Abstract
Background:
The progression of acute limb ischemia (ALI) is being significantly influenced by changes in immune system function. The study aimed to determine the dominant immune cell responses (Th1 or Th2) in ALI patients by measuring serum levels of IL-4, IL-12, and IFN-γ. Previous studies indicate altered cytokine levels in cerebral ischemia, but there is no prior research on these cytokines in ALI patients.
Methods:
This study involved 34 patients with ALI and 34 healthy controls. Blood samples were analyzed for hematological factors such as erythrocyte sedimentation rate (ESR), white blood cell (WBC) count, red blood cell (RBC) count, platelet (Plt) count, hemoglobin (Hb), and hematocrit (HCT). The levels of serum cytokines IL-4, IL-12, and IFN-γ were measured in both patients and control subjects using enzyme-linked immunosorbent assay (ELISA). The statistical analyses were conducted using SPSS and GraphPad Prism.
Results:
The results showed that serum levels of IL-4 in ALI patients did not significantly differ from those in control groups. Acute limb ischemia exhibited significantly elevated levels of IL-12 and IFN-γ compared to healthy individuals. In addition, no correlation between the production of cytokines and the hematological parameters was found.
Conclusions
Th1 responses are believed to play a role in the pathogenesis of ALI, but further research is needed to fully understand their exact role.
Key Words: Fibrinogen, ORF-9b protein, SARS-CoV-2, Serum Albumin
Introduction
The condition known as acute limb ischemia (ALI) occures when a thrombus or an embolus prevents blood flow to a limb. Severe ischemia can lead to serious emergencies, such as limb loss and death. However, early intervention can achieve limb salvage by restoring perfusion (1). In medical terminology, ALI is defined as a condition in which individuals have had symptoms of limb ischemia for less than two weeks. Individuals may exhibit the "6 Ps": pain, paralysis, paresthesia, pulselessness, poikilothermia,and pallor. A key part of the pathophysiology of ALI is the complex inflammatory interactions that happen in the vascular bed after an acute ischemia event (2). Most inflammatory lesions involve lymphocytes, which are drawn to the affected area for a duration of 24 to 48 hours by chemokines, cytokines, and other stimuli. Furthermore, lymphocytes control immunity by secreting immunoglobulins, inducing cytotoxicity, triggering apoptosis and balancing cytokines (3-5). Ischemia impacts both innate and adaptive immunity, but its impact on immune system components is unclear, possibly due to limited tissue damage (6, 7).
Based on the pattern of cytokine production, T cells can be divided into four effector types: Th1, Th2, Th17, and regulatory T cells (Treg) (8, 9). The main cytokine of Th1 cells is IFN-γ, which also inhibits the differentiation of Th2 cells while causing Th0 cells to differentiate into Th1 cells, indicating a positive feedback loop. Moreover, macrophages, NK cells, cytotoxic T cells, and mucosal epithelial cells all release IFN-γ (10-13). Lambertsen et al.'s study revealed that IFN-γ increases ischemia-induced brain damage independently of TNF or through synergistic neurotoxic interactions of IFN-γ and TNF-α (14).
Th2 cells further stimulate IL-4, initiating a positive feedback loop of increased IL-4 production. IL-4 decreases the production of Th1 cells, IFN-γ, IL-12, and macrophages (15, 16). Tissue macrophages are crucial in the processes of wound healing and chronic inflammation. IL-4 levels in extravascular tissues increase the number of alternatively activated macrophages (M2 cells) (17, 18). The increase in M2 is linked to its secretion, which in turn reduces pathological inflammation (19). IL-4 regulates pro-inflammatory mediator secretion and decreases anti-inflammatory immune cell secretion (20). The study by Kim et al. found that patients with acute cerebral ischemia showed increased serum levels of IL-4 (21).
Dendritic cells are major sources of IL-12, which induces Th1 immune deviation by stimulating TNF-α and IFN-γ secretion from T and NK cells and decreasing IL-4-mediated IFN-γ inhibition (22). IL-12 exhibits anti-angiogenic activity, inhibiting the formation of new blood vessels by increasing IFN-γ production, which in turn increases the production of inducible protein-10 (IP-10) (23). IL-12 is crucial in causing hepatic ischemia/reperfusion injury by regulating IFN-γ and TNF-α production (24). Furthermore, exogenous administration of IL-12 in healthy mice activates Kupffer cell activation, increases hepatic vascular cell adhesion molecules, and accumulates of leukocytes in the liver (14).
