Abstract
Background:
Iron overload is considered an unfavorable prognosis in myelodysplastic syndrome (MDS) even in those undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT). Although iron chelation therapy has improved the prognosis of these patients to some extent, the effect has not yet been satisfactory.
Objectives:
This study aimed to investigate the impact of granulocyte colony-stimulating factor and decitabine (G-DAC)-containing conditioning in iron-overloaded MDS patients undergoing allo-HSCT.
Design:
This was a retrospective study.
Methods:
One hundred and ninety-seven patients were enrolled in this retrospective study. Based on the level of serum ferritin (SF) and conditioning regimen, all patients enrolled were divided into four groups: SF < 1000 µg/L with G-DAC conditioning (cohort 1), SF < 1000 µg/L with non-G-DAC conditioning (cohort 2), SF ⩾ 1000 µg/L with G-DAC conditioning (cohort 3), and SF ⩾ 1000 µg/L with non-G-DAC conditioning (cohort 4). The clinical features and prognosis of the four groups were analyzed.
Results:
Significant differences in the 2-year overall survival (OS), disease-free survival (DFS), and the cumulative incidence of non-relapse mortality (NRM) were observed between the four groups. Multivariate analysis revealed that SF ⩾ 1000 µg/L was a risk factor for OS, DFS, and NRM while G-DAC-containing conditioning was a protective factor. Intriguingly, when cohort 1 to cohort 4 were included in the multivariate analysis, only cohort 4 was a risk factor for OS, DFS, and NRM, cohort 3 had no difference in prognosis compared with patients with SF < 1000 µg/L.
Conclusion:
The poor prognosis of patients with iron overload may be overcome by G-DAC-containing conditioning partly.
Keywords: allo-hematopoietic stem cell transplantation, conditioning, iron overload, myelodysplastic syndrome
Introduction
Myelodysplastic syndrome (MDS) is characterized by clonal proliferation of hematopoietic stem cells (HSCs), ineffective hematopoiesis, peripheral blood cytopenia, and a high risk of progression to acute myeloid leukemia (AML). 1 Elevated serum ferritin (SF) is a common finding in MDS, a consequence of frequently administered red blood cell transfusions and/or ineffective erythropoiesis. 2 Generally, SF ⩾ 1000 µg/L is defined as iron overload. 3 Allo-hematopoietic stem cell transplantation (allo-HSCT) remains the only potentially curative option for high-risk MDS patients. However, iron overload has proven to be an independent, adverse prognostic factor for MDS patients undergoing allo-HSCT.4–6 Decreased overall survival (OS) in patients with high SF is attributable to increased post-transplantation non-relapse mortality (NRM), which may stem from post-transplant complications caused by iron overload, such as fungal infections, hepatic dysfunction, and hepatic veno-occlusive disease.6–9 In addition, iron overload is reported to be associated with relapse after transplantation in some studies.9,10
Hypomethylating agents (HMA) such as decitabine (DAC) are widely used in the treatment of MDS and AML. The combination of DAC, a cyclin-dependent demethylation drug, and granulocyte colony-stimulating factor (G-CSF), an agent that accelerates cell cycle entry, could synergistically promote the elimination of leukemic cells. 11 DAC combined with G-CSF-priming chemotherapy was reported to improve the prognosis of patients with MDS and AML.12–14 However, whether adding G-DAC to the conditioning regimen could improve the prognosis of MDS patients with iron overload undergoing allo-HSCT has not been reported. So in this study, we investigate whether a G-DAC-containing conditioning could improve survival in iron-overloaded patients.
Patients and methods
The retrospective study included patients diagnosed with MDS and sAML between April 2016 and June 2021 who underwent allo-HSCT at our institution. Patients were enrolled in this study if they met the following criteria: (1) older than 14 years old; (2) with available SF level at pre-transplant; (3) at the time of measurement, patients had to be in good clinical with normal C-reactive protein; (3) Haematopoietic Cell Transplantation-Comorbidity Index (HCT-CI) ⩽ 2. Patients were excluded from the study if they were: (1) diagnosed with therapy-related MDS or overlap syndromes, such as MDS/myeloproliferative neoplasms; (2) afflicted with uncontrolled infections.
Based on the level of SF and whether the conditioning regimen included G-DAC or not, the patients were divided into four groups: SF < 1000 µg/L with G-DAC conditioning (cohort 1), SF < 1000 µg/L with non-G-DAC conditioning (cohort 2), SF ⩾ 1000µg/L with G-DAC conditioning (cohort 3), and SF ⩾ 1000 µg/L with non-G-DAC conditioning (cohort 4).
Diagnoses were based on the World Health Organization (WHO) 2016 criteria. Risk stratification was categorized according to the Revised International Prognostic Scoring System for MDS. 15 Follow-up data came from medical records, telephone follow-ups, and Chinese Public Security Bureau records.
Treatment algorithm
Based on the clinical practice guidelines for MDS,16,17 patients with lower-risk MDS were treated with either immunoregulatory therapy or supportive care alone. If patients had transfusion dependence, they would be given HMA or allo-HSCT when appropriate donors existed. The patients with higher-risk MDS were treated with allo-HSCT if they were candidates for the treatment and had the appropriate donors, with or without pre-transplant cytoreductive treatments (including HMA, chemotherapy combined with HMA, and AML-like chemotherapy). If they were not suitable for HSCT, they would be given cytoreductive treatments or only supportive care according to their physical condition.
All transplant patients received busulfan-based myeloablation conditioning regimens. G-DAC-containing conditioning included G-DAC-BuCy (G-CSF, 5 µg/kg/day subcutaneously, days −17 to −10; DAC, 20 mg/m2/day intravenously, days −14 to −10; busulfan, 3.2 mg/kg/day intravenously, days −7 to −4; cyclophosphamide, 60 mg/kg/day intravenously, days −3 to −2) and G-DAC-BF (G-CSF, 5 µg/kg/day subcutaneously, days −17 to −10; DAC, 20 mg/m2/day intravenously, days −14 to −10; busulfan, 3.2 mg/kg/day intravenously, days −6 to −3; fludarabine, 30 mg/m2/day intravenously, days −7 to −3). Non-G-DAC-containing conditioning comprised the same dose and duration of BuCy as G-DAC-containing conditioning. The conditioning regimen was selected according to the clinical trial protocol for patients enrolled in the clinical trial, and according to the age and status of patients who did not. A human leukocyte antigen (HLA)-matched sibling donor (MSD) was the first choice, followed by an HLA-matched unrelated donor (MUD). If both donor types were unavailable, patients would receive transplantation from an HLA-haploidentical donor (HID). Generally, HID patients were transplanted with a combination of bone marrow (BM) and peripheral blood stem cell (PBSC) grafts, whereas MSD or MUD patients received PBSC grafts. For some older people with HID, 30 mL of umbilical cord blood stem cells were added to facilitate engraftment. A combination of cyclosporin A, methotrexate, and mycophenolate was administered to patients receiving a transplant from an MSD. Cyclosporin A, methotrexate, and antithymocyte immunoglobulin were administered to patients receiving a transplant from a MUD, and these in combination with mycophenolate were administered to patients receiving a transplant from an HID for graft-versus-host disease (GVHD) prophylaxis. Infection prophylaxis was described in our previous reports.14,18
Definitions and statistical analysis
This study mainly focused on OS, disease-free survival (DFS), NRM, and relapse. OS was calculated from the date of transplantation to either the date of death or, in surviving patients, the date of last follow-up. DFS was evaluated from the time of transplantation to the time of relapse or death or censored at the last follow-up. NRM was defined as death due to any cause without relapse. Relapse includes hematological relapse, cytogenetic relapse, and molecular relapse. Hematological relapse was defined as the presence of >5% marrow blasts and/or reappearance of major myelodysplastic features associated with cytopenia (or worsening of previous cytopenia). 19 The relapse of molecular and/or cellular genetics refers to the reappearance of cellular or molecular genetic abnormalities in patients who have previously achieved complete remission at the level of cellular or molecular genetics.
