Abstract
1. Using the ventral surgical approach in vivo, extracellular recordings were made from seventy-nine cells in the supraoptic nucleus of urethane-anaesthetized male, virgin female or lactating female rats while stimulating the pituitary stalk. Cells were classed according to their spontaneous firing activity as: continuous (putative oxytocin), phasic (putative vasopressin) and silent. 2. Stimulation of the neural stalk produced an excitation (up to 25 ms poststimulus) in eleven of the seventy-nine antidromically identified magnocellular neurones, consistent with the existence of excitatory collaterals or dendritic contacts between such cells. In these recordings a second spike could frequently be seen, following the antidromic spike, with a variable latency. Such spikes consistently collided with subsequent antidromically evoked spikes. Poststimulus excitation was only seen in silent and continuously firing (putative oxytocin) cells, suggesting that oxytocin and vasopressin cells have different connections. 3. Excitatory connections were seen more frequently in lactating females (8 out of 22 cells) than in males (1 out of 15 cells) or virgin females (2 out of 10 cells), and thus may make an important contribution to the bursts of firing which precede reflex milk ejection.
Full text
PDF
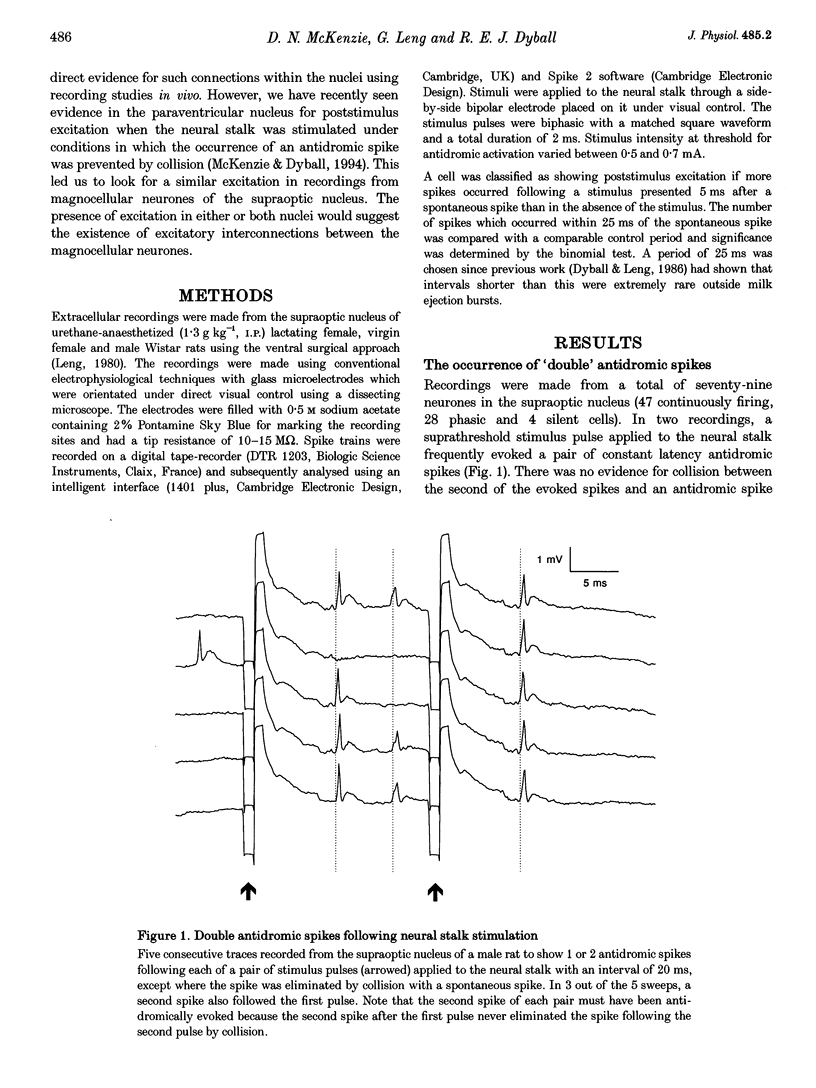
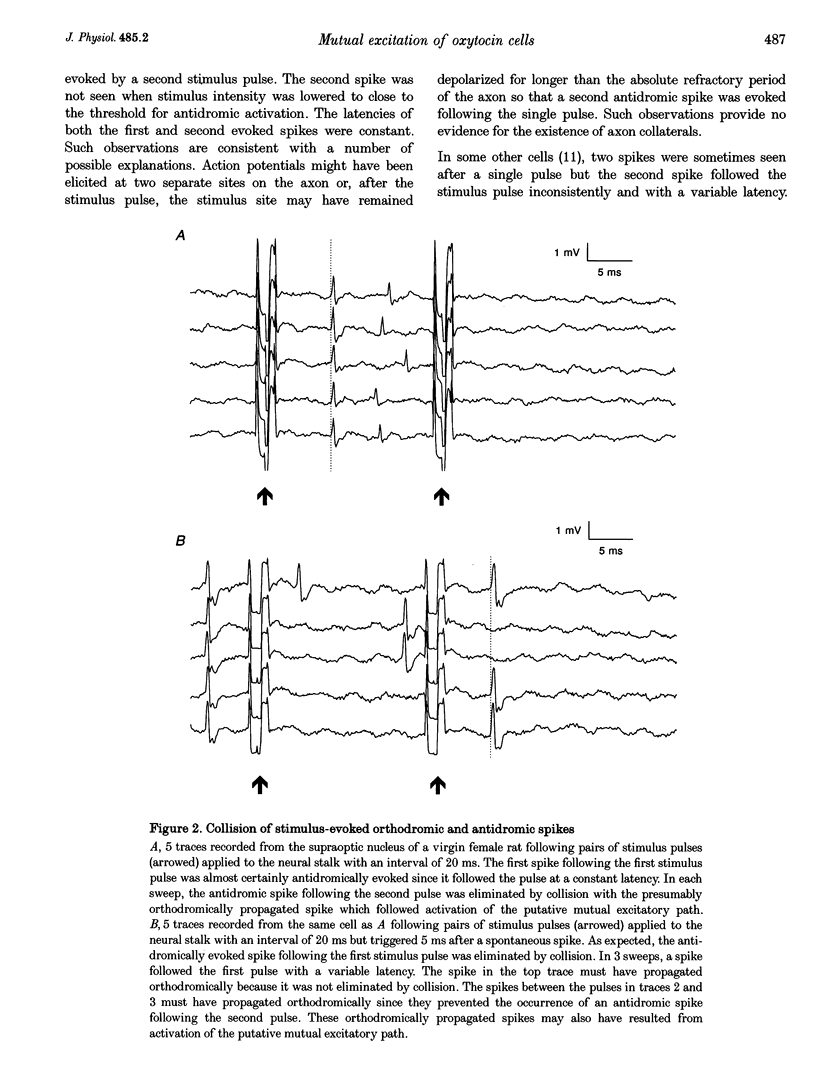
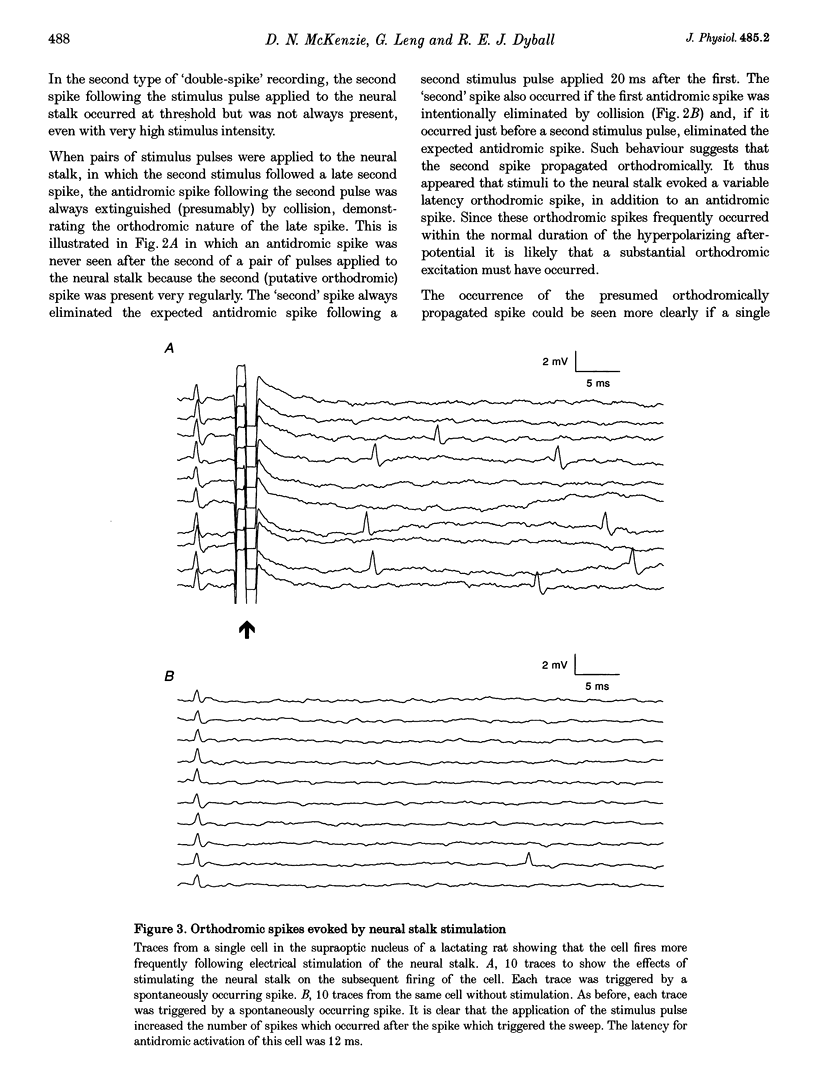
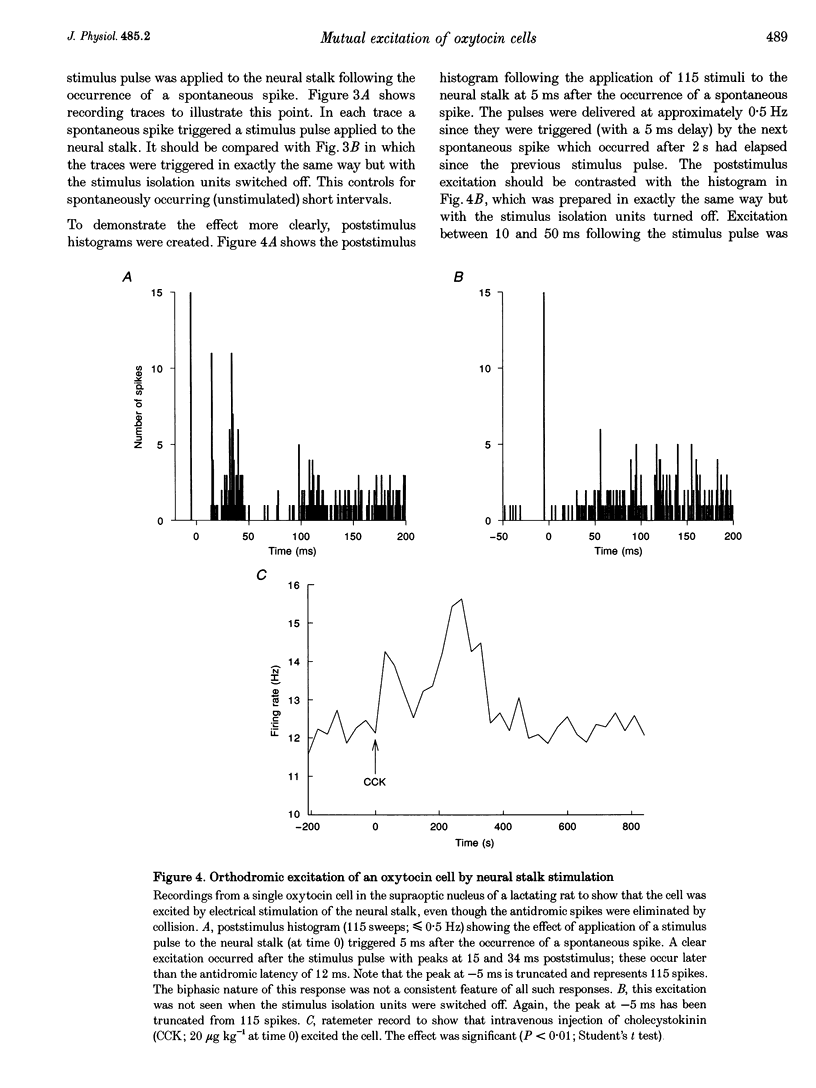



