Abstract
Background:
In extremely preterm infants, persistence of cardioventilatory events is associated with long-term morbidity. Therefore, the objective was to characterize physiologic growth curves of apnea, periodic breathing, intermittent hypoxemia, and bradycardia in extremely preterm infants during the first few months of life.
Methods:
The Prematurity-Related Ventilatory Control study included 717 preterm infants <29 weeks gestation. Waveforms were downloaded from bedside monitors with a novel sharing analytics strategy utilized to run software locally, with summary data sent to the Data Coordinating Center for compilation.
Results:
Apnea, periodic breathing, and intermittent hypoxemia events rose from day 3 of life then fell to near-resolution by 8-12 weeks of age. Apnea/intermittent hypoxemia were inversely correlated with gestational age, peaking at 3-4 weeks of age. Periodic breathing was positively correlated with gestational age peaking at 31-33 weeks postmenstrual age. Females had more periodic breathing but less intermittent hypoxemia/bradycardia. White infants had more apnea/periodic breathing/intermittent hypoxemia. Infants never receiving mechanical ventilation followed similar postnatal trajectories but with less apnea and intermittent hypoxemia, and more periodic breathing.
Conclusion:
Cardioventilatory events peak during the first month of life but the actual postnatal trajectory is dependent on the type of event, race, sex and use of mechanical ventilation.
INTRODUCTION
In preterm infants, apnea, periodic breathing, intermittent hypoxemia, and bradycardia are associated with prolonged hospitalization and long-term morbidity. These events are likely to represent developmental immaturity of the control of respiratory physiology. And they may impact long-term outcomes1–11 and care needs differently12. Natural histories of these events relative to gestational, chronological, and postmenstrual ages, sex and race, may provide insights into the postnatal maturation of ventilatory control. And in turn, those insights may guide anticipatory management that will improve clinical outcomes of infants born extremely prematurely.
Maturational changes of apnea with bradycardia and desaturation (ABD) events13 or of periodic breathing and intermittent hypoxemia1,14 in preterm infants during early postnatal life have been reported. However, these were single-site studies that did not isolate the maturational trajectories of the individual physiologic components. Moreover, they encompassed lower thresholds for oxygen saturation targets compared to the current American Academy of Pediatrics (AAP) recommended target of 90-95%15, which are known to affect the incidence of events16. As a final distinction, they did not account for the effect of sex and race on the maturational trajectory of cardiorespiratory events.
Building on these earlier publications, the Prematurity-Related Ventilatory Control (Pre-Vent) study was an NIH-funded cooperative RFA agreement among 5 Clinical Research Centers (CRC) and one Data Coordinating Center (LDCC)17. It was designed to study a multi-site cohort of >500 preterm infants born before 29 weeks gestation. Pre-Vent provided a standardized and large-scale analysis of continuous bedside monitoring recordings. It included daily documentation of clinical determinants that might affect breathing, oxygen levels and heart rate using current AAP-recommended oxygen saturation targets. The objective of this study was to describe the most up-to-date data on the physiologic trajectories of the incidence (number of events/day) and exposure (number of minutes/day) of individual physiologic measures. These measures included apnea, periodic breathing, intermittent hypoxemia, and bradycardia analyzed by gestational age, chronological age and postmenstrual age and the effects of sex and race. The pre-determined endpoint was 36 weeks postmenstrual age. We hypothesized that immature breathing events would follow different race- and sex-dependent maturational time courses resulting in distinct patterns with resolution as infants approach term-corrected age.
METHODS
Data collection.
Pre-Vent specific clinical data collection forms were designed from a centralized REDCap database system (see Supplement). Maternal and infant medical records were reviewed daily for demographics, respiratory support, medications, and co-morbidity data. Physiologic waveforms were obtained using standard-of-care continuous clinical impedance monitoring and acquisition systems (see Supplement).
Physiologic data analytics.
To avoid transferring patient data, a sharing analytics strategy was devised. Software was written to convert data to a common HDF5 format18, detect events19–22 and retrieve summary data. Each site ran the software locally with summary data sent to the LDCC for final compilation (strategy details in Supplement).
Definitions of cardioventilatory events.
We used time series methods to identify breathing pauses. Apnea was defined as a 20 second or longer respiratory pause21. Periodic breathing was detected using a previously published algorithm22 . This entailed two mother wavelets with six cycles using a sine window to weight the middle of the wavelets more heavily than the ends and allowed detection of periodic breathing with as few as three cycles. We defined periodic breathing as three cycles or more cycles of breathing with regularly spaced pauses using this wavelet transform analysis. Intermittent hypoxemia was detected as hemoglobin saturation (SpO2) <80% (IH80) or <90% (IH90) for 10 to 300 seconds23. This upper limit was used to distinguish IH from prolonged changes in baseline oxygenation1. Bradycardia was defined as heart rate less than 80 bpm for 5 seconds or longer.
Trajectories.
Physiologic variables are presented as median daily frequency, percentile curves and heat maps as functions of chronological age and gestational age. Apnea and periodic breathing were quantified only for non-intubated days; intermittent hypoxemia and bradycardia were documented during all modes of respiratory support. Daily 25th, 50th (median), 75th, and 90th percentile values were plotted for incidence and exposure of each event type from day of enrollment until 36 weeks 0 days postmenstrual age. Only days with ≥12 hours of data available were included. Median/percentile curves were smoothed using local regression method LOWESS (R stats package) with span equal to 25% of the data. At least 30 infants were required to have measurements on a particular day for inclusion in these curves. Thus, plots began at day 3 of life since few infant had recorded data on days 0-2 of life.
Statistical analysis.
Sample percentiles were calculated as summary statistics for the cardiorespiratory monitor data analytics and used to detect and characterize longitudinal immature breathing patterns. To test the effect of gestational age, sex and race on physiologic trajectories, linear mixed effects models were developed using all available daily measurements made in the first 8 weeks of life for each infant. The physiologic response variable was standardized using the rankit transformation of the percentile of each infant compared to other infants at same day of life. The fixed effects were sex, race (white versus non-white), gestational age, day of life and whether infant was mechanically ventilated. The random effects were day of life grouped by infant.
Waiver of consent, consent, and institutional review board approval.
Institutional Review Board (IRB) approval was obtained at all sites. Waiver of consent was approved by the LDCC IRB and 3 of 5 CRC IRBs, while 2 CRC sites obtained consent. Oversight was provided by an observational safety monitoring board, appointed by the National Heart, Lung, Blood Institute (NHLBI).
RESULTS
Demographics of the 717 infants that met inclusion criteria are reported in Table 1 and Supplement Figure 1. Study population by gestational age and postnatal week, indicating enrolled infants alive, in NICU and up to 36 weeks post-menstrual age is shown in Supplement Figure 2. Availability of NICU data was 80% of potentially recorded time for respiratory and ECG waveforms, and 82% of potentially recorded time for heart rate and SpO2 data, with 37,000 of 46,000 NICU days analyzed. Data from all 5 clinical sites are included in the analysis and figures, providing representation of management and care across the sites.
Table 1.