The chi-square or Fisher’s exact test was used for categorical variables and the Mann–Whitney U test was used for numerical variables. OS and DFS were estimated using the Kaplan–Meier method and compared using the log-rank test. Cumulative incidence of relapse (CIR), NRM, and GVHD were calculated by accounting for competing risks. Relapse and NRM were competing risks for each other. Competing risks for GVHD included death without GVHD and relapse. Multivariate analysis was performed using the Cox proportional hazards model. Only variables with p < 0.10 were included in the multivariate model. All statistical tests were two-sided, and p < 0.05 was considered statistically significant. We used SPSS v23.0 (IBM, Armonk, NY, USA) and R v4.0.3 (R Foundation, Vienna, Austria) for data analysis.
Results
Patients’ baseline and treatment characteristics
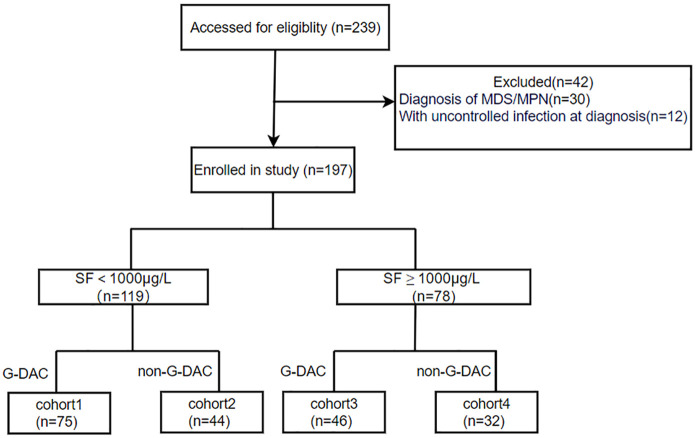
There were 197 patients enrolled in this study, with 119 patients having SF < 1000 µg/L and 78 patients with SF ⩾ 1000 µg/L. Of these, 121 patients received G-DAC conditioning and 76 received non-G-DAC conditioning. The entire cohort included 75 in cohort 1, 44 in cohort 2, 46 in cohort 3, and 32 in cohort 4. The study flow diagram is shown in Figure 1. Patients and transplant characteristics are detailed in Table 1, which shows no differences between cohorts 1 and 2, cohorts 3 and 4, as well as among the four groups (all p ⩾ 0.05).
Figure 1.
Flow diagram.
Table 1.
Patients’ baseline and transplant characteristics (n = 197).
| Variable | SF < 1000 µg/L | SF ⩾ 1000 µg/L | p | ||||
|---|---|---|---|---|---|---|---|
| G-DAC | Non-G-DAC | p1 | G-DAC | Non-G-DAC | p2 | ||
| Cohort 1 (n = 75) | Cohort 2 (n = 44) | Cohort 3 (n = 46) | Cohort 4 (n = 32) | ||||
| Age(years), median, range | 48 (16–67) | 43.5 (14–63) | 0.354 | 42.5 (23–66) | 48.5 (17–66) | 0.670 | 0.421 |
| Gender, n (%) | 0.851 | 0.058 | 0.050 | ||||
| Male | 41 (54.7) | 25 (56.8) | 24 (52.2) | 24 (75.0) | |||
| Female | 34 (45.3) | 19 (43.2) | 22 (47.8) | 8 (25.0) | |||
| Diagnosis according to WHO (2016), n (%) | 0.755 | 0.117 | 0.214 | ||||
| MDS-EB-1 | 42 (56.0) | 27 (61.4) | 23 (50.0) | 22 (68.8) | |||
| MDS-EB-2 | 18 (24.0) | 8 (18.2) | 6 (13.0) | 5 (15.6) | |||
| Secondary acute myeloid leukemia evolving from MDS | 15 (20.0) | 9 (20.5) | 17 (37.0) | 5 (15.6) | |||
| HCT-CI | 0.447 | 0.506 | 0.872 | ||||
| 0 | 45 (60.0) | 23 (52.3) | 27 (58.7) | 18 (56.3) | |||
| 1–2 | 30 (40.0) | 21 (47.7) | 19 (41.3) | 14 (43.7) | |||
| Performance score, n (%) | 0.451 | 0.495 | 0.533 | ||||
| 0 | 40 (53.3) | 20 (45.5) | 25 (54.3) | 20 (62.5) | |||
| 1–2 | 35 (46.7) | 24 (54.5) | 21 (45.7) | 12 (37.5) | |||
| Cytogenetic subgroups | 0.109 | 0.391 | 0.220 | ||||
| Good | 45 (60.0) | 29 (65.9) | 30 (65.2) | 18 (56.3) | |||
| Intermediate | 17 (22.7) | 3 (6.8) | 12 (26.1) | 8 (25.0) | |||
| Poor | 6 (8.0) | 4 (9.1) | 2 (4.3) | 1 (3.1) | |||
| Very poor | 7 (9.3) | 8 (18.2) | 2 (4.3) | 5 (15.6) | |||
| IPSS-R risk group, n (%) | 0.764 | 0.488 | 0.498 | ||||
| Low/intermediate | 21 (28.0) | 10 (22.7) | 9 (19.6) | 8 (25.0) | |||
| High | 26 (34.7) | 15 (34.1) | 19 (41.3) | 9 (28.1) | |||
| Very high | 28 (37.3) | 19 (43.2) | 18 (39.1) | 15 (46.9) | |||
| Pre-transplant treatment | 0.451 | 0.243 | 0.110 | ||||
| No cytoreductive treatments | 40 (53.3) | 26 (59.1) | 16 (34.8) | 16 (50.0) | |||
| Cytoreductive treatments | 35 (46.7) | 18 (40.9) | 30 (65.2) | 16 (50.0) | |||
| HMA | 15 (42.9) | 8 (44.4) | 11 (36.7) | 10 (62.5) | |||
| Chemotherapy combined with HMA | 19 (54.3) | 9 (50.0) | 15 (50.0) | 6 (37.5) | |||
| AML-like chemotherapy | 1 (2.9) | 1 (5.6) | 4 (13.3) | 0 (0.0) | |||
| Time between diagnosis and HSCT | 0.902 | 0.155 | 0.181 | ||||
| ⩽6 months | 57 (76.0) | 33 (75.0) | 27 (58.7) | 24 (75.0) | |||
| >6 months | 18 (24.0) | 11 (25.0) | 19 (41.3) | 8 (25.0) | |||
| BM blast at transplantation (%), median, range | 7 (0–41.5) | 6 (0–27.0) | 0.978 | 6 (0–37.5) | 6.5 (0–38.0) | 0.200 | 0.353 |
| Remission status at transplant, n (%) | 0.562 | 0.812 | 0.896 | ||||
| CR | 30 (40.0) | 15 (34.1) | 18 (39.1) | 11 (34.3) | |||
| No CR | 45 (60.0) | 29 (65.9) | 28 (60.9) | 21 (65.6) | |||
| Transplant modality | 0.226 | 0.056 | 0.101 | ||||
| MSD | 32 (42.7) | 21 (47.7) | 28 (60.9) | 11 (34.4) | |||
| HID | 41 (54.7) | 19 (43.2) | 16 (34.8) | 17 (53.1) | |||
| MUD | 2 (2.7) | 4 (9.1) | 2 (4.3) | 4 (12.5) | |||
| Stem cell source | 0.573 | 0.214 | 0.513 | ||||
| PB + BM | 30 (40.0) | 20 (45.5) | 16 (34.8) | 13 (40.6) | |||
| PB | 27 (36.0) | 17 (38.6) | 20 (43.5) | 8 (25.0) | |||
| PB + UCB | 18 (24.0) | 7 (15.9) | 10 (21.7) | 11 (34.4) | |||
p, p value between cohorts 1, 2, 3, and 4; p1, p value between cohort 1 and cohort 2; p2, p value between cohort 3 and cohort 4.
AML, acute myeloid leukemia; BM, bone marrow; UCB, Umbilical Cord Blood; CR, complete remission; MDS, myelodysplastic syndrome; MDS-EB, MDS with excess of blasts; HMA, hypomethylating agents; HID, haploidentical donor; HLA-MSD, human leukocyte antigen–matched sibling donor; IPSS-R, Revised International Prognostic Scoring System; MUD, matched unrelated donor; PB, peripheral blood.