Elevated ESR, HB, Plt, erythrocyte, and leukocyte counts, along with cytokine induction, significantly impact ischemic damage and clinical prognosis in acute inflammation (25). Therefore, we examined the relationship between serum cytokine levels and blood parameters secreted in response to acute inflammation in patients with ALI.
Materials and Methods
Patients
The study analyzed 34 ALI patients admitted to Velayat Hospital, Mashhad University of Medical Science, Iran, between February and August 2022 and compared them with 34 control age-matched subjects. The study has been approved by the Ethical Committee for Mashhad University of Medical Sciences under the code IR.MUMS.MEDICAL.REC.1400.464. All inpatients who participated in the study were given written informed consent after explaining the study's purpose.
Adult patients aged 18 years or older, demonstrating adequate cognitive ability to participate, and presenting clinically with first ALI symptoms were the main requirements for inclusion. The symptoms of acute artery occlusion, which included pain, pallor, coldness, and a lack of pulse, were classified as ALI if they lasted for two weeks. The exclusion criteria encompassed dissatisfaction with study participation, age below 18 years, kidney and heart failure, Buerger's disease, vasculitis, popliteal artery entrapment syndrome, cancer, receiving thrombolytic therapy, pregnancy, high blood pressure, and unclear symptom onset. Clinical and demographic data of patients were gathered about the patients, including age, sex, clinical symptoms, presentation of upper and lower limb acute ischemia, level of upper and lower limb ischemia (lower limb: from the top of the thigh down, below the knee down, from the ankle down, and upper limb: from the shoulder down, from the elbow down, from the wrist down), other diseases, history of smoking and drug use, history of medication use, and other conditions.
Control subjects
Control serum samples were prepared by collecting blood from 34 healthy donors at Mashhad University of Medical Sciences in Iran. To create a biobank comparable to the serum from our ALI patients, we selected healthy donors with an even distribution of male and female individuals aged between 18 and 70 years. None of the donors were taking immunosuppressive drugs.
Blood collection and serum preparation
Blood samples were taken from all healthy subjects and patients. For cytokine measurements, blood was collected in 6-ml serum gel tubes with a clot activator (FL Medical, Italy) and stored for 30-120 minutes at room temperature before centrifugation at 1372 G for 5 minutes. Subsequently, the serum samples were immediately stored at -70 °C for cytokine analysis.
Hematological parameter measurement
Blood samples were immediately analyzed for hematology using heparin sodium (1%) anticoagulation. Blood counts for RBC, WBC, Plt, Hb, and HCT were determined using the Sysmex XP 300 Automated Hematology Analyzer (Sysmex Corporation, China), expressed as a percentage of total blood volume, and replicated counts were made for each sample.
Cytokine measurement
The concentrations of IL-4, IL-12, and IFN-γ were measured in both patients and healthy subjects using commercial ELISA kits (Invitrogen, Frederick, Maryland, USA) following the manufacturer's instructions. Intra- and inter-assay coefficients for three cytokines were less than 3% and 8%, respectively. Sensitivities of determination for IL-4, IL-12, and IFN-γ were 0.1, 2.1, and 4.0 pg/ml, respectively. All samples were analyzed in duplicate.
Statistical analysis
The means were compared using the independent sample t-test. Pearson's correlation analysis was used to examine the correlations between serum cytokine levels and hematological parameters. All data were expressed as the mean ± standard deviation (SD). The statistical software SPSS 16.0 (SPSS Inc., USA) was utilized to analyze the data. A value of P < 0.05 was considered statistically significant.