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourque C. W., Renaud L. P. Membrane properties of rat magnocellular neuroendocrine cells in vivo. Brain Res. 1991 Feb 1;540(1-2):349–352. doi: 10.1016/0006-8993(91)90535-4. [DOI] [PubMed] [Google Scholar]
- Bourque C. W. Transient calcium-dependent potassium current in magnocellular neurosecretory cells of the rat supraoptic nucleus. J Physiol. 1988 Mar;397:331–347. doi: 10.1113/jphysiol.1988.sp017004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles J. A., Poulain D. A. Extracellular K+ in the supraoptic nucleus of the rat during reflex bursting activity by oxytocin neurones. J Physiol. 1991 Aug;439:383–409. doi: 10.1113/jphysiol.1991.sp018672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss J. J., Kelly J. S. Recurrent inhibition of antidromically identified rat supraoptic neurones. J Physiol. 1972 Jan;220(1):87–103. doi: 10.1113/jphysiol.1972.sp009696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyball R. E., Leng G. Regulation of the milk ejection reflex in the rat. J Physiol. 1986 Nov;380:239–256. doi: 10.1113/jphysiol.1986.sp016283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund-Mercier M. J., Richard P. Electrophysiological evidence for facilitatory control of oxytocin neurones by oxytocin during suckling in the rat. J Physiol. 1984 Jul;352:447–466. doi: 10.1113/jphysiol.1984.sp015302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gähwiler B. H., Dreifuss J. J. Phasically firing neurons in long-term cultures of the rat hypothalamic supraoptic area: pacemaker and follower cells. Brain Res. 1979 Nov 9;177(1):95–103. doi: 10.1016/0006-8993(79)90920-x. [DOI] [PubMed] [Google Scholar]