Engraftment and GVHD
All patients achieved hematopoietic reconstitution, except for 1 patient in cohort 4 who died of intracranial hemorrhage. Of the 196 evaluable patients, the 30-day incidence of neutrophil engraftment was 98.3% (95% CI: 95.8–99.6) and 98.7% (95% CI: 95.5–99.9) in patients with SF < 1000 µg/L and SF ⩾ 1000 µg/L, respectively (p = 0.338), and it was 98.3% (95% CI: 95.8–99.6) and 98.7% (95% CI: 95.3–99.9) in the G-DAC and non-G-DAC groups, respectively (p = 0.410). The 30-day incidence of neutrophil engraftment was 98.6% (95% CI: 95.3–99.9), 97.7% (95% CI: 95.2–99.9), 97.8% (95% CI: 92.4–99.9), and 90.1% (95% CI: 80.0–99.9) in cohorts1, 2, 3, and 4, respectively (p = 0.527). The 30-day incidence of platelet engraftment was 92.4% (95% CI: 87.5–97.3) and 88.3% (95% CI: 80.9–95.7) in patients with SF < 1000 µg/L and SF ⩾ 1000 µg/L, respectively (p = 0.038), and it was 95.0% (95% CI: 91.0–99.0) and 84.0% (95% CI: 75.5–92.5) in the G-DAC and non-G-DAC groups, respectively (p = 0.042). The 30-day incidence of platelet engraftment was 96.0% (95% CI: 91.2–99.9), 86.3% (95% CI: 76.3–96.8), 93.4% (95% CI: 91.7–99.9), and 78.1% (95% CI: 63.1–93.1) in cohorts 1, 2, 3, and 4, respectively (p = 0.035). Cohort 4 had lower incidence of platelet engraftment than those in cohort 1 to cohort 3 (p = 0.008, p = 0.039, and p = 0.018, respectively), while it was no significantly different between cohort 1 and cohort 2; cohort 1 and cohort 3; cohort 2 and cohort 3 (p = 0.407, p = 0.386, p = 0.295, respectively).
The cumulative incidence of grade II–IV acute GVHD (aGVHD) at day +100 was 38.2% (95% CI: 29.3–47.1) in the SF < 1000 µg/L group and 45.3% (95% CI: 35.0–55.6) in the SF ⩾ 1000 µg/L group, respectively (p = 0.034). And it was 39.5% (95% CI: 35.0–44.0) and 46.2% (95% CI: 45.5–65.8) in the G-DAC and non-G-DAC groups, respectively (p = 0.046). Additionally, it was 36.4% (95% CI: 21.9–50.8), 40.6% (95% CI: 26.3–52.0), 41.5% (95% CI: 26.2–56.8), and 52.4% (95% CI: 34.9–69.9) in cohorts 1, 2, 3, and 4, respectively (p = 0.041). The cohort 4 had higher incidence of grade II–IV aGVHD at day +100 than those in cohort 1 to cohort 3 (p = 0.009, p = 0.014, and p = 0.040, respectively), while it was no significantly different between cohort 1 and cohort 2; cohort 1 and cohort 3; cohort 2 and cohort 3 (p = 0.317, p = 0.521, p = 0.128, respectively). To investigate the impact of SF and conditioning regimens on engraftment and aGVHD, we conducted a Cox analysis. The results indicate that SF ⩾ 1000 µg/L was an unfavorable risk factor for platelet engraftment (hazard ratio (HR): 1.362, p = 0.007) and aGVHD (HR: 2.747, p = 0.027), whereas receipt of G-DAC conditioning served as a protective factor for platelet engraftment (HR: 0.529, p = 0.032) and aGVHD (HR: 0.694, p = 0.019; Supplemental Table 1). Eighty-three of 166 patients surviving more than 100 days developed chronic GVHD (cGVHD). The overall cumulative incidence of cGVHD at 2 years did not differ significantly between the four groups (cohort 1: 44.6%, 95% CI: 32.6–56.6; cohort 2: 42.9%, 95% CI: 26.2–59.5; cohort 3: 43.6%, 95% CI: 27.8–59.4; and cohort 4: 47.2%, 95% CI: 31.1–63.3; p = 0.591)
Infections
The overall incidence of bacterial infections after allo-HSCT was similar between the four groups (74.7% vs 63.6% vs 80.4% vs 78.1%, p = 0.296). Moreover, there was also no increased incidence of post-transplant fungal infections between the four groups (10.7% vs 22.7% vs 13.0% vs 18.8%, p = 0.304).
OS and DFS
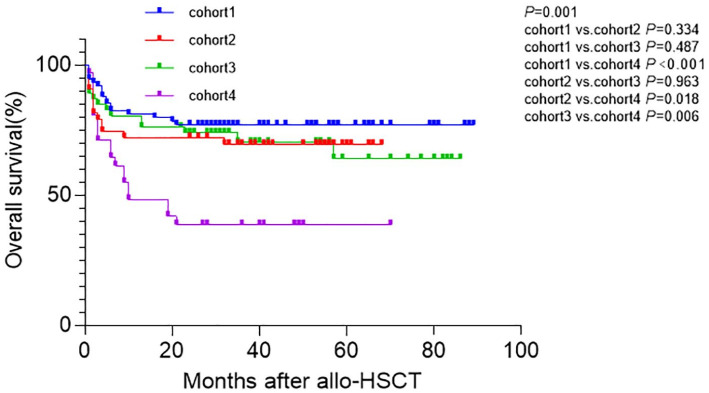
In the entire cohort, the 2-year OS for patients with SF < 1000 µg/L and SF ⩾ 1000 µg/L were 73.9% (95% CI: 66.1–81.7) and 58.9% (95% CI: 47.9–69.9), respectively (p = 0.022). The 2-year OS for patients receiving G-DAC and non-G-DAC conditioning was 75.2% (95% CI: 67.6–82.8) and 56.6% (95% CI: 45.4–67.8), respectively (p = 0.006). In all patients, 66 patients died during follow-up, including 18, 14, 14, and 20 patients in cohort 1, cohort 2, cohort 3, and cohort 4 respectively. Two-year OS post-transplantation in cohort 1, cohort 2, cohort 3, cohort 4 were 77.0% (95% CI: 66.4–85.6), 72.1% (95% CI: 57.0–84.0), 73.8% (95% CI: 61.1–86.5), and 38.7% (95% CI: 20.6–54.4), respectively (p = 0.001). The 2-year OS in cohort 4 was significantly shorter than those in cohort 1, cohort 2, and cohort 3 (p < 0.001, p = 0.018, and p = 0.006, respectively), while it was similar between cohort 1 and cohort 2; cohort 1 and cohort 3; cohort 2 and cohort 3 (p = 0.334, p = 0.487, p = 0.963, respectively; Figure 2).
Figure 2.
OS between the four cohorts. Two-year OS post-transplantation in cohort 1, cohort 2, cohort 3, cohort 4 were 77.0% (95% CI: 66.4–85.6), 72.1% (95% CI: 57.0–84.0%), 73.8% (95% CI: 61.1–86.5), and 38.7% (95% CI: 20.6–54.4), respectively (p = 0.001). The 2-year OS in cohort 4 was significantly shorter than those in cohort 1, cohort 2, and cohort 3 (p < 0.001, p = 0.018, p = 0.006, respectively), while it was similar between cohort 1 and cohort 2; cohort 1 and cohort 3; cohort 2 and cohort 3 (p = 0.334, p = 0.487, p = 0.963, respectively.
OS, overall survival.
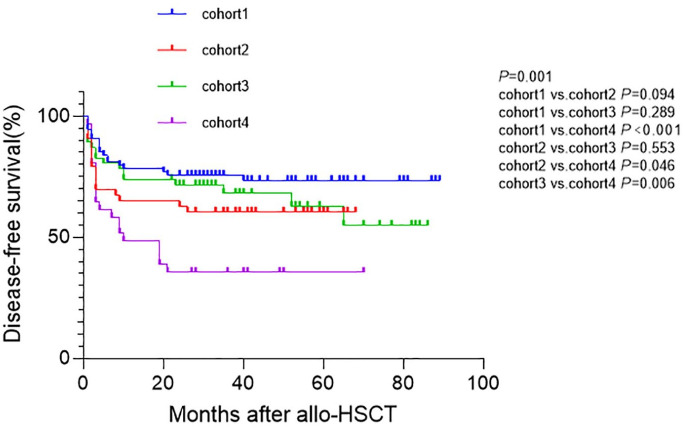
Similarly, the 2-year DFS for patients with SF < 1000 µg/L and SF ⩾ 1000 µg/L were 69.7% (95% CI: 61.4–77.9) and 56.4% (95% CI: 45.4–63.4), respectively (p = 0.039). The 2-year DFS for patients receiving G-DAC and non-G-DAC conditioning were 73.5% (95% CI: 65.7–81.3) and 50.0% (95% CI: 38.3–61.2), respectively (p = 0.001). Two-year DFS post-transplantation in cohorts1, 2, 3, and 4 were 75.7% (95% CI: 64.9–84.5), 62.8% (95% CI: 46.7–75.7), 71.7% (95% CI: 58.6–84.8), and 35.5% (95% CI: 17.9–50.9), respectively (p = 0.001). The 2-year DFS in cohort 4 was shorter than those in cohort 1, cohort 2, and cohort 3 (p < 0.001, p = 0.046, p = 0.006, respectively), while it was similar between cohort 1 and cohort 2; cohort 1 and cohort 3; cohort 2 and cohort 3 (p = 0.094, p = 0.289, p = 0.553, respectively; Figure 3).