Results
The analysis of all clinical, experimental, and demographic data for ALI patients showed that the mean age and gender distribution did not significantly differ between the AIS and control groups (Table 1). The study involved 21 male and 13 female patients, with 23 aged over 65 years and 11 under 65 years. It also included 14 patients with lower extremity ischemia, including two above the knee and five below the knee, and 20 patients with upper extremity ischemia, including three above the arm, 10 below the elbow, and 7 below the wrist. The mean WBC, RBC, and Plt counts in ALI patients were 9.81 ± 4.19 (103/mcL), 4.02 ± 0.93 (106/mcL), and 258.5 ± 130 (103/mcL), respectively. The mean Hb, HCT, and ESR levels were recorded as 11.33 ± 3.02 (g/dL), 33.51 ± 7.57 (%), and 43.8 ± 34.8 (mm), respectively. Additionally, our findings showed that the mean levels of IL-4 in serum of ALI patients did not significantly change (1.7 ± 0.2 pg/ml, P = 0.6) compared to the healthy control group (1.68 ± 0.19 pg/ml). Patients showed significantly higher mean concentrations of IL-12 (37.09 ± 2.4 pg/ml, P < 0.001) compared to healthy subjects (29.41 ± 2.08 pg/ml). The patients also exhibited significantly higher mean IFN-γ levels (14.51 ± 2.23 pg/ml, P < 0.001) compared to the control group (9.36 ± 1.14 pg/ml) (Figure. 1). Additionally, significant positive correlations (P < 0.001) were found between Hb and RBC count, Hb and HCT, and ESR and Plt in ALI patients. Significant negative correlations were observed between ESR and HCT (P = 0.033), ESR and Hb (P = 0.034), and ESR and RBC count (P = 0.013).
Table 1.
Clinical and hematological variables of the acute limb ischemia (ALI) patients and cytokine serum levels of these and control subjects.
| Characteristic | ALI patients (n=34) | Control subject/Reference interval (n=34) |
|---|---|---|
| Gender, n (%) | ||
| Man | 21 (61.76%) | 21 (61.76%) |
| Woman | 13 (38.23%) | 13 (38.23%) |
| Age | ||
| Man | 70.4±3.5a | 71.04±3.4 |
| Woman | 54.8±9.2 | 53.46±9.3 |
| <65 | 11 | 11 |
| >65 | 23 | 23 |
| Upper extremity, n (%) | ||
| Above the arm | 3 (8.8%) | - |
| Below the elbow | 10 (29.4%) | |
| Below the ankle | 7 (20.5%) | |
| Lower extremity, n (%) | ||
| Above the knee | 2 (5.8%) | - |
| Below the elbow | 7 (20.5%) | |
| Below the ankle | 5 (14.7%) | |
| WBC (103/mcL) | 9.81±4.19 | 4.5-11 |
| RBC (106/mcL) | 4.02±0.93 | 4.2-6.1 |
| Plt (103/mcL) | 258.5±130 | 150-400 |
| ESR (mm/h) | 43.88±34.8 | <30 |
| Hb (g/dL) | 11.33±3.02 | 12-18 |
| HCT (%) | 33.51±7.57 | 36-50 |
*The 21 patients were male, and 13 patients were female. The 20 patients (3 above the arm ischemia, 10 below the elbow ischemia and 7 from below the wrist ischemia) suffered from upper extremity ischemia and 14 patients (2 above the knee ischemia, 7 below the knee ischemia and 5 the ankle ischemia) suffered from lower extremity ischemia.
Data are shown as mean ± standard deviation.
Fig. 1.
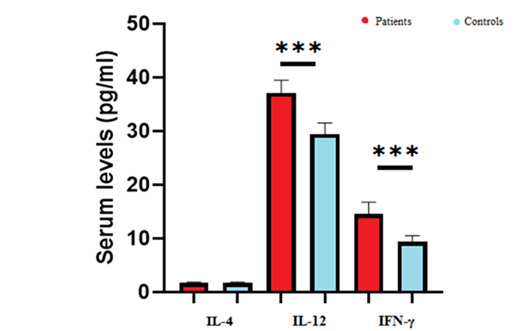
Comparing serum levels of IL-4, IL-12 and IFN-γ in ALI patients compared to healthy patients. Student's t-test was used for comparison between groups. Comparison of IL-4 levels in ALI patients were not changed significantly compared to healthy patients’ group (P=0.6). The serum levels of IL-12 were increased significantly in ALI patients compared to healthy patients. The levels of IFN-γ were also enhanced in ALI patients compared to healthy patients. ***P<0.001. ALI patients are shown with red color and healthy patients are shown with blue color.