Cohort Characteristics
| n | N | |
|---|---|---|
| Birth weight (grams), mean (SD) | 717 | 871 (259) |
| <10th percentile weight (Fenton) | 717 | 81 (11.3) |
| Gestational age (weeks), mean (SD) | 717 | 26.4 (1.7) |
| 22 0/7-22 6/7 weeks | 717 | 11 (1.5%) |
| 23 0/7-23 6/7 weeks | 717 | 68 (9.5%) |
| 24 0/7-24 6/7 weeks | 717 | 66 (9.2%) |
| 25 0/7-25 6/7 weeks | 717 | 132 (18.4%) |
| 26 0/7-26 6/7 weeks | 717 | 126 (17.6%) |
| 27 0/7-27 6/7 weeks | 717 | 128 (17.9%) |
| 28 0/7-28 6/7 weeks | 717 | 186 (25.9%) |
| Sex (female) | 717 | 351 (49.0%) |
| Multiple gestation | 717 | 165 (23.0%) |
| Born outside study center | 717 | 64 (8.9%) |
| Clinical chorioamnionitis | 591 | 13 (2.2%) |
| Histological chorioamnionitis | 591 | 212 (35.9%) |
| Antenatal steroids: None | 696 | 59 (8.5%) |
| Antenatal steroids: Complete courses only, mean (SD) | 694 | 0.76 (0.58) |
| Mode of birth: C-section | 717 | 486 (67.8%) |
| Infant race: | ||
| Black | 717 | 354 (49.4%) |
| White | 717 | 326 (45.5%) |
| Asian | 717 | 13 (1.8%) |
| North American Indian/Native Alaskan | 717 | 3 (0.4%) |
| Other | 717 | 2 (0.3%) |
| Unknown | 717 | 19 (2.6%) |
| Infant ethnicity: Hispanic | 717 | 118 (16.5%) |
| Maternal age (yrs), mean (SD) | 717 | 29.4 (6.1) |
| Baby’s health insurance: Medicaid/Public | 712 | 450 (63.2%) |
| Baby’s health insurance: Private | 712 | 238 (33.4%) |
| Maternal hypertension | 622 | 250 (40.2%) |
| Maternal hypertension: Pre-existing | 607 | 139 (22.9%) |
| Maternal diabetes mellitus: Gestational diabetes | 603 | 32 (5.3%) |
| Maternal diabetes mellitus: Pre-existing | 599 | 36 (6.0%) |
| Maternal asthma during pregnancy | 597 | 62 (10.4%) |
| Antepartum hemorrhage | 597 | 96 (16.1%) |
| Medications to prolong pregnancy | 677 | 243 (35.9%) |
| Maternal tobacco smoking | 611 | 73 (11.9%) |
| Maternal opiates (oral/iv) or sedatives or other illicit drugs * | 613 | 77 (12.6%) |
| Apgar at 1 minute, mean (SD) | 704 | 4.3 (2.4) |
| Apgar at 5 minutes, mean (SD) | 704 | 6.5 (2.0) |
| Resuscitation at birth: Bag & mask (NIPPV in REDCap) | 712 | 548 (77.0%) |
| Resuscitation at birth: Intubation | 712 | 384 (53.9%) |
| Resuscitation at birth: Chest compression | 712 | 14 (2.0%) |
| Resuscitation at birth: Epinephrine | 712 | 4 (0.6) |
| Prophylactic indomethacin, ibuprofen, acetaminophen | 710 | 268 (37.7%) |
Figures 1–4 plot the frequencies (number events/day) and exposures (minutes/day) of apnea (Fig 1), periodic breathing (Fig 2), intermittent hypoxemia (thresholds of 80% and 90%)(Figs 3, 4), and bradycardia (Fig 5) as a function of developmental maturation. Because apnea and periodic breathing could not be measured during mechanical ventilation, those days were not included in the analysis. All days were included for intermittent hypoxemia and bradycardia regardless of respiratory support.
Figure 1.
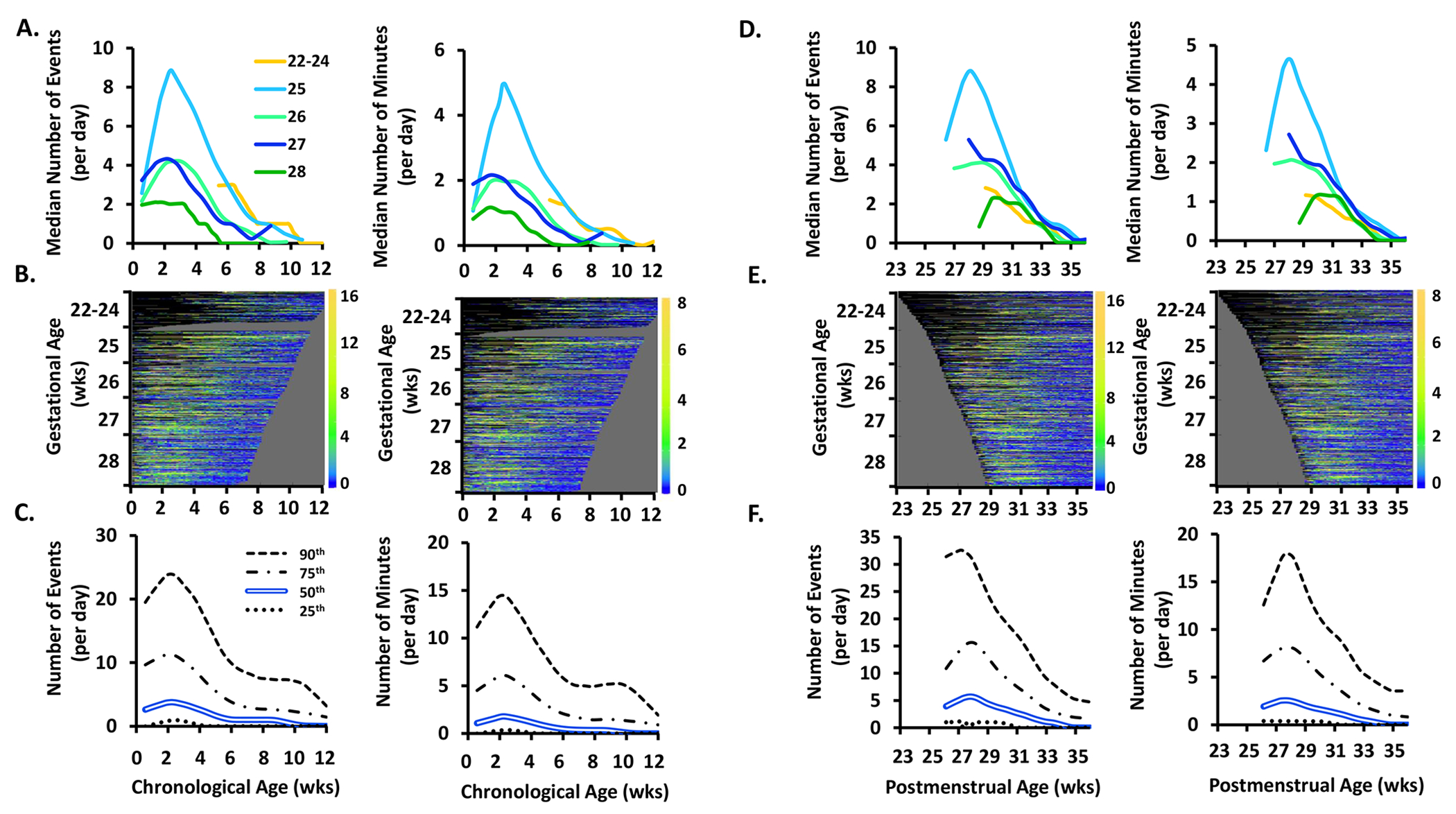
The frequency (number of events/day) and exposure (number of minutes/day) of apnea events by chronological age, postmenstrual age, and gestational age.
Top Row. Data plotted by chronological age are shown in A. and by postmenstrual age in D. Within A. and D., apnea frequency is shown on the left and apnea exposure is shown on the right. Infants are grouped by gestational age as noted in the within-figure key. Days with mechanical ventilation were excluded from apnea and periodic breathing quantifications. Due to the high rate of mechanical ventilation in the 22 to 24 gestational age infants, values were not available during the first 5 weeks of life in this youngest infant cohort.