Figure 3.
DFS between the four cohorts. Two-year DFS post-transplantation in cohort 1, cohort 2, cohort 3, cohort 4 were 75.7% (95% CI: 64.9–84.5), 62.8% (95% CI: 46.7–75.7), 71.7% (95% CI: 58.6–84.8), and 35.5% (95% CI: 17.9–50.9), respectively (p = 0.001). The 2-year DFS in cohort 4 was shorter than those in cohort 1, cohort 2, and cohort 3 (p < 0.001, p = 0.046, p = 0.006, respectively), while it was similar between cohort 1 and cohort 2; cohort 1 and cohort 3; cohort 2 and cohort 3 (p = 0.094, p = 0.289, p = 0.553, respectively).
DFS, disease-free survival.
In multivariate analysis, SF ⩾ 1000 µg/L and complex karyotype were unfavorable risk factors for OS (HR: 1.777, p = 0.022 and HR: 2.316; p = 0.005, respectively) and DFS (HR: 1.687, p = 0.025 and HR: 2.381, p = 0.002, respectively), while receiving G-DAC conditioning was a protective factor for OS (HR: 0.522, p = 0.009) and DFS (HR: 0.500, p = 0.003; Table 2). To further understand the combined effect in these patients, we included groups in the multivariate analysis but did not include SF and conditioning. Multivariate analysis revealed that complex karyotype (OS—HR: 2.206, p = 0.010; DFS—HR: 2.381, p = 0.002) and cohort 4 (OS—HR: 3.806, p = 0.000; HR: 2.539, p = 0.012; HR: 2.542, p = 0.012; DFS—HR: 3.673, p = 0.000; HR: 1.753, p = 0.049; HR: 2.466, p = 0.011) were unfavorable risk factors for OS and DFS (Table 3).
Table 2.
Univariate/multivariate analysis of outcomes by serum ferritin and conditioning.
| Variable | OS | DFS | NRM | Relapse | ||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multicariate | Univariate | Multivariate | Univariate | Multicariate | Univariate | Multivariate | |
| p | p | p | p | |||||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||||
| Gender, male vs female | 0.273 0.758 (0.463–1.243) |
— | 0.420 0.828 (0.523–1.311) |
— | 0.071 0.577 (0.318–1.049) |
0.135 1.594(0.865–2.938) |
0.261 1.563 (0.718–3.403) |
— |
| Age | 0.584 1.006 (0.986–1.026) |
— | 0.809 1.002 (0.984–1.021) |
— | 0.912 0.999 (0.976–1.022) |
— | 0.570 1.010 (0.977–1.043) |
— |
| Cytogenic, noncomplex vs complex karyotypes | 0.008 2.137 (1.201–3.804) |
0.005 2.316 (1.291–4.156) |
0.004 2.234 (1.299–3.842) |
0.002 2.381 (1.375–4.121) |
0.183 1.637 (0.793–3.378) |
— | 0.002 3.714 (1.610–8.566) |
0.001 4.204(1.809–9.769) |
| Serum ferritin, <1000 vs ⩾1000 (µg/L) | 0.038 1.734 (1.069–2.811) |
0.022 1.777 (1.087–2.905) |
0.045 1.589 (1.010–2.499) |
0.025 1.687 (1.067–2.667) |
0.077 1.658 (0.946–2.903) |
0.036 1.972 (1.004–3.231) |
0.333 1.465 (0.677–3.173) |
— |
| Previous therapy for MDS, no cytoreductive treatments vs cytoreductive treatments | 0.847 1.049 (0.647–1.699) |
— | 0.559 1.145 (0.727–1.803) |
— | 0.506 0.826 (0.470–1.450) |
— | 0.062 2.210 (0.960–5.089) |
0.032 2.505 (1.080–5.807) |
| HLA type | ||||||||
| MUD | ref | ref | ref | ref | ||||
| MSD | 0.918 1.056 (0.373–2.987) |
— | 0.716 1.211 (0.431–3.403) |
— | 0.988 0.991 (0.296–3.310) |
— | 0.545 1.872 (0.246–14.243) |
— |
| HID | 0.980 1.013 (0.357–2.877) |
— | 0.734 1.197 (0.425–3.367) |
— | 0.893 1.086 (0.327–3.607) |
— | 0.686 1.525 (0.197–11.818) |
— |
| Stem cell source | ||||||||
| PB + BM | ref | ref | ref | ref | ||||
| PB | 0.962 1.013 (0.591–1.737) |
— | 0.595 0.871 (0.522–1.451) |
— | 0.486 0.802 (0.431–1.493) |
— | 0.939 1.036 (0.421–2.550) |
— |
| PB + BM + UCB | 0.754 0.900 (0.465–1.740) |
— | 0.672 0.877 (0.476–1.613) |
— | 0.269 0.637 (0.286–1.418) |
— | 0.402 1.522 (0.570–4.061) |
— |
| Time between diagnosis and HSCT, <6 vs ⩾6 months | 0.246 0.711 (0.400–1.265) |
— | 0.317 0.763 (0.449–1.296) |
— | 0.319 0.711 (0.363–1.391) |
— | 0.739 0.863 (0.362–2.055) |
— |
| Conditioning, non-G-DAC group vs G-DAC group | 0.007 0.509 (0.313–0.829) |
0.009 0.522 (0.320–0.851) |
0.002 0.490 (0.311–0.773) |
0.003 0.500 (0.317–0.789) |
0.003 0.420 (0.238–0.741) |
0.004 0.436 (0.247–0.770) |
0.293 0.657 (0.300–1.437) |
— |
BM, bone marrow; UCB, UCB, Umbilical Cord Blood; DAC, decitabine; DFS, disease-free survival; HID, haploidentical donor; HLA-MSD, human leukocyte antigen–matched sibling donor; HR, hazard ratio; HSCT, hematopoietic stem cell transplantation; MDS, myelodysplastic syndrome; MUD, matched unrelated donor; NRM, non-relapse mortality; OS, overall survival; PB, peripheral blood.
Table 3.
Univariate/multivariate analysis of outcomes by groups.