- Hatton G. I., Cobbett P., Salm A. K. Extranuclear axon collaterals of paraventricular neurons in the rat hypothalamus: intracellular staining, immunocytochemistry and electrophysiology. Brain Res Bull. 1985 Feb;14(2):123–132. doi: 10.1016/0361-9230(85)90072-3. [DOI] [PubMed] [Google Scholar]
- Hatton G. I., Yang Q. Z., Smithson K. G. Synaptic inputs and electrical coupling among magnocellular neuroendocrine cells. Brain Res Bull. 1988 Jun;20(6):751–755. doi: 10.1016/0361-9230(88)90087-1. [DOI] [PubMed] [Google Scholar]
- Leng G., Dyball R. E. Intercommunication in the rat supraoptic nucleus. Q J Exp Physiol. 1983 Jul;68(3):493–504. doi: 10.1113/expphysiol.1983.sp002742. [DOI] [PubMed] [Google Scholar]
- Leng G. Rat supraoptic neurones: the effects of locally applied hypertonic saline. J Physiol. 1980 Jul;304:405–414. doi: 10.1113/jphysiol.1980.sp013332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G., Way S., Dyball R. E. Identification of oxytoxin cells in the rat supraoptic nucleus by their response to cholecystokinin injection. Neurosci Lett. 1991 Jan 28;122(2):159–162. doi: 10.1016/0304-3940(91)90847-m. [DOI] [PubMed] [Google Scholar]
- Leng G., Yamashita H., Dyball R. E., Bunting R. Electrophysiological evidence for a projection from the arcuate nucleus to the supraoptic nucleus. Neurosci Lett. 1988 Jun 29;89(2):146–151. doi: 10.1016/0304-3940(88)90371-0. [DOI] [PubMed] [Google Scholar]