Middle Row. B. and E. show heat maps in which each colored row represents an individual infant, sorted by gestational age on the y-axis. Dark blue indicates 0 or few events while orange indicates a high incidence of events, with the actual number of events shown in the corresponding color code on the right Y-axis of each heat map. Black indicates no available data (days on mechanical ventilation were excluded for apnea and periodic breathing heat maps and also denoted in black). Grey haystack at the right indicates data truncation when an infant reached 36 weeks 6 days postmenstrual age. Yellow color indicates a higher frequency or exposure of events. The right edges of the heat maps plotted by chronological age (A.) are irregular because of varying times of death or discharge. The left edges of the heat maps plotted by postmenstrual age (B.) are irregular because of varying times of birth.
Bottom Row. C. and F. shows results for the entire cohort as the median (double blue line), 25th (dotted line), 75th (dot and dashed line) and 90th (dashed line) percentiles.
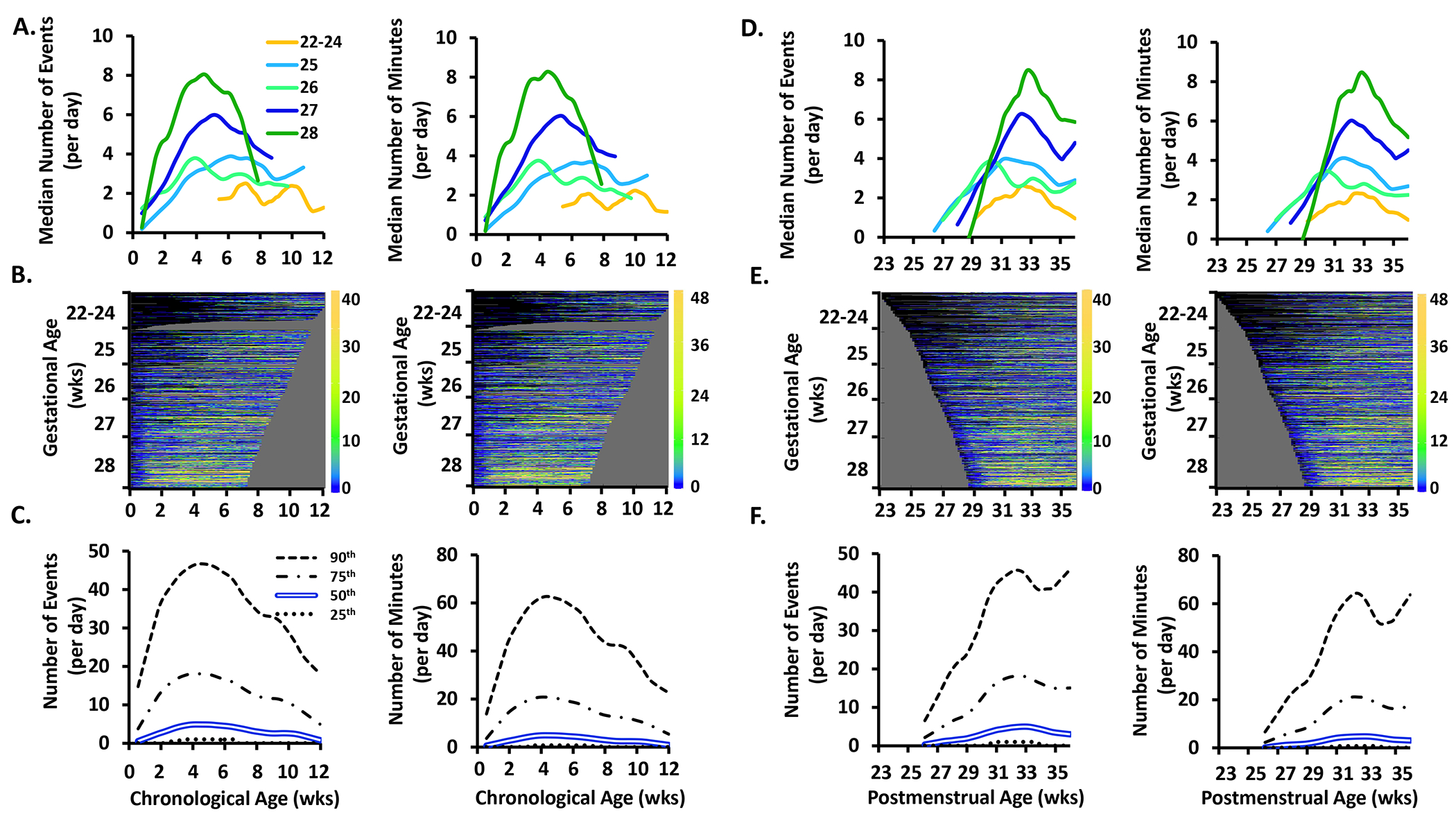
Figure 4.
The frequency (number of events/day) and exposure (number of minutes/day) of intermittent hypoxemia less than 90% events by chronological age, postmenstrual age, and gestational age. See Figure 1 for further details of each row. Similar to IH <80%, yellow is less prominent at the bottom of the heat map signifying that less premature infants have more IH <90% than more premature infants.
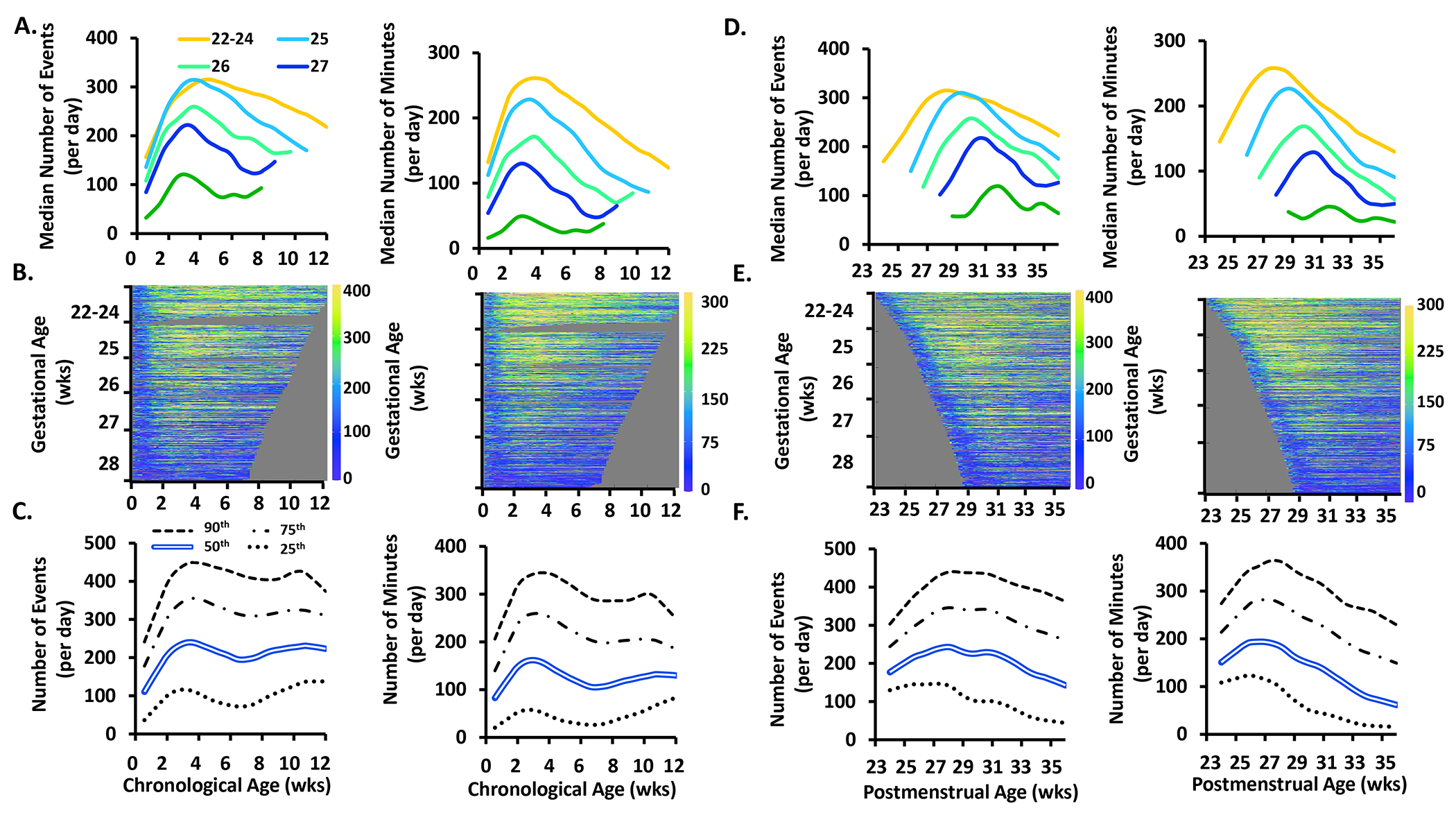
Figure 2.