Table 2.
Pearson correlation coefficient (upper value) and p-value (lower value) of the hematological parameters and cytokine levels in ALI patients.
| IL4 | 1 | ||||||||
| IL-12 | 0.03 | 1 | |||||||
| 0.842 | |||||||||
| IFN-γ | 0.09 | 0.14 | 1 | ||||||
| 0.596 | 0.439 | ||||||||
| ESR | 0.04 | -0.02 | -0.20 | 1 | |||||
| 0.841 | 0.906 | 0.321 | |||||||
| Hb | -0.27 | -0.05 | 0.24 | -0.42 | 1 | ||||
| 0.162 | 0.794 | 0.209 | 0.034 | ||||||
| WBC | 0.19 | -0.10 | -0.27 | -0.05 | -0.21 | 1 | |||
| 0.347 | 0.604 | 0.171 | 0.797 | 0.285 | |||||
| RBC | -0.21 | -0.12 | 0.25 | -0.50 | 0.91 | -0.11 | 1 | ||
| 0.303 | 0.544 | 0.227 | 0.013 | <0.001 | 0.595 | ||||
| HCT | -0.27 | -0.05 | 0.25 | -0.42 | 0.98 | -0.11 | 0.93 | 1 | |
| 0.172 | 0.785 | 0.208 | 0.033 | <0.001 | 0.572 | <0.001 | |||
| Plt | 0.09 | -0.07 | -0.19 | 0.70 | -0.14 | 0.22 | -0.04 | -0.11 | 1 |
| 0.650 | 0.741 | 0.355 | <0.001 | 0.501 | 0.295 | 0.833 | 0.590 | ||
| IL-4 | IL-12 | IFN-γ | ESR | Hb | WBC | RBC | HCT | Plt | |
Significant positive correlation was found between ESR and Plt, Hb and RBC count, Hb and HCT (P<001) in ALI patients. Significant negative correlation was found between ESR and HCT (P=0.033), ESR and Hb (P=0.034), ESR and RBC count (P=0.013).
Fig. 2.
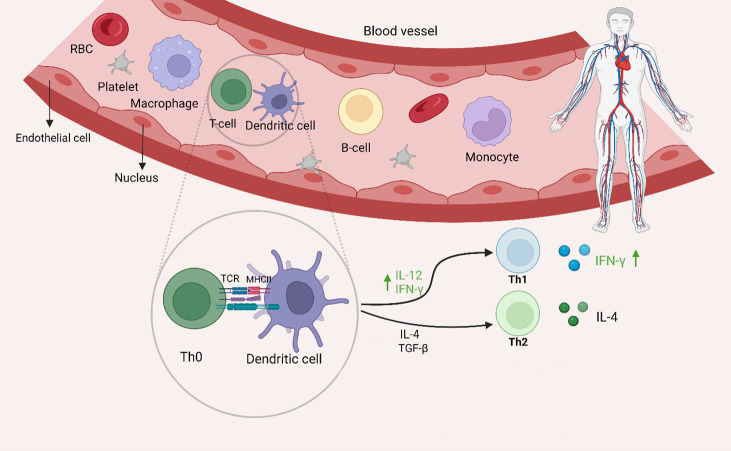
Cytokines production in the serum of ALI patients. The interaction between dendritic cell (DC) and T cell leads to T cell activation and immune response including ALI diseases. The MHC-antigen complex in the surface of DC activates the TCR and the T cell cytokines secretion. The serum levels of IL-4 in ALI patients were not changed compared to control group. The serum levels of IFN-γ and IL-12 were increased in patients compared to healthy control group, that induce Th1 immune response. *The figure was created with BioRender.com.
