9 or 5.4 mM) to determine the ionic selectivity of i(f) in the ventricle. The results suggest that this current was carried by both sodium and potassium ions. 4. The plots of the time constants of i(f) activation against voltage were 'bell shaped' in both canine ventricular and Purkinje myocytes. The curve for the ventricular myocytes was shifted about 30 mV in the negative direction. In both ventricular and Purkinje myocytes, the fully activated I-V relationship exhibited outward rectification in 5.4 mM extracellular K+. 5. Calyculin A (0.5 microM) increased i(f) by shifting its activation to more positive potentials in ventricular myocytes. Protein kinase inhibition by H-7 (200 microM) or H-8 (100 microM) reversed the positive voltage shift of i(f) activation. This effect of calyculin A also occurred when the permeabilized patch was used for whole-cell recording. 6. These results indicate i(f) is present in ventricular myocytes. If shifted to more positive potentials i(f) could play a role in ischaemia-induced ventricular arrhythmias. The negative shift of i(f) in the ventricle might play a role in differentiating non-pacing regions of the heart from those regions that pace.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brochu R. M., Clay J. R., Shrier A. Pacemaker current in single cells and in aggregates of cells dissociated from the embryonic chick heart. J Physiol. 1992 Aug;454:503–515. doi: 10.1113/jphysiol.1992.sp019276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert G., Carmeliet E., Vereecke J. Single cardiac Purkinje cells: general electrophysiology and voltage-clamp analysis of the pace-maker current. J Physiol. 1984 Apr;349:643–661. doi: 10.1113/jphysiol.1984.sp015179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F., Cohen I. S., DiFrancesco D., Rosen M. R., Tromba C. Effects of protein kinase inhibitors on canine Purkinje fibre pacemaker depolarization and the pacemaker current i(f). J Physiol. 1991;440:367–384. doi: 10.1113/jphysiol.1991.sp018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F., Cohen I. S. Mechanism of acetylcholine action on pacemaker current (i(f)) in canine Purkinje fibers. Pflugers Arch. 1992 Mar;420(3-4):389–392. doi: 10.1007/BF00374474. [DOI] [PubMed] [Google Scholar]
- Cohen I. S., Datyner N. B., Gintant G. A., Mulrine N. K., Pennefather P. Properties of an electrogenic sodium-potassium pump in isolated canine Purkinje myocytes. J Physiol. 1987 Feb;383:251–267. doi: 10.1113/jphysiol.1987.sp016407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I. S., Falk R. T., Mulrine N. K. Actions of barium and rubidium on membrane currents in canine Purkinje fibres. J Physiol. 1983 May;338:589–612. doi: 10.1113/jphysiol.1983.sp014691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Production of membrane potential changes in the frog's heart by inhibitory nerve impulses. Nature. 1955 Jun 11;175(4467):1035–1035. doi: 10.1038/1751035a0. [DOI] [PubMed] [Google Scholar]
- Datyner N. B., Gintant G. A., Cohen I. S. Versatile temperature controlled tissue bath for studies of isolated cells using an inverted microscope. Pflugers Arch. 1985 Mar;403(3):318–323. doi: 10.1007/BF00583607. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. A study of the ionic nature of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:377–393. doi: 10.1113/jphysiol.1981.sp013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., Ferroni A. Delayed activation of the cardiac pacemaker current and its dependence on conditioning pre-hyperpolarizations. Pflugers Arch. 1983 Mar 1;396(3):265–267. doi: 10.1007/BF00587866. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol. 1993;55:455–472. doi: 10.1146/annurev.ph.55.030193.002323. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. The cardiac hyperpolarizing-activated current, if. Origins and developments. Prog Biophys Mol Biol. 1985;46(3):163–183. doi: 10.1016/0079-6107(85)90008-2. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991 May 9;351(6322):145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D., Tromba C. Inhibition of the hyperpolarization-activated current (if) induced by acetylcholine in rabbit sino-atrial node myocytes. J Physiol. 1988 Nov;405:477–491. doi: 10.1113/jphysiol.1988.sp017343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earm Y. E., Shimoni Y., Spindler A. J. A pace-maker-like current in the sheep atrium and its modulation by catecholamines. J Physiol. 