The frequency (number of events/day) and exposure (number of minutes/day) of periodic breathing events by chronological age, postmenstrual age, and gestational age. See Figure 1 for further details of each row. Yellow is more prominent at the bottom of the heat map signifying that less premature infants have more periodic breathing than more premature infants.
Figure 3.
The frequency (number of events/day) and exposure (number of minutes/day) of intermittent hypoxemia less than 80% events by chronological age, postmenstrual age, and gestational age. See Figure 1 for further details of each row. Yellow is less prominent at the bottom of the heat map signifying that less premature infants have more IH <80% than more premature infants.
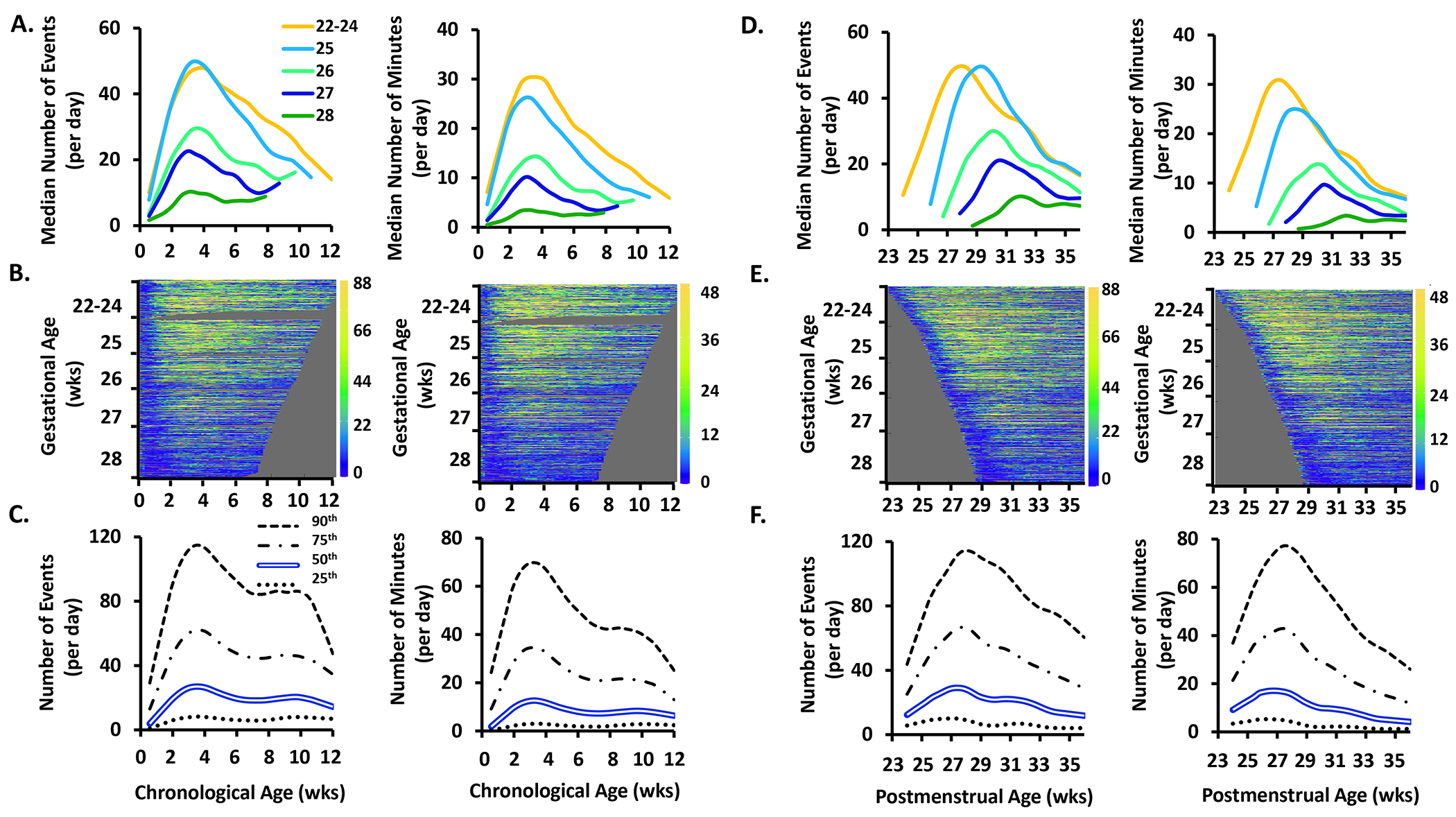
Figure 5.