Discussion
Our findings revealed that patients had higher serum levels of IL-12 and IFN-γ when compared to a healthy control group, both of which induce a Th1 immune response. Although a few studies have looked at cytokine production in ALI patients, none have investigated into Th1 cytokine production (26). A growing body of evidence suggests that inflammation plays a critical role in the pathogenesis of acute ischemic stroke (27-29). Yassin et al. assayed plasma concentrations of TNF-α in male Wistar rats and found a significant elevation of TNF-α levels in animals who were affected by 3 hours of bilateral hind limb ischemia and 1 hour of reperfusion compared to the control group (30). Yu et al. discovered increased Th17 and Th1 cells in acute ischemic stroke patients compared to controls, but not Th2 cells.
Furthermore, they found a link between Th1 and Th17 cells and the severity of acute ischemic stroke (31). Bellavia et al. investigated the Th1/Th2 balance to assess the peripheral immune response to cerebral ischemia. The results revealed that there was no change towards Th1 (32). Ormstad et al. observed higher blood levels of IL-12 in acute ischemic stroke patients, which is consistent with our findings (27). According to Zaremba et al., serum IL-12 levels were higher in stroke patients than in controls. The authors also reported that IL-12 levels exhibited a strong connection with ESR values in stroke patients (33). Some animal studies have found IL-12 involvement in cardiac ischemia-reperfusion injury (34-36). Yan et al. discovered that dectin-2 deficient mice can reduce IL-12 expression and alleviate Th1 immune responses, potentially protecting against cardiac ischemia-reperfusion injury (37, 38). Ischemic brain injury may be exacerbated by IL-12. IL-12 can boost the production and activity of several pro-inflammatory chemokines and cytokines (39). After retinal ischemia, animals were given a neutralizing IFN-γ antibody, which enhanced retinal ganglion cell survival (40). IL-4 can increase Th2 differentiation by activating the transcription factors GATA3 and STAT6 (41). To date, there have been no investigations into ALI patients' secretion of IL-4. In our investigation, there was no significant difference in IL-4 levels between ALI patients and controls.
The present study found a positive correlation between ESR and Plt count in ALI patients, while a negative correlation was found with Hb, ESR, and RBC, indicating that Hb, Plt, RBC, and WBC count are key predictors of ESR. ESR levels are affected by a variety of confounding circumstances. Several investigations have found a substantial relationship between ESR levels and RBC, Hb, and HCT (28), as well as platelet count (29). Elevated ESR levels should not be used as a direct indicator of inflammation in ALI but should be considered alongside other strong inflammation markers (42, 43). The study suggests that elevated serum levels of IL-12 and IFN-γ, but not IL-4, may indicate a Th1 dominant response in ALI patients.
The study suggests that the Th1 response may be involved in the pathogenesis of ALI, as serum levels of IFN-γ and IL-12 increased in ALI patients compared to healthy controls. To better understand immune system alterations in ALI patients, a larger study population and assaying other cytokines and immunological markers are recommended.
Funding
This work was supported by the Vascular and Endovascular Surgery Research Center, Mashhad University of Medical Sciences, Mashhad, Iran [Grant Number: 992062].
Conflict of interest
No potential conflict of interest was reported by the authors.
Acknowledgments
The authors would like to acknowledge Vascular and Endovascular Surgery Research center, Mashhad University of Medical Sciences, Mashhad, Iran.
