Abstract
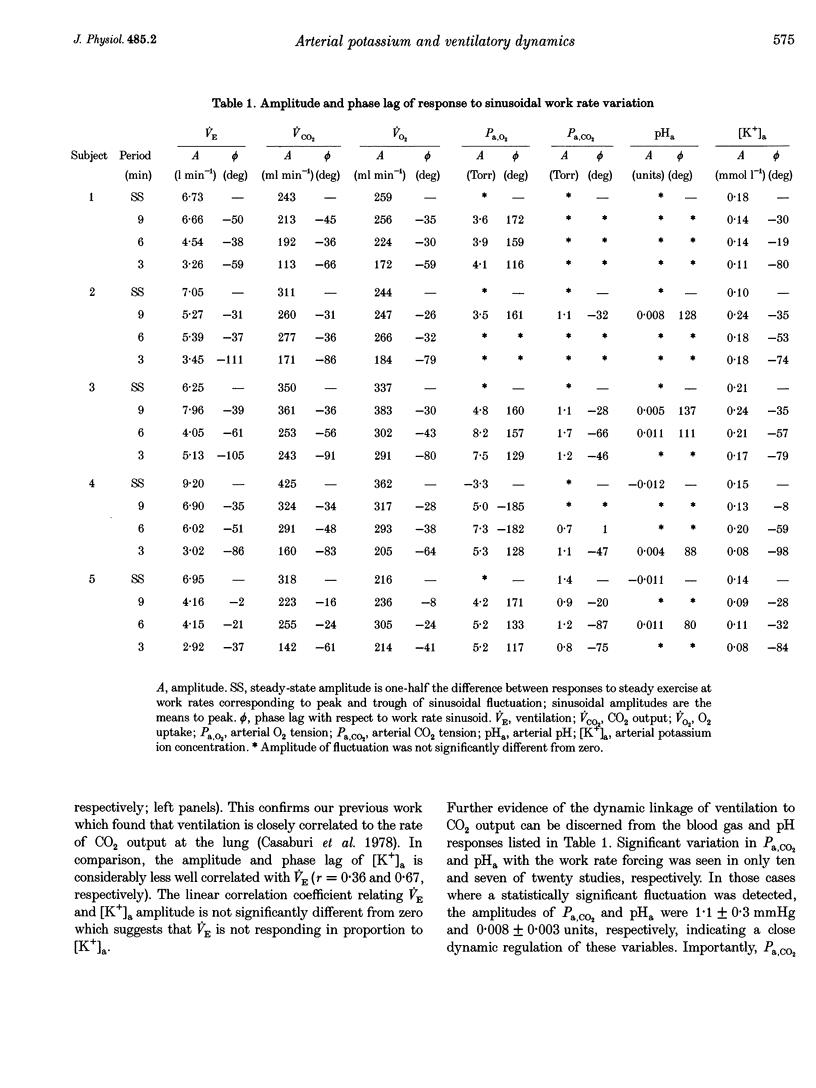
1. The mechanisms underlying the exercise hyperpnoea have been difficult to define. Recently it has been suggested that exercise ventilation (VE) changes in proportion to changes in arterial potassium concentration ([K+]a). Similar VE and [K+]a time courses following work rate changes have been cited as supporting evidence. This study compared [K+]a and VE dynamics during moderate exercise in man. 2. We observed VE and gas exchange responses in five healthy men to sinusoidal work rate variation between 25 and approximately 105 W. Tests of approximately 30 min duration were performed at sinusoidal periods of 9, 6 and 3 min and in the steady state. In each test, during two or three sine periods, arterial blood was sampled (24 per test) and analysed for [K+] and blood gases. Response amplitude and phase (relative to work rate) were determined for each variable. 3. [K+]a fluctuated in response to sinusoidal work rate forcing with mean-to-peak amplitude averaging 0.15 mmol 1(-1). However, among tests, VE amplitude and phase were not highly correlated with [K+]a (r = 0.36 and 0.67, respectively). Further, average [K+]a amplitude in the 9 and 6 min sinusoidal studies tended to exceed the steady-state amplitude, while average VE amplitude fell progressively with increasing forcing frequency. The dissimilar dynamics of [K+]a and VE seem inconsistent with a major role for [K+]a as a proportional controller of ventilation during non-steady state moderate exercise in man. 4. Among tests, VE and CO2 output (VCO2) amplitude and phase were closely correlated (r = 0.87 and 0.94, respectively). Further, arterial CO2 pressure (Pa,CO2) and arterial pH(pHa) did not fluctuate significantly in ten of twenty and thirteen of twenty studies, respectively. In tests where sinusoidal fluctuation was detected, amplitude averaged 1.1 mmHg and 0.008 units, respectively. Thus VE demonstrated a close dynamic coupling to CO2 output, with consequent tight regulation of Pa,CO2 and pHa.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Band D. M., Linton R. A., Kent R., Kurer F. L. The effect of peripheral chemodenervation on the ventilatory response to potassium. Respir Physiol. 1985 May;60(2):217–225. doi: 10.1016/0034-5687(85)90105-7. [DOI] [PubMed] [Google Scholar]
- Band D. M., Linton R. A. The effect of potassium on carotid body chemoreceptor discharge in the anaesthetized cat. J Physiol. 1986 Dec;381:39–47. doi: 10.1113/jphysiol.1986.sp016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver W. L., Wasserman K., Whipp B. J. On-line computer analysis and breath-by-breath graphical display of exercise function tests. J Appl Physiol. 1973 Jan;34(1):128–132. doi: 10.1152/jappl.1973.34.1.128. [DOI] [PubMed] [Google Scholar]
- Busse M. W., Maassen N., Konrad H. Relation between plasma K+ and ventilation during incremental exercise after glycogen depletion and repletion in man. J Physiol. 1991 Nov;443:469–476. doi: 10.1113/jphysiol.1991.sp018845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse M. W., Scholz J., Saxler F., Maassen N., Böning D. Relationship between plasma potassium and ventilation during successive periods of exercise in men. Eur J Appl Physiol Occup Physiol. 1992;64(1):22–25. doi: 10.1007/BF00376434. [DOI] [PubMed] [Google Scholar]