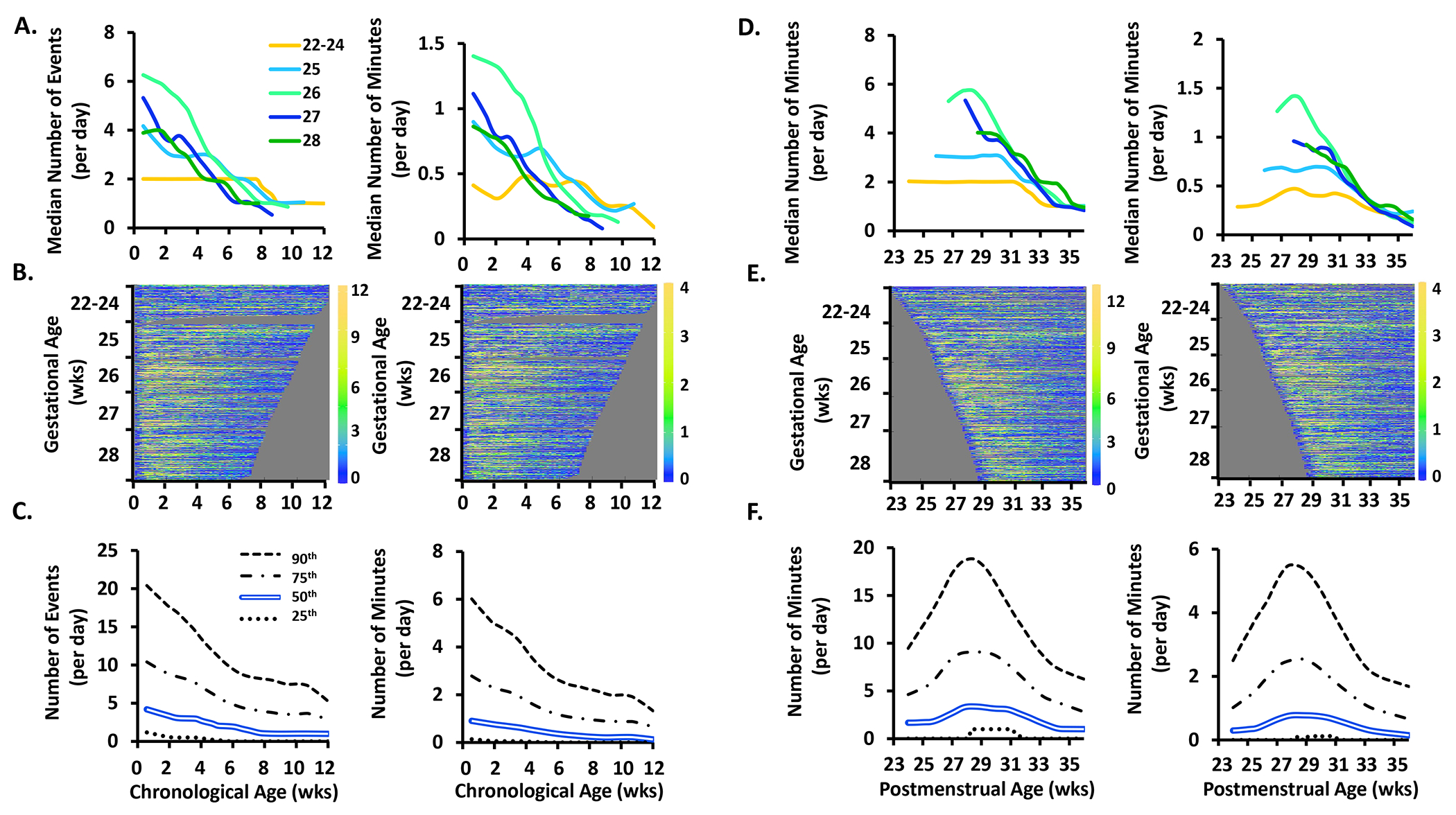
The frequency (number of events/day) and exposure (number of minutes/day) of bradycardia events by chronological age, postmenstrual age, and gestational age. See Figure 1 for further details of each row.
Apnea.
Frequency and exposure of apnea increased over the first 2-3 weeks of life then decreased or resolved by 8-12 weeks chronological age (Fig 1A–C) or 34 weeks postmenstrual age (Fig 1D–F). Frequency of apnea fell with increasing gestational age (p<0.00001, Fig 1A). Both frequency and exposure of apnea peaked at 2.5 weeks chronological age regardless of gestational age, with the highest peak of 9 events and 5 minute/day in infants of 25 weeks gestational age (Figs 1A and 1D). Heat maps give details of individual infant patterns and emphasize the increased number of days on mechanical ventilation in the youngest infants (Figs 1B and 1E), with each line representing an individual infant. The heat maps indicate which patients were part of the analysis at different time points. Almost all infants of 22-24 weeks gestational age were on mechanical ventilation during the first 5 weeks of life and thus did not substantially contribute to the apnea analysis during that time.
Periodic Breathing.
The frequency and exposure of periodic breathing increased over the first 4 weeks of life followed by a decrease or plateau by 8-12 weeks chronological age (Fig 2A–C) or 33 weeks postmenstrual age (Fig 2D–F). In contrast to apnea, there was a significant increase in periodic breathing frequency with increasing gestational age (p<0.00001) (Fig 2A). In addition, the age of peak periodic breathing was more variable between gestational age groups ranging from 4.0-6.5 weeks chronological age (Fig 2A) and 31.0-33.0 weeks postmenstrual age (Fig 2D), with the highest peak of 8 events and 8.3 minutes/day at 4.5 weeks of age in the 28 weeks gestational age infants (Figs 2A and 2D). As with apnea, most infants of 22-24 weeks gestational age were on mechanical ventilation during the first 5 weeks of life and thus did not substantially contribute to the periodic breathing analysis during that time.
Intermittent Hypoxemia.
The frequency and exposure of intermittent hypoxemia episodes <80% and <90% increased over the first 3 weeks of life (Figs 3 and 4A–C, respectively) and then fell. The frequency of intermittent hypoxemia episodes decreased with increasing gestational age (p<0.00001) (Figs 3A and 4A). Intermittent hypoxemia <80% peaked in all gestational age groups around 3.5 weeks chronological age with a corresponding postmenstrual age of 27.5-31.5 weeks (Figs 3A and 3D), while intermittent hypoxemia <90% peaked at 2.5-3.5 weeks chronological age with a corresponding postmenstrual age of 28.5-31.5 weeks (Figs 4A and 4D). Although there was a subsequent decrease in intermittent hypoxemia events, at 8.5 weeks of age the frequency of intermittent hypoxemia events <90% remained substantially elevated with 217 events/day in contrast to 19 events/day for intermittent hypoxemia <80% (Figs 3C and 4C).
Bradycardia.
Among infants with gestational age ≥25 weeks, the frequency and exposure of bradycardia events fell after the first week of life, with resolution by 8-10 weeks (Fig 5A) or 33 weeks postmenstrual age (Fig 5D). In the youngest infants of 22-24 weeks gestation, the frequency of bradycardia remained constant with a median of 2 events and <0.5 minutes/day (Figs 5A and 5D).
Demographic factors.
There were no sex differences in apnea frequency, but females had more periodic breathing and less intermittent hypoxemia and bradycardia (Fig 6 and Supplement Table 1). White infants (n=326) had an overall higher frequency of apnea during the monitoring period, in addition to periodic breathing, and intermittent hypoxemia, with no differences in bradycardia compared to non-white infants (Fig 6 and Supplement Table 1). Exposure (minutes/day) of apnea, intermittent hypoxemia, periodic breathing, and bradycardia followed similar trajectories to the frequency data.
Figure 6.