References
- 1.Obara H, Matsubara K, Kitagawa Y. Acute Limb Ischemia. Ann Vasc Dis. 2018;11(4):443–448. doi: 10.3400/avd.ra.18-00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narula N, Dannenberg AJ, Olin JW, Bhatt DL, Johnson KW, Nadkarni G, et al. Pathology of Peripheral Artery Disease in Patients With Critical Limb Ischemia. J Am Coll Cardiol. 2018;72(18):2152–2163. doi: 10.1016/j.jacc.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Tanzadehpanah H, Lotfian E, Avan A, Saki S, Nobari S, Mahmoodian R, et al. Role of SARS-COV-2 and ACE2 in the pathophysiology of peripheral vascular diseases. Biomed Pharmacother. 2023;166:115321. doi: 10.1016/j.biopha.2023.115321. [DOI] [PubMed] [Google Scholar]
- 4.Mahaki H, Saeed Modaghegh MH, Nasr Isfahani Z, Amir Daddost R, Molaei P, Ahmadyousefi Y, et al. The role of peptide-based tumor vaccines on cytokines of adaptive immunity: a review. Int J Peptide Res Therap. 2021;27:2527–42. [Google Scholar]
- 5.Tanzadehpanah H, Modaghegh MHS, Mahaki H. Key biomarkers in cerebral arteriovenous malformations: Updated review. J Gene Med. 2023;25(12):e3559. doi: 10.1002/jgm.3559. [DOI] [PubMed] [Google Scholar]
- 6.DeLong JH, Ohashi SN, O'Connor KC, Sansing LH. Inflammatory Responses After Ischemic Stroke. Semin Immunopathol. 2022;44(5):625–648. doi: 10.1007/s00281-022-00943-7. [DOI] [PubMed] [Google Scholar]
- 7.Molaei P, Mahaki H, Manoochehri H, Tanzadehpanah H. Binding Sites of Anticancer Drugs on Human Serum Albumin (HSA): A Review. Protein Pept Lett. 2022;29(8):651–675. doi: 10.2174/0929866529666220426124834. [DOI] [PubMed] [Google Scholar]
- 8.Mahaki H, Tanzadehpanah H, Jabarivasal N, Sardanian K, Zamani A. A review on the effects of extremely low frequency electromagnetic field (ELF-EMF) on cytokines of innate and adaptive immunity. Electromagn Biol Med. 2019;38(1):84–95. doi: 10.1080/15368378.2018.1545668. [DOI] [PubMed] [Google Scholar]
- 9.Hagag S, Kodous A, Shaaban HA. Molecular and Immunohistochemical Alterations in Breast Cancer Patients in Upper Egypt. Rep Biochem Mol Biol. 2023;11(4):532–546. doi: 10.52547/rbmb.11.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahaki H, Jabarivasal N, Sardanian K, Zamani A. Effects of Various Densities of 50 Hz Electromagnetic Field on Serum IL-9, IL-10, and TNF-α Levels. Int J Occup Environ Med. 2020;11(1):24–32. doi: 10.15171/ijoem.2020.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahaki H, Jabarivasal N, Sardarian K, Zamani A. The effects of extremely low-frequency electromagnetic fields on c-Maf, STAT6, and RORα expressions in spleen and thymus of rat. Electromagn Biol Med. 2019;38(2):177–183. doi: 10.1080/15368378.2019.1608832. [DOI] [PubMed] [Google Scholar]
- 12.Sardarian K, Maghsood AH, Farimani M, Hajiloii M, Saidijam M, Farahpour M, et al. Detection of Toxoplasma gondii B1 gene in placenta does not prove congenital toxoplasmosis. Hum Antibodies. 2019;27(1):31–35. doi: 10.3233/HAB-180346. [DOI] [PubMed] [Google Scholar]
- 13.Tanzadehpanah H, Bahmani A, Hosseinpour Moghadam N, Gholami H, Mahaki H, Farmany A, Saidijam M. Synthesis, anticancer activity, and β-lactoglobulin binding interactions of multitargeted kinase inhibitor sorafenib tosylate (SORt) using spectroscopic and molecular modelling approaches. Luminescence. 2021;34(1):117–128. doi: 10.1002/bio.3929. [DOI] [PubMed] [Google Scholar]
- 14.Lambertsen KL, Gregersen R, Meldgaard M, Clausen BH, Heibøl EK, Ladeby R, et al. A role for interferon-gamma in focal cerebral ischemia in mice. J Neuropathol Exp Neurol. 2004;63(9):942–55. doi: 10.1093/jnen/63.9.942. [DOI] [PubMed] [Google Scholar]