| Variable | OS | DFS | NRM | Relapse | ||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multicariate | Univariate | Multicariate | Univariate | Multicariate | Univariate | Multicariate | |
| p | p | p | p | |||||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||||
| Gender, male vs female | 0.273 0.758 (0.463–1.243) |
— | 0.420 0.828 (0.523–1.311) |
— | 0.071 0.577 (0.318–1.049) |
0.162 1.365 (0.741–2.935) |
0.261 1.563 (0.718–3.403) |
— |
| Age | 0.584 1.006 (0.986–1.026) |
— | 0.809 1.002 (0.984–1.021) |
— | 0.912 0.999 (0.976–1.022) |
— | 0.570 1.010 (0.977–1.043) |
— |
| Cytogenic, noncomplex vs complex karyotypes | 0.008 2.137 (1.201–3.804) |
0.010 2.206 (1.210–4.022) |
0.004 2.234 (1.299–3.842) |
0.002 2.381 (1.385–4.309) |
0.183 1.637 (0.793–3.378) |
— | 0.002 3.714 (1.610–8.566) |
0.000 5.936 (2.257–9.617) |
| Previous therapy for MDS, no cytoreductive treatments vs cytoreductive treatments | 0.847 1.049 (0.647–1.699) |
— | 0.559 1.145 (0.727–1.803) |
— | 0.506 0.826 (0.470–1.450) |
— | 0.062 2.210 (0.960–5.089) |
0.017 3.147 (1.229–8.055) |
| HLA type | ||||||||
| MUD | ref | ref | ref | ref | ||||
| MSD | 0.918 1.056 (0.373–2.987) |
— | 0.716 1.211 (0.431–3.403) |
— | 0.988 0.991 (0.296–3.310) |
— | 0.545 1.872 (0.246–14.243) |
— |
| HID | 0.980 1.013 (0.357–2.877) |
— | 0.734 1.197 (0.425–3.367) |
— | 0.893 1.086 (0.327–3.607) |
— | 0.686 1.525 (0.197–11.818) |
— |
| Stem cell source | ||||||||
| PB + BM | ref | ref | ref | ref | ||||
| PB | 0.962 1.013 (0.591–1.737) |
— | 0.595 0.871 (0.522–1.451) |
— | 0.486 0.802 (0.431–1.493) |
— | 0.939 1.036 (0.421–2.550) |
— |
| PB + BM + UCB | 0.754 0.900 (0.465–1.740) |
— | 0.672 0.877 (0.476–1.613) |
— | 0.269 0.637 (0.286–1.418) |
— | 0.402 1.522 (0.570–4.061) |
— |
| Time between diagnosis and HSCT, <6 vs ⩾6 months | 0.246 0.711 (0.400–1.265) |
— | 0.317 0.763 (0.449–1.296) |
— | 0.319 0.711 (0.363–1.391) |
— | 0.739 0.863 (0.362–2.055) |
— |
| Group | ||||||||
| Cohort 2 vs cohort 1 | 0.312 1.434 (0.713–2.885) |
— | 0.101 1.721 (0.910–3.255) |
— | 0.265 1.580 (0.707–1.218) |
— | 0.198 1.991 (0.698–5.680) |
— |
| Cohort 3 vs cohort 1 | 0.463 1.299 (0.646–2.611) |
— | 0.370 1.351 (0.700–2.608) |
— | 0.960 1.023 (0.424–2.468) |
— | 0.189 1.975 (0.716–5.448) |
— |
| Cohort 4 vs cohort 1 | 0.000 3.259 (1.718–6.182) |
0.000 3.806 (1.932–7.498) |
0.000 3.164 (1.707–5.863) |
0.000 3.673 (1.910–7.064) |
0.000 3.870 (1.870–8.009) |
0.000 3.910 (1.820–8.399) |
0.347 1.809 (0.525–6.226) |
— |
| Cohort 3 vs cohort 2 | 0.811 0.914 (0.435–1.919) |
— | 0.457 0.774 (0.394–1.521) |
— | 0.400 0.676 (0.272–1.682) |
— | 0.867 0.917 (0.331–2.541) |
— |
| Cohort 4 vs cohort 2 | 0.018 2.817 (1.101–4.347) |
0.012 2.539 (1.223–5.272) |
0.046 1.729 (0.924–3.271) |
0.049 1.753 (1.004–3.897) |
0.028 2.245 (1.050–4.802) |
0.026 2.495 (1.113–5.596) |
0.873 0.904 (0.263–3,110) |
— |
| Cohort 4 vs cohort 3 | 0.006 2.657 (1.328–5.319) |
0.012 2.542 (1.225–5.273) |
0.006 2.552 (1.312–4.962) |
0.011 2.466 (1.230–4.945) |
0.002 3.625 (1.556–8.442) |
0.009 3.260 (1.344–7.910) |
0.752 1.220 (0.355–4.187) |
— |
BM, bone marrow; CB, cord blood; DFS, disease-free survival; HID, haploidentical donor; HLA-MSD, human leukocyte antigen–matched sibling donor; HR, hazard ratio; HSCT, hematopoietic stem cell transplantation; MDS, myelodysplastic syndrome; MUD, matched unrelated donor; NRM, non-relapse mortality; OS, overall survival; PB, peripheral blood.
Non-relapse mortality
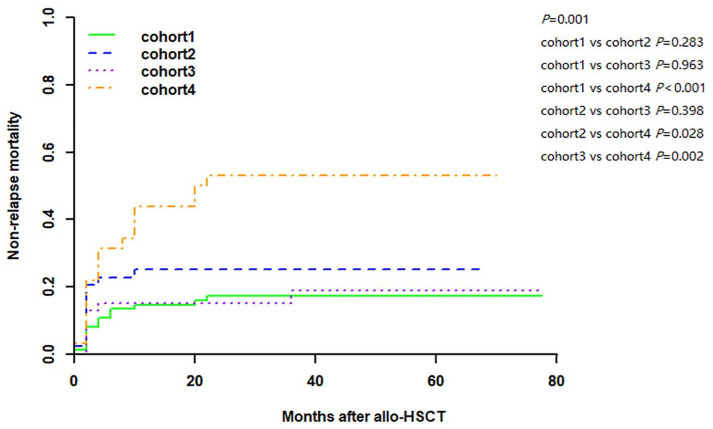
In the entire cohort, the 2-year NRM for patients with SF < 1000 µg/L and SF ⩾ 1000 µg/L were 20.2% (95% CI: 13.0–27.4) and 30.8% (95% CI: 20.5–41.1), respectively (p = 0.046). The 2-year NRM for patients receiving G-DAC and non-G-DAC conditioning was 16.5% (95% CI: 9.8–23.2) and 36.8% (95% CI: 25.9–47.7), respectively (p = 0.002). Of the 197 patients, 66 patients died including 17 patients who died of relapse and 49 patients who died without relapse, and patients had a cumulative incidence of 2-year NRM of 17.3% (95% CI: 8.7–26.0), 25.0% (95% CI: 12.1–37.9), 15.2% (95% CI: 4.7–25.7), and 53.1% (95% CI: 35.5–70.7) in cohort 1, cohort 2, cohort 3, and cohort 4, respectively (p = 0.001). Cohort 4 had a higher NRM than cohort 1, cohort 2, and cohort 3 (p < 0.001, p = 0.028, and p = 0.002, respectively), while it was similar between cohort 1 and cohort 2; cohort 1 and cohort 3; cohort 2 and cohort 3 (p = 0.283, p = 0.963, p = 0.398, respectively; Figure 4).
Figure 4.
Cumulative incidence of non-relapse mortality. Patients had a cumulative incidence of 2-year NRM of 17.3% (95% CI: 8.7–26.0), 25.0% (95% CI: 12.1–37.9), 15.2% (95% CI: 4.7–25.7), and 53.1% (95% CI: 35.5–70.7) in cohort 1, cohort 2, cohort 3, and cohort 4, respectively (p = 0.001). Cohort 4 had a higher NRM than cohort 1, cohort 2, and cohort 3 (p < 0.001, p = 0.028, p = 0.002, respectively), while it was similar between the cohort 1 and cohort 2; cohort 1 and cohort 3; cohort 2 and cohort 3 (p = 0.283, p = 0.963, p = 0.398, respectively).
NRM, non-relapse mortality.
In multivariate analysis by SF and conditioning, SF ⩾ 1000 µg/L was an unfavorable risk factor for NRM (HR: 1.972, p = 0.036), and receiving G-DAC conditioning was a protective factor for NRM (HR: 0.436, p = 0.004; Table 2). In multivariate analysis by groups, cohort 4 was an unfavorable risk factor for NRM (HR: 3.910, p = 0.000; HR: 2.495, p = 0.026; HR: 3.260, p = 0.009; Table 3). In addition, to understand the potential factors of higher NRM in cohort 4, the causes of NRM were analyzed. For all the patients, a descriptive analysis of the cause of death was given in Table 4. Most patients succumbed to infections and patients in cohort 4 had a higher mortality of GVHD-related than other cohorts.
Table 4.
Causes of non-relapse mortality in different groups.
| Variable | Cohort 1 (n = 75) | Cohort 2 (n = 44) | Cohort 3 (n = 46) | Cohort 4 (n = 32) | p |
|---|---|---|---|---|---|
| GVHD-related, N (%) | 2 (2.7) | 2 (4.5) | 3 (6.5) | 7 (21.9) | 0.008 |
| Infections, N (%) | 8 (10.7) | 7 (15.9) | 4 (8.7) | 9 (28.1) | 0.089 |
| Hemorrhage, N (%) | 0 (0.0) | 1 (2.3) | 0 (0.0) | 1 (3.1) | 0.201 |
| TMA, N (%) | 1 (1.3) | 0 (0.0) | 1 (2.2) | 0 (0.0) | 0.689 |
| Unknown, N (%) | 2 (2.7) | 1 (2.3) | 0 (0.0) | 0 (0.0) | 0.777 |
GVHD, graft-versus-host disease; TMA, thrombotic microangiopathy.