- Mason W. T., Ho Y. W., Hatton G. I. Axon collaterals of supraoptic neurones: anatomical and electrophysiological evidence for their existence in the lateral hypothalamus. Neuroscience. 1984 Jan;11(1):169–182. doi: 10.1016/0306-4522(84)90221-5. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982 Apr;7(4):773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Theodosis D. T., Chapman D. B., Montagnese C., Poulain D. A., Morris J. F. Structural plasticity in the hypothalamic supraoptic nucleus at lactation affects oxytocin-, but not vasopressin-secreting neurones. Neuroscience. 1986 Mar;17(3):661–678. doi: 10.1016/0306-4522(86)90038-2. [DOI] [PubMed] [Google Scholar]
- Theodosis D. T., Montagnese C., Rodriguez F., Vincent J. D., Poulain D. A. Oxytocin induces morphological plasticity in the adult hypothalamo-neurohypophysial system. Nature. 1986 Aug 21;322(6081):738–740. doi: 10.1038/322738a0. [DOI] [PubMed] [Google Scholar]
- Wakerley J. B., Lincoln D. W. The milk-ejection reflex of the rat: a 20- to 40-fold acceleration in the firing of paraventricular neurones during oxytocin release. J Endocrinol. 1973 Jun;57(3):477–493. doi: 10.1677/joe.0.0570477. [DOI] [PubMed] [Google Scholar]
- Yang Q. Z., Hatton G. I. Direct evidence for electrical coupling among rat supraoptic nucleus neurons. Brain Res. 1988 Oct 25;463(1):47–56. doi: 10.1016/0006-8993(88)90525-2. [DOI] [PubMed] [Google Scholar]