- 15.Raphael I, Nalawade S, Eagar TN, Forsthuber TG. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2015;74(1):5–17. doi: 10.1016/j.cyto.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yousefzadeh H, Jabbari Azad F, Rastin M, Banihashemi M, Mahmoudi M. Expression of Th1 and Th2 Cytokine and Associated Transcription Factors in Peripheral Blood Mononuclear Cells and Correlation with Disease Severity. Rep Biochem Mol Biol. 2017;6(1):102–111. [PMC free article] [PubMed] [Google Scholar]
- 17.Yan W, Li T, Yin T, Hou Z, Qu K, Wang N, et al. M2 macrophage-derived exosomes promote the c-KIT phenotype of vascular smooth muscle cells during vascular tissue repair after intravascular stent implantation. Theranostics. 2020;10(23):10712–10728. doi: 10.7150/thno.46143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sardarian K, Maghsood AH, Farimani M, Hajiloii M, Saidijam M, Rezaeepoor M, et al. Evaluation of Toxoplasma gondii B1 gene in Placental Tissues of Pregnant Women with Acute Toxoplasmosis. Adv Biomed Res. 2018;7:119. doi: 10.4103/abr.abr_58_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson EA, Darrah PA, Foulds KE, Hoffer E, Caffrey-Carr A, Norenstedt S, et al. Monocytes Acquire the Ability to Prime Tissue-Resident T Cells via IL-10-Mediated TGF-β Release. Cell Rep. 2019;28(5):1127–1135.e4. doi: 10.1016/j.celrep.2019.06.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bobinski F, Teixeira JM, Sluka KA, Santos ARS. Interleukin-4 mediates the analgesia produced by low-intensity exercise in mice with neuropathic pain. Pain. 2018 Mar;159(3):437–450. doi: 10.1097/j.pain.0000000000001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Liu J, Zhao S, Zhang H, Cai W, Cai M, et al. Interleukin-4 Is Essential for Microglia/Macrophage M2 Polarization and Long-Term Recovery After Cerebral Ischemia. Stroke. 2016;47(2):498–504. doi: 10.1161/STROKEAHA.115.012079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chyuan IT, Lai JH. New insights into the IL-12 and IL-23: From a molecular basis to clinical application in immune-mediated inflammation and cancers. Biochem Pharmacol. 2020;175:113928. doi: 10.1016/j.bcp.2020.113928. [DOI] [PubMed] [Google Scholar]
- 23.Agnihotri SK, Kumar B, Jain A, Anjali A, Negi MPS, Sachan R, et al. Clinical Significance of Circulating Serum Levels of sCD95 and TNF-α in Cytoprotection of Cervical Cancer. Rep Biochem Mol Biol. 2022;10(4):711, 721. doi: 10.52547/rbmb.10.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmadvand H, Yalameha B, Adibhesami G, Nasri M, Naderi N, Babaeenezhad E, Nouryazdan N. The Protective Role of Gallic Acid Pretreatment On Renal Ischemia-reperfusion Injury in Rats. Rep Biochem Mol Biol. 2019;8(1):42, 48. [PMC free article] [PubMed] [Google Scholar]
- 25.Kaur A. Erythrocyte Sedimentation Rate: Its Determinants and Relationship with Risk Factors Involved in Ischemic Stroke. Korean J Clin Lab Sci. 2022;54(1):1, 8. [Google Scholar]
- 26.Ege T, Us MH, Sungun M, Duran E. Cytokine response in lower extremity ischaemia/reperfusion. J Int Med Res. 2004;32(2):124, 31. doi: 10.1177/147323000403200204. [DOI] [PubMed] [Google Scholar]
- 27.Ormstad H, Aass HC, Lund-Sørensen N, Amthor KF, Sandvik L. Serum levels of cytokines and C-reactive protein in acute ischemic stroke patients, and their relationship to stroke lateralization, type, and infarct volume. J Neurol. 2011;258(4):677–85. doi: 10.1007/s00415-011-6006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiotti N, Giansante C, Ponte E, Delbello C, Calabrese S, Zacchi T, et al. Atherosclerosis and inflammation. Patterns of cytokine regulation in patients with peripheral arterial disease. Atherosclerosis. 1999;145(1):51–60. doi: 10.1016/s0021-9150(99)00013-1. [DOI] [PubMed] [Google Scholar]