- Casaburi R., Whipp B. J., Wasserman K., Beaver W. L., Koyal S. N. Ventilatory and gas exchange dynamics in response to sinusoidal work. J Appl Physiol Respir Environ Exerc Physiol. 1977 Feb;42(2):300–301. doi: 10.1152/jappl.1977.42.2.300. [DOI] [PubMed] [Google Scholar]
- Casaburi R., Whipp B. J., Wasserman K., Stremel R. W. Ventilatory control characteristics of the exercise hyperpnea as discerned from dynamic forcing techniques. Chest. 1978 Feb;73(2 Suppl):280–283. doi: 10.1378/chest.73.2_supplement.280. [DOI] [PubMed] [Google Scholar]
- Engeman R. M., Swanson G. D., Jones R. H. Optimal frequency locations for estimating model parameters in studies on respiratory control. Comput Biomed Res. 1983 Dec;16(6):531–536. doi: 10.1016/0010-4809(83)90039-3. [DOI] [PubMed] [Google Scholar]
- GRODINS F. S., JAMES G. Mathematical models of respiratory regulation. Ann N Y Acad Sci. 1963 Jun 24;109:852–868. doi: 10.1111/j.1749-6632.1963.tb13510.x. [DOI] [PubMed] [Google Scholar]
- Krogh A., Lindhard J. The regulation of respiration and circulation during the initial stages of muscular work. J Physiol. 1913 Oct 17;47(1-2):112–136. doi: 10.1113/jphysiol.1913.sp001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin P., Linton R. A., Band D. M. Effects of potassium and lactic acid on ventilation in anaesthetized cats. Respir Physiol. 1994 Feb;95(2):171–179. doi: 10.1016/0034-5687(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Medbø J. I., Sejersted O. M. Plasma potassium changes with high intensity exercise. J Physiol. 1990 Feb;421:105–122. doi: 10.1113/jphysiol.1990.sp017935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky H. J., Ransnas L. A. Fitting curves to data using nonlinear regression: a practical and nonmathematical review. FASEB J. 1987 Nov;1(5):365–374. [PubMed] [Google Scholar]
- Paterson D. J. Potassium and ventilation in exercise. J Appl Physiol (1985) 1992 Mar;72(3):811–820. doi: 10.1152/jappl.1992.72.3.811. [DOI] [PubMed] [Google Scholar]
- Paterson D. J., Robbins P. A., Conway J. Changes in arterial plasma potassium and ventilation during exercise in man. Respir Physiol. 1989 Dec;78(3):323–330. doi: 10.1016/0034-5687(89)90107-2. [DOI] [PubMed] [Google Scholar]
- Qayyum M. S., Barlow C. W., O'Connor D. F., Paterson D. J., Robbins P. A. Effect of raised potassium on ventilation in euoxia, hypoxia and hyperoxia at rest and during light exercise in man. J Physiol. 1994 Apr 15;476(2):365–372. doi: 10.1113/jphysiol.1994.sp020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELDINGER S. I. Catheter replacement of the needle in percutaneous arteriography; a new technique. Acta radiol. 1953 May;39(5):368–376. doi: 10.3109/00016925309136722. [DOI] [PubMed] [Google Scholar]
- Sue D. Y., Wasserman K., Moricca R. B., Casaburi R. Metabolic acidosis during exercise in patients with chronic obstructive pulmonary disease. Use of the V-slope method for anaerobic threshold determination. Chest. 1988 Nov;94(5):931–938. doi: 10.1378/chest.94.5.931. [DOI] [PubMed] [Google Scholar]
- Whipp B. J., Ward S. A., Lamarra N., Davis J. A., Wasserman K. Parameters of ventilatory and gas exchange dynamics during exercise. J Appl Physiol Respir Environ Exerc Physiol. 1982 Jun;52(6):1506–1513. doi: 10.1152/jappl.1982.52.6.1506. [DOI] [PubMed] [Google Scholar]
- Whipp B. J., Wasserman K., Casaburi R., Juratsch C. E., Weissman M. L., Stremel R. W. Ventilatory control characteristics of conditions resulting in isocapnic hyperpnea. Adv Exp Med Biol. 1978;99:355–365. doi: 10.1007/978-1-4613-4009-6_38. [DOI] [PubMed] [Google Scholar]