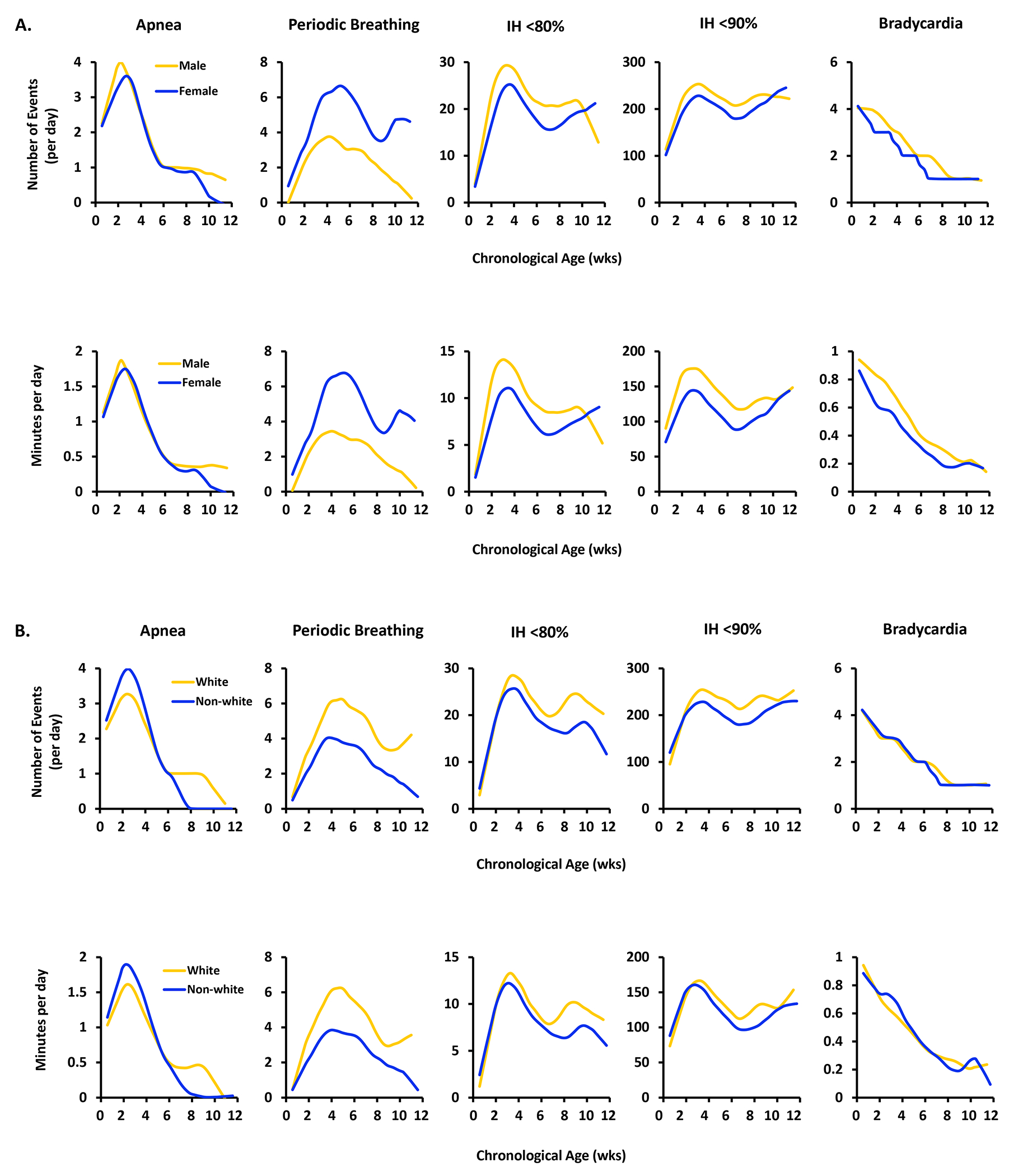
A. The median frequency (number of events/day) (upper row) and exposure (minutes/day) (lower row) of apnea, periodic breathing, intermittent hypoxemia less than 80% and less than 90% and bradycardia events by sex and chronological age.
B. The median frequency (number of events/day) (upper row) and exposure (minutes/day) (lower row) of apnea, periodic breathing, intermittent hypoxemia less than 80% and less than 90% and bradycardia events by race and chronological age.
Caffeine and Mechanical Ventilation
More than 97% of infants received caffeine at some point during their hospital stay, starting at a mean of 2.3±7.8 days of life and lasting for a mean of 56.7±25.4 days. Therefore, we were unable to assess the effect of caffeine on the frequency of events. To assess whether infants coming off mechanical ventilation accounted for the increase in cardioventilatory events during the first month of life, infants who were intubated at least once were compared to infants who were never intubated. Infants never receiving mechanical ventilation followed similar postnatal frequency and exposure trajectories but with a smaller magnitude of apnea and intermittent hypoxemia and a larger magnitude of periodic breathing and initially bradycardia (Supplement Fig 3). Only extubated infants could contribute to apnea and periodic breathing analyses. Therefore, the proportion of infants intubated by postnatal week is provided in Supplement Fig 4.
DISCUSSION
We studied the postnatal trajectories of ventilatory control in the multicenter Pre-Vent study of 717 infants born before 29 weeks gestation across 5 NICUs. Our major findings are that central apnea, periodic breathing, and intermittent hypoxemia episodes peaked in the first few weeks after birth and then diminished. Importantly, the findings for apnea and periodic breathing differed. Apnea was triggered by birth, peaked around 3 weeks of chronological age, and was most frequent in the more premature infants. In contrast, periodic breathing aligned better with postmenstrual age, peaked around 32 weeks, and was more frequent in the more mature infants. Temporal patterns were consistent for all gestational ages, regardless of sex and race, and even for infants never on mechanical ventilation. However, females had more periodic breathing and less intermittent hypoxemia, while white infants had more apnea, periodic breathing, and intermittent hypoxemia.
The distinction between the time courses of apnea and periodic breathing points to different maturational mechanisms. Earlier studies -- smaller, or from a single site -- found differences of timing and duration of periodic breathing and central apnea13,14,24,25. Here, the differences are made vivid by the nature of the cohort -- large, multicenter, and diverse -- and the quantitative detection and analysis of apnea and periodic breathing episodes.
Apnea is a major focus of clinical neonatal practice with potential mechanisms likely to involve multiple factors including reduced central chemoreceptor CO2 sensitivity, reduced or enhanced peripheral chemoreceptor sensitivity, low lung volumes, upper airway reflexes, and immature neuromuscular control of upper airway patency26–30. We found an initial rise of apnea over the first 2-3 postnatal weeks followed by a fall. The time-until-resolution varied inversely with gestational age at birth, consistent with previous studies13,31,32. This temporal pattern was present in infants who were never intubated (although smaller in magnitude) and is likely to be physiological and not due to mixing of data from infants who were recently extubated or about to be intubated.
While periodic breathing, with its short respiratory pauses, may be associated with brief bradycardia or oxygen desaturation33–35, the clinical view is that it reflects ventilatory control immaturity and is benign. One framework for understanding the mechanism is loop gain, a concept that describes the stability of feedback control systems36. In the case of periodic breathing, loop gain is a function of central and peripheral chemoreceptor sensitivity, circulatory delays, and the effectiveness of pulmonary gas exchange as well as lung volume36. A high loop gain is associated with more ventilatory control instability and more periodic breathing. In full-term infants, loop gain is low after birth and increases steadily until about 2-4 weeks of age, slowly declining thereafter36. This trajectory may represent postnatal maturation of peripheral chemoreceptor function as well as stabilization of end-expiratory lung volume36. In infants who were never intubated, we found more periodic breathing with varying onset depending on gestational age. Periodic breathing peaked around 32 weeks postmenstrual age, or 4-7 weeks chronological age, similar to a large single-site study14.
Although apnea typically determines acute interventions, physiologic compromise from intermittent hypoxemia may have more impact on outcomes. Aside from adverse outcomes on neurocognitive development and retinopathy of prematurity, chronic intermittent hypoxemia in neonatal animal studies has been associated with enhanced carotid chemoreceptor sensitivity to hypoxia, upper airway muscle weakness later in life, altered respiratory mechanics and other physiological derangements23,37–39 The low incidence of intermittent hypoxemia in week 1, rise to a peak incidence at 3-4 weeks, and slow decline thereafter confirms similar longitudinal profiles in a previous small study of 79 extremely preterm infants23. In the current study, the intermittent hypoxemia trajectories followed those of apnea rather than periodic breathing. One of the most striking findings was that the number of intermittent hypoxemia events <90% persisted at relatively high levels, with the youngest infants showing over 200 events/day and 100 minutes/day at 12 weeks. Whether this sustained increase in mildly hypoxemic events contributes to poor outcomes is currently unknown.
Oxygen saturation values are often averaged over various time windows before reporting which can affect the oxygen saturation profile40. In this study, the averaging times were 8-10 seconds as commonly practiced in many clinical units. Likewise, lower oxygen saturation targets have been associated with fewer intermittent hypoxemia episodes16. In this study, the NICUs used the AAP-recommended oxygen saturation target of 90-95%15. Finally, a clinically useful threshold of a high-risk intermittent hypoxemia event is not agreed upon. Previous studies have shown a relationship between severe intermittent hypoxemia <80% and morbidity while the NEOPROM trials41 suggest that milder fluctuations <90% may also be important. Therefore, we analyzed two thresholds; <80% to represent severe hypoxemia and <90% to represent times when the infant was below the AAP target of 90 to 95% and thereby still produced alarms. We found lower rates of intermittent hypoxemia events <80% than previously reported1,4,5,42 possibly reflecting longer averaging times, higher oxygen saturation targets, or less severe lung disease.