1983 Sep;342:569–590. doi: 10.1113/jphysiol.1983.sp014869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Mathias R. T., Cohen I. S., Baldo G. J. Isoprenaline, Ca2+ and the Na(+)-K+ pump in guinea-pig ventricular myocytes. J Physiol. 1992 Apr;449:689–704. doi: 10.1113/jphysiol.1992.sp019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H. Modulation by intracellular Ca2+ of the hyperpolarization-activated inward current in rabbit single sino-atrial node cells. J Physiol. 1989 Feb;409:121–141. doi: 10.1113/jphysiol.1989.sp017488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth O., Noble D., Tsien R. W. Adrenaline: mechanism of action on the pacemaker potential in cardiac Purkinje fibers. Science. 1968 Nov 22;162(3856):916–917. doi: 10.1126/science.162.3856.916. [DOI] [PubMed] [Google Scholar]
- Isenberg G. Cardiac Purkinje fibres: [Ca2+]i controls the potassium permeability via the conductance components gK1 and gK2. Pflugers Arch. 1977 Oct 19;371(1-2):77–85. doi: 10.1007/BF00580775. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Calcium currents of isolated bovine ventricular myocytes are fast and of large amplitude. Pflugers Arch. 1982 Oct;395(1):30–41. doi: 10.1007/BF00584965. [DOI] [PubMed] [Google Scholar]
- Ishihara H., Ozaki H., Sato K., Hori M., Karaki H., Watabe S., Kato Y., Fusetani N., Hashimoto K., Uemura D. Calcium-independent activation of contractile apparatus in smooth muscle by calyculin-A. J Pharmacol Exp Ther. 1989 Jul;250(1):388–396. [PubMed] [Google Scholar]
- Mathias R. T., Cohen I. S., Oliva C. Limitations of the whole cell patch clamp technique in the control of intracellular concentrations. Biophys J. 1990 Sep;58(3):759–770. doi: 10.1016/S0006-3495(90)82418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol. 1984 Aug;353:1–50. doi: 10.1113/jphysiol.1984.sp015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A., Irisawa H., Kokobun S., Kotake H., Nishimura M., Watanabe Y. Slow current systems in the A-V node of the rabbit heart. Nature. 1980 May 22;285(5762):228–229. doi: 10.1038/285228a0. [DOI] [PubMed] [Google Scholar]
- Oliva C., Cohen I. S., Mathias R. T. Calculation of time constants for intracellular diffusion in whole cell patch clamp configuration. Biophys J. 1988 Nov;54(5):791–799. doi: 10.1016/S0006-3495(88)83017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H., Sperelakis N. Identification of the hyperpolarization-activated inward current in young embryonic chick heart myocytes. J Dev Physiol. 1991 Apr;15(4):247–252. [PubMed] [Google Scholar]
- Toda N., Shimamoto K. The influence of sympathetic stimulation on transmembrane potentials in the S-A node. J Pharmacol Exp Ther. 1968 Feb;159(2):298–305. [PubMed] [Google Scholar]
- Tsien R. W. Mode of action of chronotropic agents in cardiac Purkinje fibers. Does epinephrine act by directly modifying the external surface charge? J Gen Physiol. 1974 Sep;64(3):320–342. doi: 10.1085/jgp.64.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara K., Irisawa H. Inward current activated during hyperpolarization in the rabbit sinoatrial node cell. Pflugers Arch. 1980 May;385(1):11–19. doi: 10.1007/BF00583909. [DOI] [PubMed] [Google Scholar]
- Yu H., Chang F., Cohen I. S. Pacemaker current exists in ventricular myocytes. Circ Res. 1993 Jan;72(1):232–236. doi: 10.1161/01.res.72.1.232. [DOI] [PubMed] [Google Scholar]
- Yu H., Chang F., Cohen I. S. Phosphatase inhibition by calyculin A increases i(f) in canine Purkinje fibers and myocytes. Pflugers Arch. 1993 Mar;422(6):614–616. doi: 10.1007/BF00374010. [DOI] [PubMed] [Google Scholar]
- Zaza A., Maccaferri G., Mangoni M., DiFrancesco D. Intracellular calcium does not directly modulate cardiac pacemaker (if) channels. Pflugers Arch. 1991 Dec;419(6):662–664. doi: 10.1007/BF00370312. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Lipsius S. L. Properties of the pacemaker current (If) in latent pacemaker cells isolated from cat right atrium. J Physiol. 1992;453:503–523. doi: 10.1113/jphysiol.1992.sp019242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ginneken A. C., Giles W. Voltage clamp measurements of the hyperpolarization-activated inward current I(f) in single cells from rabbit sino-atrial node. J Physiol. 1991 Mar;434:57–83. doi: 10.1113/jphysiol.1991.sp018459. [DOI] [PMC free article] [PubMed] [Google Scholar]