Relapse
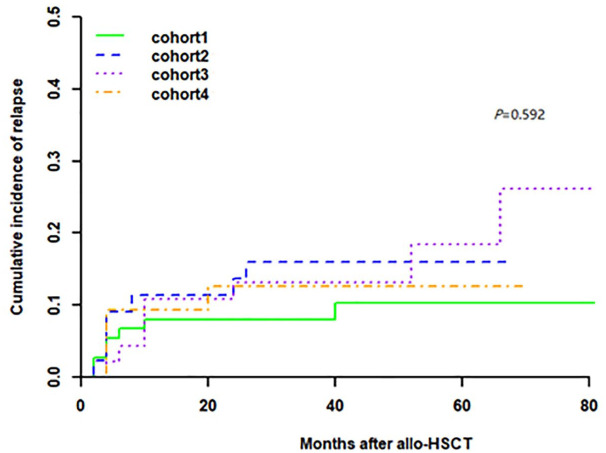
In the entire cohort, the 2-year relapse rate for patients with SF < 1000 µg/L and SF ⩾ 1000 µg/L were 10.1% (95% CI: 4.6–15.5) and 12.9% (95% CI: 5.4–20.3%), respectively (p = 0.466). The 2-year relapse rate for patients receiving G-DAC and non-G-DAC conditioning was 9.9% (95% CI: 4.6–15.3) and 13.2% (95% CI: 5.5–20.8), respectively (p = 0.585). By the time of follow-up, 26 patients experienced relapse at a median time of 5.5 (range: 2.0–65.0) months post-transplantation, including 7 (9.3%), 7 (15.9%), 8 (17.4%), 4 (12.5%) patients in cohort 1, cohort 2, cohort 3, and cohort 4 groups, respectively. There was no difference in 2-year CIR post-transplantation in the four groups (cohort 1: 8%, 95% CI: 1.8–14.2; cohort 2: 13.6%, 95% CI: 3.4–23.9; cohort 3:13.1%, 95% CI: 3.2–23.0; cohort 4: 12.5%, 95% CI: 8.1–24.2, p = 0.592; Figure 5).
Figure 5.
Cumulative incidence of relapse. There was no difference in 2-year CIR post-transplantation in the four groups (cohort 1: 8%, 95% CI: 1.8–14.2; cohort 2: 13.6%, 95% CI: 3.4–23.9; cohort 3:13.1%, 95% CI: 3.2–23.0; cohort 4: 12.5%, 95% CI: 8.1–24.2, p = 0.592).
In multivariate analysis, complex karyotype and receiving cytoreductive treatments before allo-HSCT were risk factors for relapse whether enrolled in SF and conditioning (HR: 4.204, p = 0.001 and HR: 2.505, p = 0.032, respectively; Table 2) or groups (HR: 5.936, p = 0.000 and HR: 3.147, p = 0.017, respectively; Table 3).
Discussion
In this retrospective study of 197 patients enrolled, 119 patients had SF < 1000 µg/L and 78 patients with SF ⩾ 1000 µg/L. Of these, 121 patients received G-DAC conditioning and 76 received non-G-DAC conditioning. We found that iron overload was a significant contributor to NRM for MDS patients undergoing allo-HSCT. SF ⩾ 1000µg/L may be associated with inferior OS and DFS, which were consistent with previous studies.4,6,9,20,21 In addition, we found that G-DAC-containing conditioning could reduce NRM of MDS patients with iron overload. The improvement in NRM translated into a survival advantage. To the best of our knowledge, this is the first study reported to date that has analyzed the impact of G-DAC-containing conditioning on MDS patients with iron overload undergoing allo-HSCT.
Decreased OS in MDS patients with iron overload was attributable to increased NRM. How can iron overload potentially mediate NRM in the post-transplant period? In fact, increased oxidative stress caused by reactive iron species as well as consecutive and permanent tissue damage has been proposed as a possible reason for the association between iron overload and aGVHD by some researchers.5,22,23 Our data showed that patients with SF ⩾ 1000 µg/L had a higher cumulative incidence of aGVHD, as well as being an unfavorable risk factor for aGVHD, in agreement with previous research,5,24 which may play an important role in increased oxidative stress and higher NRM. Besides, oxidative stress plays an important role in the mechanism of iron overload, affecting not only HSCs but also the stromal component of the hematopoietic niche.25–28 According to our data, patients with SF ⩾ 1000 µg/L had a lower incidence of platelet engraftment, and iron overload was an unfavorable risk factor for platelet engraftment, which was also observed in basic research.29,30 Abnormal BM microenvironment and altered function of BM mesenchymal stromal cells might be implicated in delayed platelet engraftment. Apart from causing oxidative stress, iron is also an essential cofactor for the growth of a number of opportunistic bacteria and fungi, and free iron could increase the susceptibility to infections by impairing cellular immunity and inhibiting chemotaxis and phagocytosis,31–34 and therefore it is intriguing to speculate that infections may be more common in patients with iron overload. However, in contrast to previously published studies,5,6,9 no increased incidence of post-transplant infections was observed in our results. We think one possible reason for these conflicting results is the difference in sample size and patients’ characteristics between these earlier studies and the current study. Another possible reason is that with wide applications of antibacterial and antifungal drugs in the prophylaxis and therapy of infections, the incidence and mortality of bacterial and fungal infections post-transplantation decrease markedly. Based on these considerations, it is understandable that MDS patients with iron overload had a higher NRM.
Second, and most importantly we could show that G-DAC-containing conditioning may have a significant impact on post-transplant outcome of MDS patients with iron overload. Our data shows that G-DAC-containing conditioning was associated with superior OS, DFS, and lower NRM compared to those with non-G-DAC conditioning. In the subgroup analysis of patients with SF ⩾ 1000 µg/L, specifically cohorts 3 and 4, the addition of G-DAC conditioning in cohort 3 improved the prognosis of the patients in this group, eliminating statistical differences in OS, DFS, and NRM between them and those with SF < 1000 µg/L. In contrast, iron-overloaded MDS patients with non-G-DAC conditioning (cohort 4) had worse OS, DFS, and NRM than the other three groups, which may imply that the prognosis of patients with iron overload could be improved with the use of G-DAC-containing conditioning. To our knowledge, this is the first retrospective report that demonstrates the impact of G-DAC-containing conditioning on MDS patients with iron overload.
Why G-DAC-containing conditioning affected the survival of these patients remains unclear. The Nrf2/ARE signaling pathway is a cell survival response pathway in response to environmental stresses. The study by Chen et al. 35 showed that hydrogen peroxide (H2O2) could activate the Nrf2/ARE signaling pathway in zebrafish and it could inhibit the expression of methyltransferase. Besides, it showed that there was a synergistic effect between H2O2 and methyltransferase inhibitors. As we all know, H2O2 is one of the vital components in oxidative stress, so the iron-overloaded patients undergoing G-DAC-containing conditioning may benefit from the synergistic effect of H2O2 produced by oxidative stress in patients with iron overload and DAC. Similarly, Mpakou et al. 36 reported that DAC acted synergistically with bortezomib, an oxidative stress-inducing agent in Kasumi-1 AML cells in vitro. These results provide an explanation that the improvement in survival in patients with iron overload undergoing G-DAC-containing conditioning may result from a synergistic effect of DAC with certain substances in a pathway or certain drug. Our study found that G-DAC-containing conditioning could reduce the incidence of grades II–IV aGVHD. Previous studies have shown that G-CSF can reduce the incidence of aGVHD, which may be related to the immunoregulatory effects of G-CSF on T-cells.37–39 Additionally, DAC was also found to decrease the incidence of GVHD40,41 that is likely due to increased numbers of regulatory T-cells. In patients with SF ⩾ 1000µg/L, cohort 4 had a higher mortality rate related to GVHD compared to cohort 3. In other words, cohort 3 had a lower NRM, which may be because they benefited from a lower incidence of GVHD mediated by G-DAC. As previously discussed, patients with iron overload have an increased incidence of aGVHD. Therefore, G-DAC-containing conditioning may improve the prognosis of iron-overloaded patients by reducing the aGVHD. Moreover, our data showed that G-DAC-conditioning had a higher incidence of platelet engraftment, which is in line with previous study, 14 and might be related to the improvement of BM microenvironment by DAC, such as megakaryocytes, endothelial cells, and cytokines associated with megakaryocytes migration and endothelial cell injury, 42 which could also be a reason for a lower NRM in patients with G-DAC conditioning. In summary, these factors may all contribute to the better prognosis in iron-overloaded patients undergoing G-DAC conditioning compared to those without.