Numerous aspects of the ventilatory control system change rapidly after birth and there is a growing appreciation that the newborn period is a critical window during which environmental stressors can alter postnatal trajectories of ventilatory control. For example, plasma serotonin concentrations in premature newborns are low at birth and decline further over the first week of life43. Serotonin modulates respiration and plays a critical role in cardioventilatory homeostasis44. In addition, in neonatal rodents, gamma aminobutyric acid (GABA) undergoes an excitatory-to-inhibitory switch within the first week of life45. If or when this occurs in preterm infants is unknown46,47. Birth is also followed by a resetting of peripheral chemoreceptor sensitivity to adjust from the in utero hypoxic environment to the higher arterial oxygen tension after birth. Ventilatory control system maturation is genetically regulated but ventilatory control neurons and neural pathways also exhibit phenotypic plasticity, during critical periods, in response to environmental stressors such as hyperoxia and intermittent hypoxia48,49. Ventilatory control instability, for example, may cause chronic intermittent hypoxemia, which can irreversibly increase carotid chemoreceptor sensitivity, leading to increased ventilatory control instability50. In contrast, intermittent hyperoxia in neonatal animal models can blunt the hypoxic ventilatory response, which may persist into adulthood51,52. Finally, underlying lung disease and low lung volumes may further exacerbate intermittent hypoxia and increase loop gain, potentially resulting in more periodic breathing and chronic intermittent hypoxemia53. Taken together, these results may lead to insights into potential mechanisms for the extremely preterm-born infant physiologic phenotype.
Demographic factors may also affect maturation of ventilatory control. An early investigation of breathing characteristics in 40 infants who died of sudden infant death syndrome and 607 healthy infants found a greater frequency of apnea in males54. A subsequent study of >24,000 preterm infants revealed more females with apnea but the difference was of only marginal significance (p=0.058)55. These authors also reported that females spent less time on caffeine, suggesting that maturation of respiratory control may occur more rapidly in females, resulting in less intermittent hypoxemia55. And a recent study of >900 infants <34 weeks gestation reported no difference between sexes in apnea when accompanied by both hypoxemia and bradycardia56. These conflicting findings may represent variations in detection and definitions of events. For example, Nagraj et al did not separate apnea from intermittent hypoxemia and bradycardia events. We found no sex differences in the frequency of apnea. However, females had more periodic breathing and less intermittent hypoxemia, suggesting that females exhibit a more rapid maturation of ventilatory control following birth. In addition, white infants had more apnea, periodic breathing and intermittent hypoxemia. These results from the Pre-Vent study suggest that both sex and race may play a role in ventilatory control. However, the cause of racial differences in ventilatory control are unknown but could be due to differences in prenatal maternal environment, treatment disparities or severity of respiratory disease. These are all beyond the scope of this study and worthy of further examination.
Though these results in >700 extremely premature infants represent the spectrum of five NICUs in the Pre-Vent study, we identify several limitations. First, their generalizability may be limited by variability in clinical practice, bedside monitor manufacturers, and data acquisition practices at other sites. Second, we do not report on apnea and periodic breathing in ventilated infants because mechanical breaths can preclude detection of these events, limiting the number of infants included for analysis, especially at the youngest gestational ages for the first several weeks of life. Third, periods of non-invasive ventilation were included in the analyses, but such support could alter the cardioventilatory patterns yet include some of the youngest infants. Fourth, intermittent hypoxemia and bradycardia can occur during periodic breathing, apnea, hypoventilation and motion57 and in infants on mechanical ventilation58, therefore intermittent hypoxemia and bradycardia were reported during all modes of ventilation, representing the full cohort. Fifth, the results of this multicenter study also represent a population treated with current caffeine protocols, a well-known treatment for apnea of prematurity and intermittent hypoxemia59. Over 97% of infants were on caffeine for a mean duration of 57 days. Therefore, the frequency of cardioventilatory events in untreated extremely preterm infants may be substantially higher12. Sixth, though the Pre-Vent cohort exceeded the RFA expectation of 500 infants by >40%, the variation by site for waiver of consent to multi-site study enrollment (3 sites) vs. required consent for multi-/single-site enrollment (2 sites), coupled with recruitment issues related to the COVID-19 pandemic, resulted in an additional 273 eligible babies who were not enrolled because parents were not/could not be approached or declined, so we cannot rule out small effects of this enrollment strategy. Lastly, these metrics may not correspond to traditional bedside clinically acquired measures but may provide insight for future clinical monitoring. To advance these findings and tools at the bedside requires automated data analysis with algorithms that are not currently available outside the research setting.
In conclusion, in this large cohort of preterm infants <29 weeks gestation, the first week of life is marked by more frequency and severity of apnea and intermittent hypoxemia events that peaks by 4 weeks of life and is inversely correlated with gestational age. In contrast, periodic breathing peaks later for the most premature infants, while bradycardia is most frequent immediately after birth. These transitory manifestations of immaturity of cardioventilatory control may require intervention but do not necessarily imply structural heart or lung disease or the need for long-term support. Knowledge of typical longitudinal patterns of physiologic instability can impact clinical care by helping to identify individual patients that may be outliers and can serve as the basis for further research into the links between physiologic biomarkers and outcomes.
Supplementary Material
Impact Response Statement.
Physiologic curves of cardiorespiratory events in extremely preterm-born infants offer 1)objective measures to assess individual patient courses and 2)guides for research into control of ventilation, biomarkers and outcomes.
Presented are updated maturational trajectories of apnea, periodic breathing, intermittent hypoxemia, and bradycardia in 717 infants born <29 weeks gestation from the multi-site NHLBI-funded Pre-Vent study.
Cardioventilatory events peak during the first month of life but the actual postnatal trajectory is dependent on the type of event, race, sex and use of mechanical ventilation.
Different time courses for apnea and periodic breathing suggest different maturational mechanisms.
Acknowledgements
The National Institutes of Health (NIH) and the National Heart, Lung, and Blood Institute (NHLBI) provided grant support through cooperative agreements. While NHLBI staff had input into study design, conduct, analysis, and manuscript drafting, the content and views expressed in this article are solely the responsibility of the authors and do not necessarily represent the official views of NIH or the U.S. Department of Health and Human Services.