- 29.Jaffer U, Wade RG, Gourlay T. Cytokines in the systemic inflammatory response syndrome: a review. HSR Proc Intensive Care Cardiovasc Anesth. 2010;2(3):161–75. [PMC free article] [PubMed] [Google Scholar]
- 30.Yassin MM, Harkin DW, Barros D'Sa AA, Halliday MI, Rowlands BJ. Lower limb ischemia-reperfusion injury triggers a systemic inflammatory response and multiple organ dysfunction. World J Surg. 2002;26(1):115–21. doi: 10.1007/s00268-001-0169-2. [DOI] [PubMed] [Google Scholar]
- 31.Yu S, Cui W, Han J, Chen J, Tao W. Longitudinal change of Th1, Th2, and Th17 cells and their relationship between cognitive impairment, stroke recurrence, and mortality among acute ischemic stroke patients. J Clin Lab Anal. 2022;36(7):e24542. doi: 10.1002/jcla.24542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellavia S, Scala I, Rizzo PA, Brunetti V, Broccolini A, Della Marca G, et al. Th1/Th2 polarization of peripheral immune response in atherothrombotic and cardioembolic stroke: a prospective study. Sci Rep. 2022;12(1):16384. doi: 10.1038/s41598-022-20515-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zaremba J, Losy J. Interleukin-12 in acute ischemic stroke patients. Folia Neuropathol. 2006;44(1):59–66. [PubMed] [Google Scholar]
- 34.Halladin NL, Ekeløf S, Alamili M, Bendtzen K, Lykkesfeldt J, Rosenberg J, Gögenur I. Lower limb ischaemia and reperfusion injury in healthy volunteers measured by oxidative and inflammatory biomarkers. Perfusion. 2015;30(1):64–70. doi: 10.1177/0267659114530769. [DOI] [PubMed] [Google Scholar]
- 35.Yassin MM, Barros D'Sa AA, Parks G, Abdulkadir AS, Halliday I, Rowlands BJ. Mortality following lower limb ischemia-reperfusion: a systemic inflammatory response? World J Surg. 1996;20(8):961–6. doi: 10.1007/s002689900144. discussion 966-7. [DOI] [PubMed] [Google Scholar]
- 36.Jalkanen J, Hollmén M, Maksimow M, Jalkanen S, Hakovirta H. Serum cytokine levels differ according to major cardiovascular risk factors in patients with lower limb atherosclerosis. Cytokine. 2019;114:74–80. doi: 10.1016/j.cyto.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Ye J, Wang Y, Wang Z, Liu L, Yang Z, Wang M, et al. Roles and Mechanisms of Interleukin-12 Family Members in Cardiovascular Diseases: Opportunities and Challenges. Front Pharmacol. 2020;11:129. doi: 10.3389/fphar.2020.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan X, Zhang H, Fan Q, Hu J, Tao R, Chen Q, et al. Dectin-2 Deficiency Modulates Th1 Differentiation and Improves Wound Healing After Myocardial Infarction. Circ Res. 2017;120(7):1116–1129. doi: 10.1161/CIRCRESAHA.116.310260. [DOI] [PubMed] [Google Scholar]
- 39.Zhu H, Hu S, Li Y, Sun Y, Xiong X, Hu X, Chen J, Qiu S. Interleukins and Ischemic Stroke. Front Immunol. 2022;13:828447. doi: 10.3389/fimmu.2022.828447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho K-S, Vu THK, Doesburg D, Chen DF. Suppression of Interferon-gamma protects retinal ischemia-induced neuron death. Invest Ophth Vis Sci. 2014;55(13):1897–46. [Google Scholar]
- 41.Leung S, Liu X, Fang L, Chen X, Guo T, Zhang J. The cytokine milieu in the interplay of pathogenic Th1/Th17 cells and regulatory T cells in autoimmune disease. Cell Mol Immunol. 2010;7(3):182–9. doi: 10.1038/cmi.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagelkerken L. Role of Th1 and Th2 cells in autoimmune demyelinating disease. Braz J Med Biol Res. 1998;31(1):55–60. doi: 10.1590/s0100-879x1998000100007. [DOI] [PubMed] [Google Scholar]
- 43.Chou FC, Shieh SJ, Sytwu HK. Attenuation of Th1 response through galectin-9 and T-cell Ig mucin 3 interaction inhibits autoimmune diabetes in NOD mice. Eur J Immunol. 2009;39(9):2403, 11. doi: 10.1002/eji.200839177. [DOI] [PubMed] [Google Scholar]