Our results showed that G-DAC-containing conditioning reduced NRM, while previous studies involving G-DAC or G-CSF primarily focused on reducing relapse mortality.14,43,44 Comparatively, our study included a relatively higher proportion of elderly patients, which might explain the higher NRM in our study compared to the prospective ones. It is also noteworthy to mention that DAC could ameliorate primary iron overload in MDS in a previous study. 45 Combined with the aforementioned finding that patients with SF ⩾ 1000µg/L have a lower incidence of platelet engraftment and a higher incidence of GVHD, and the role of DAC and G-CSF in reducing GVHD and promote platelet engraftment, the survival benefit observed in our patients is attributed to the G-DAC regimen’s ability to reduce NRM. First and foremost, the major limitation of this study was that it is a retrospective analysis. Therefore, further prospective multicenter studies will be required to validate our findings in the future. Besides, although most iron-overloaded patients were administered with iron chelation therapy, the initial time of treatment, course of treatment, and dosage of drugs may vary a lot in clinical practice. However, we did not bring these factors into the analysis. In addition, the mechanistic evidence will also need to be validated in future studies.
Conclusion
Iron overload at pre-transplantation was an independent unfavorable risk factor for OS, DFS, and NRM in MDS patients undergoing allo-HSCT. In addition, G-DAC-containing conditioning might be important to improve survival by reducing NRM for MDS patients with iron overload undergoing allo-HSCT.
Supplemental Material
Supplemental material, sj-doc-1-tah-10.1177_20406207241292451 for The impact of granulocyte colony-stimulating factor and decitabine-containing conditioning in myelodysplastic syndrome patients with iron overload undergoing allogeneic hematopoietic stem cell transplantation: a retrospective study by Wenshu Zhao, Xiangzong Zeng, Danqi Pan, Li Xuan, Zhiping Fan, Fen Huang, Na Xu, Jing Sun, Qifa Liu and Min Dai in Therapeutic Advances in Hematology
Acknowledgments
We sincerely thank all colleagues in the Department of Hematology, Nanfang Hospital, for their help in this study.
Footnotes
ORCID iDs: Wenshu Zhao  https://orcid.org/0000-0001-7451-9022
https://orcid.org/0000-0001-7451-9022
Xiangzong Zeng  https://orcid.org/0000-0001-6759-9909
https://orcid.org/0000-0001-6759-9909
Danqi Pan  https://orcid.org/0000-0002-8222-9700
https://orcid.org/0000-0002-8222-9700
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Wenshu Zhao, Department of Hematology, Nanfang Hospital, Southern Medical University, Guangzhou, China.
Xiangzong Zeng, Department of Hematology, The Affiliated Qingyuan Hospital (Qingyuan People’s Hospital), Guangzhou Medical University, Guangzhou, China.
Danqi Pan, Department of Hematology, The First Affiliated Hospital of Jinan University, Jinan University, Guangzhou, China.
Li Xuan, Department of Hematology, Nanfang Hospital, Southern Medical University, Guangzhou, China.
Zhiping Fan, Department of Hematology, Nanfang Hospital, Southern Medical University, Guangzhou, China.
Fen Huang, Department of Hematology, Nanfang Hospital, Southern Medical University, Guangzhou, China.
Na Xu, Department of Hematology, Nanfang Hospital, Southern Medical University, Guangzhou, China.
Jing Sun, Department of Hematology, Nanfang Hospital, Southern Medical University, Guangzhou, China.
Qifa Liu, Department of Hematology, Nanfang Hospital, Southern Medical University, Guangzhou, China.
Min Dai, Department of Hematology, Nanfang Hospital, Southern Medical University, Guangzhou, 510515, China.
Declarations
Ethics approval and consent to participate: The work was conducted in compliance with Institutional Review Board/Human Subjects Research Committee requirements. The present study was approved by the Institutional Review Board of Nanfang Hospital, Southern Medical University, and was conducted in accordance with the Declaration of Helsinki (2021015). Due to the retrospective nature of this study, informed consent was not required.
Consent for publication: Not applicable.
Author contributions: Wenshu Zhao: Conceptualization; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Xiangzong Zeng: Conceptualization; Formal analysis; Investigation; Methodology.
Danqi Pan: Conceptualization; Formal analysis; Investigation; Methodology.
Li Xuan: Data curation.
Zhiping Fan: Data curation.
Fen Huang: Data curation.
Na Xu: Data curation.
Jing Sun: Data curation.
Qifa Liu: Data curation.
Min Dai: Conceptualization; Data curation; Funding acquisition; Methodology; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by the National Natural Science Foundation of China (Grant No. 81500149).
The authors declare that there is no conflict of interest.
Availability of data and materials: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127: 2391–2405. [DOI] [PubMed] [Google Scholar]
- 2. Hoeks M, Yu G, Langemeijer S, et al. Impact of treatment with iron chelation therapy in patients with lower-risk myelodysplastic syndromes participating in the European MDS registry. Haematologica 2020; 105: 640–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Knovich MA, Storey JA, Coffman LG, et al. Ferritin for the clinician. Blood Rev 2009; 23: 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Armand P, Kim HT, Cutler CS, et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood 2007; 109: 4586–4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pullarkat V, Blanchard S, Tegtmeier B, et al. Iron overload adversely affects outcome of allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2008; 42: 799–805. [DOI] [PubMed] [Google Scholar]
- 6. Kataoka K, Nannya Y, Hangaishi A, et al. Influence of pretransplantation serum ferritin on nonrelapse mortality after myeloablative and nonmyeloablative allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2009; 15: 195–204. [DOI] [PubMed] [Google Scholar]
- 7. Kontoyiannis DP, Chamilos G, Lewis RE, et al. Increased bone marrow iron stores is an independent risk factor for invasive aspergillosis in patients with high-risk hematologic malignancies and recipients of allogeneic hematopoietic stem cell transplantation. Cancer 2007;110: 1303–1306. [DOI] [PubMed] [Google Scholar]
- 8. Kamble RT, Selby GB, Mims M, et al. Iron overload manifesting as apparent exacerbation of hepatic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2006; 12: 506–510. [DOI] [PubMed] [Google Scholar]
- 9. Penack O, Peczynski C, van der Werf S, et al. Association of serum ferritin levels before start of conditioning with mortality after alloSCT – a prospective, non-interventional study of the EBMT transplant complications working party. Front Immunol 2020; 11: 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cremers EMP, de Witte T, de Wreede L, et al. A prospective non-interventional study on the impact of transfusion burden and related iron toxicity on outcome in myelodysplastic syndromes undergoing allogeneic hematopoietic cell transplantation. Leuk Lymphoma 2019; 60: 2404–2414. [DOI] [PubMed] [Google Scholar]
- 11. Hackanson B, Daskalakis M. Decitabine. Recent Results Cancer Res 2014; 201: 269–297. [DOI] [PubMed] [Google Scholar]
- 12. Xu Q, Li Y, Jing Y, et al. Epigenetic modifier gene mutations-positive AML patients with intermediate-risk karyotypes benefit from decitabine with CAG regimen. Int J Cancer 2020; 146: 1457–1467. [DOI] [PubMed] [Google Scholar]
- 13. Hong M, Zhu H, Sun Q, et al. Decitabine in combination with low-dose cytarabine, aclarubicin and G-CSF tends to improve prognosis in elderly patients with high-risk AML. Aging 2020; 12: 5792–5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xuan L, Dai M, Jiang E, et al. The effect of granulocyte-colony stimulating factor, decitabine, and busulfan-cyclophosphamide versus busulfan-cyclophosphamide conditioning on relapse in patients with myelodysplastic syndrome or secondary acute myeloid leukaemia evolving from myelodysplastic syndrome undergoing allogeneic haematopoietic stem-cell transplantation: an open-label, multicentre, randomised, phase 3 trial. Lancet Haematol 2023; 10: e178–e190. [DOI] [PubMed] [Google Scholar]
- 15. Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012; 120: 2454–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Greenberg PL, Stone RM, Al-Kali A, et al. Myelodysplastic syndromes, version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017; 15: 60–87. [DOI] [PubMed] [Google Scholar]
- 17. Steensma DP. Myelodysplastic syndromes current treatment algorithm 2018. Blood Cancer J 2018; 8: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu S, Huang F, Fan Z, et al. Haploidentical versus HLA-matched sibling transplantation for refractory acute leukemia undergoing sequential intensified conditioning followed by DLI: an analysis from two prospective data. J Hematol Oncol 2020; 13: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen Y, Huang F, Xuan L, et al. Upfront transplantation may have better outcomes than pretransplant cytoreductive therapy for treating patients with MDS-EB-1 or MDS-EB-2. Int J Cancer 2021; 149: 1109–1120. [DOI] [PubMed] [Google Scholar]
- 20. Wermke M, Schmidt A, Middeke JM, et al. MRI-based liver iron content predicts for nonrelapse mortality in MDS and AML patients undergoing allogeneic stem cell transplantation. Clin Cancer Res 2012; 18: 6460–6468. [DOI] [PubMed] [Google Scholar]
- 21. Kikuchi S, Kobune M, Iyama S, et al. Prognostic significance of serum ferritin level at diagnosis in myelodysplastic syndrome. Int J Hematol 2012; 95: 527–534. [DOI] [PubMed] [Google Scholar]
- 22. Nogai A, Shi Y, Pérez-Hernandez D, et al. Organ siderosis and hemophagocytosis during acute graft-versus-host disease. Haematologica 2016; 101: e344–e346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Küpesiz FT, Hazar V, Eker N, et al. Retrospective evaluation of relationship between iron overload and transplantation complications in pediatric patient who underwent allogeneic stem cell transplantation due to acute leukemia and myelodysplastic syndrome. J Pediatr Hematol oncology 2020; 42: e315–e320 [DOI] [PubMed] [Google Scholar]
- 24. Atilla E, Toprak SK, Demirer T. Current review of iron overload and related complications in hematopoietic stem cell transplantation. Turk J Haematol 2017; 34: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Angelucci E, Cianciulli P, Finelli C, et al. Unraveling the mechanisms behind iron overload and ineffective hematopoiesis in myelodysplastic syndromes. Leuk Res 2017; 62: 108–115. [DOI] [PubMed] [Google Scholar]
- 26. Puliyel M, Mainous AG, Berdoukas V, et al. Iron toxicity and its possible association with treatment of Cancer: lessons from hemoglobinopathies and rare, transfusion-dependent anemias. Free Radic Biol Med 2015; 79: 343–351. [DOI] [PubMed] [Google Scholar]
- 27. Zheng Q, Zhao Y, Guo J, et al. Iron overload promotes mitochondrial fragmentation in mesenchymal stromal cells from myelodysplastic syndrome patients through activation of the AMPK/MFF/Drp1 pathway. Cell Death Dis 2018; 9: 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen J, Wang H, Zhou J, et al. Advances in the understanding of poor graft function following allogeneic hematopoietic stem-cell transplantation. Ther Adv Hematol 2020; 11: 2040620720948743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Okabe H, Suzuki T, Uehara E, et al. The bone marrow hematopoietic microenvironment is impaired in iron-overloaded mice. Eur J Haematol 2014; 93: 118–128. [DOI] [PubMed] [Google Scholar]
- 30. Wu X-Q, Lin K-N, Chen M-M, et al. Iron overload as a risk factor for poor graft function following allogeneic hematopoietic stem cell transplantation. Kaohsiung J Med Sci 2020; 36: 825–833. [DOI] [PubMed] [Google Scholar]
- 31. Majhail NS, Lazarus HM, Burns LJ. Iron overload in hematopoietic cell transplantation. Bone Marrow Transplant 2008; 41: 997–1003. [DOI] [PubMed] [Google Scholar]
- 32. Dadwal SS, Tegtmeier B, Liu X, et al. Impact of pretransplant serum ferritin level on risk of invasive mold infection after allogeneic hematopoietic stem cell transplantation. Eur J Haematol 2015; 94: 235–242. [DOI] [PubMed] [Google Scholar]
- 33. Tachibana T, Tanaka M, Takasaki H, et al. Pretransplant serum ferritin is associated with bloodstream infections within 100 days of allogeneic stem cell transplantation for myeloid malignancies. Int J Hematol 2011; 93: 368–374. [DOI] [PubMed] [Google Scholar]
- 34. Sivgin S, Baldane S, Kaynar L, et al. Pretransplant iron overload may be associated with increased risk of invasive fungal pneumonia (IFP) in patients that underwent allogeneic hematopoietic stem cell transplantation (alloHSCT). Transfus Apher Sci 2013; 48: 103–108. [DOI] [PubMed] [Google Scholar]
- 35. Chen C, He M, Li X, et al. H2O2/DEM-promoted maft promoter demethylation drives Nrf2/ARE activation in zebrafish. Life (Basel, Switzerland) 2022; 12: 1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mpakou V, Spathis A, Bouhla A, et al. Synergistic inhibitory effects of low-dose decitabine in combination with bortezomib in the AML cell line Kasumi-1. Exp Ther Med 2021; 21: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xuan L, Wu X, Qiu D, et al. Regulatory γδ T cells induced by G-CSF participate in acute graft-versus-host disease regulation in G-CSF-mobilized allogeneic peripheral blood stem cell transplantation. J Transl Med 2018; 16: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. D’Aveni M, Rossignol J, Coman T, et al. G-CSF mobilizes CD34+ regulatory monocytes that inhibit graft-versus-host disease. Sci Transl Med 2015; 7: 281ra242. [DOI] [PubMed] [Google Scholar]
- 39. Sun Z-M, Liu H-L, Geng L-Q, et al. HLA-matched sibling transplantation with G-CSF mobilized PBSCs and BM decreases GVHD in adult patients with severe aplastic anemia. J Hematol Oncol 2010; 3: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li Z, Shi W, Lu X, et al. Decitabine-intensified modified busulfan/cyclophosphamide conditioning regimen improves survival in acute myeloid leukemia patients undergoing related donor hematopoietic stem cell transplantation: a propensity score matched analysis. Front Oncol 2022; 12: 844937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ma Y, Qu C, Dai H, et al. Maintenance therapy with decitabine after allogeneic hematopoietic stem cell transplantation to prevent relapse of high-risk acute myeloid leukemia. Bone Marrow Transplant 2020; 55: 1206–1208. [DOI] [PubMed] [Google Scholar]
- 42. Tang Y, Chen J, Liu Q, et al. Low-dose decitabine for refractory prolonged isolated thrombocytopenia after HCT: a randomized multicenter trial. Blood Adv 2021; 5: 1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Löwenberg B, van Putten W, Theobald M, et al. Effect of priming with granulocyte colony-stimulating factor on the outcome of chemotherapy for acute myeloid leukemia. N Engl J Med 2003; 349: 743–752. [DOI] [PubMed] [Google Scholar]
- 44. Konuma T, Ooi J, Uchida N, et al. Granulocyte colony-stimulating factor combined regimen in cord blood transplantation for acute myeloid leukemia: a nationwide retrospective analysis in Japan. Haematologica 2014; 99: e264–e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shucheng G, Chunkang C, Youshan Z, et al. Decitabine treatment could ameliorate primary iron-overload in myelodysplastic syndrome patients. Cancer Invest 2015; 33: 98–106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-doc-1-tah-10.1177_20406207241292451 for The impact of granulocyte colony-stimulating factor and decitabine-containing conditioning in myelodysplastic syndrome patients with iron overload undergoing allogeneic hematopoietic stem cell transplantation: a retrospective study by Wenshu Zhao, Xiangzong Zeng, Danqi Pan, Li Xuan, Zhiping Fan, Fen Huang, Na Xu, Jing Sun, Qifa Liu and Min Dai in Therapeutic Advances in Hematology