Participating sites collected and stored the data while the University of Virginia, the lead data and coordinating center (LDCC), analyzed the data. The co-PIs at each site had full access to her/his individual site data and take responsibility for the integrity of the raw waveforms while Drs. Randall Moorman (LDCC co-PI) and Douglas Lake (LDCC co-PI) take responsibility for the integrity of the data and accuracy of the data analysis.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following individuals, in addition to those listed as authors, participated in this study:
NIH/NHLBI
Neil Aggarwal, MD: National Institutes of Health, National Heart, Lung and Blood Institute, Division of Lung Diseases, Bethesda, MD
Lawrence Baizer, PhD: National Institutes of Health, National Heart, Lung and Blood Institute, Division of Lung Diseases, Bethesda, MD
Peyvand Ghofrani, MDE: National Institutes of Health, National Heart, Lung and Blood Institute, Division of Lung Diseases, Bethesda, MD
Aaron D. Laposky, PhD: National Institutes of Health, National Heart, Lung and Blood Institute, Division of Lung Diseases, Bethesda, MD
Aruna Natarajan, MD, PhD: National Institutes of Health, National Heart, Lung and Blood Institute, Division of Lung Diseases, Bethesda, MD
Barry Schmetter, BS: National Institutes of Health, National Heart, Lung and Blood Institute, Division of Lung Diseases, Bethesda, MD
OSMB
Estelle B. Gauda, MD (Chair): University of Toronto Hospital for Sick Children, Division of Neonatology, Toronto, Ontario
Jonathan M. Davis, MD: Tufts Clinical and Translational Science Institute, Division of Newborn Medicine, Boston, MA
Roberta L. Keller, MD: University of California, San Francisco School of Medicine, Department of Pediatrics, San Francisco CA
Robinder G. Khemani, MD: Children’s Hospital Los Angeles, Department of Anesthesiology and Critical Care Medicine, Los Angeles, CA
Renee H. Moore, PhD: Drexel University Department of Epidemiology and Biostatistics, Philadelphia, PA
Elliott M. Weiss, MD, MSME: Department of Pediatrics, University of Washington School of Medicine, Seattle, WA
University of Virginia
Amy K. Camblos, BS: University of Virginia School of Medicine, Clinical Trials Office, UVa School of Medicine, Charlottesville, VA
Gina M. Duda, BS: University of Virginia School of Medicine, Clinical Trials Office, UVa School of Medicine, Charlottesville, VA
Abigail A. Flower, PhD University of Virginia, Data Science Institute, Charlottesville, VA
Steven A. Fowler, BS: University of Virginia School of Medicine, Clinical Trials Office, UVa School of Medicine, Charlottesville, VA
Patcharin Pramoonjago, PhD: University of Virginia School of Medicine, Biorepository and Tissue Research Facility, Charlottesville, VA
Craig A. Rumpel, MS: University of Virginia School of Medicine, Biorepository and Tissue Research Facility, Charlottesville, VA
Northwestern University
Michael S. Carroll, PhD: Ann & Robert H. Lurie Children’s Hospital of Chicago and Stanley Manne Children’s Research Institute, Data Analytics and Reporting, Chicago, IL
Bradley S. Hopkins, RRT: Ann & Robert H. Lurie Children’s Hospital of Chicago, and Stanley Manne Children’s Research Institute, Pediatric Autonomic Medicine, Chicago, IL
University of Alabama at Birmingham
David Paydarfar, MD: University of Texas Austin, Department of Neurology at Dell Medical School, Austin, TX
Elisabeth Salisbury, PhD, University of Massachusetts Medical School, Department of Pediatrics and Neurology, Worcester, MA
Bradley Troxler, MD: University of Alabama at Birmingham School of Medicine, Division of Pulmonary and Sleep Medicine, Department of Pediatrics, Birmingham, AL
Washington University
Ryan Colvin, MS: Washington University School of Medicine in St. Louis, Division of General Medicine, St. Louis, MO
Joey Egan, RRT: St. Louis Children’s Hospital, Respiratory Care, St. Louis, MO
Elise Eiden, MS, Washington University School of Medicine in St. Louis, Institute for Informatics, St. Louis, MO
Jeffery Hoover, MD: Washington University School of Medicine in St. Louis, Division of Newborn Medicine, St. Louis, MO
Laura Linneman, RN: Washington University School of Medicine in St. Louis, St. Louis, MO
Daniel Mammel MD: Washington University School of Medicine in St. Louis, Division of Newborn Medicine, St. Louis, MO
Michael McLeland, PhD: St. Louis Children’s Hospital, Sleep Laboratory, St. Louis, MO
Harley Pyles, RRT: St. Louis Children’s Hospital, Respiratory Care, St. Louis, MO
Barbara Warner, MD: Washington University School of Medicine in St. Louis, Division of Newborn Medicine, St. Louis, MO
Funding Statement
Supported by NIH grants as provided below:
University of Virginia (NCT03174301): U01 HL133708, HL133708-05S1
Case Western Reserve University: U01 HL133643, The Gerber Foundation
Northwestern University: U01 HL133704
University of Alabama at Birmingham: U01 HL133536
University of Miami: U01 HL133689
Washington University: U01 HL133700
Footnotes
Competing Interests: None of the authors have financial ties to products in the study or potential/perceived conflicts of interest.
Consent Statement: Institutional Review Board (IRB) approval was obtained at all sites. Waiver of consent was approved by the LDCC IRB and 3 of 5 CRC IRBs, while 2 CRC sites obtained consent.
Data Availability Statement
The datasets generated during and analyzed during the current study are not publicly available due to embargo until after publication of the primary outcome manuscripts, at which point they will become available in the NHLBI’s Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC biolincc.nhlbi.nih.gov). The datasets are available from the corresponding author on reasonable request.
References
- 1.Di Fiore JM et al. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J Pediatr. 157,69–73(2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Fiore JM et al. Patterns of Oxygenation, Mortality, and Growth Status in the Surfactant Positive Pressure and Oxygen Trial Cohort. J Pediatr. 186,49–56 e41(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Fiore JM et al. Early inspired oxygen and intermittent hypoxemic events in extremely premature infants are associated with asthma medication use at 2 years of age. J Perinatol. 39,203–211(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poets CF et al. Association Between Intermittent Hypoxemia or Bradycardia and Late Death or Disability in Extremely Preterm Infants. JAMA. 314,595–603(2015). [DOI] [PubMed] [Google Scholar]
- 5.Fairchild KD, Nagraj VP, Sullivan BA, Moorman JR, Lake DE Oxygen desaturations in the early neonatal period predict development of bronchopulmonary dysplasia. Pediatr Res. 85,987–993(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sullivan BA, et al. Early Heart Rate Characteristics Predict Death and Morbidities in Preterm Infants. J Pediatr.174,57–62(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan BA, et al. Early Pulse Oximetry Data Improves Prediction of Death and Adverse Outcomes in a Two-Center Cohort of Very Low Birth Weight Infants. Am J Perinatol. 35,1331–1338(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sullivan BA, et al. Clinical and vital sign changes associated with late-onset sepsis in very low birth weight infants at 3 NICUs. JNPN.14,553–561(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raffay TM, et al. Neonatal intermittent hypoxemia events are associated with diagnosis of bronchopulmonary dysplasia at 36 weeks postmenstrual age. Pediatr Res. 85,318–323(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vesoulis ZA, et al. Early hypoxemia burden is strongly associated with severe intracranial hemorrhage in preterm infants. J Perinatol. 39,48–53(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fairchild KD, et al. Vital signs and their cross-correlation in sepsis and NEC: a study of 1,065 very-low-birth-weight infants in two NICUs. Pediatr Res. 81,315–321(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagraj VP, Sinkin RA, Lake DE, Moorman JR, Fairchild KD Recovery from bradycardia and desaturation events at 32 weeks corrected age and NICU length of stay: an indicator of physiologic resilience? Pediatr Res. 86,622–627(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairchild K, et al. Clinical associations of immature breathing in preterm infants: part 1-central apnea. Pediatr Res. 80,21–27(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel M, et al. Clinical associations with immature breathing in preterm infants: part 2-periodic breathing. Pediatr Res. 80,28–34(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cummings JJ, Polin RA Oxygen Targeting in Extremely Low Birth Weight Infants. Pediatrics. 138,e1–e9(2016). [DOI] [PubMed] [Google Scholar]
- 16.Di Fiore JM, et al. Low oxygen saturation target range is associated with increased incidence of intermittent hypoxemia. J Pediatr. 161,1047–1052(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dennery PA, et al. Pre-Vent: the prematurity-related ventilatory control study. Pediatr Res. 85,769–776(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laird P, et al. The critical care data exchange format: a proposed flexible data standard for combining clinical and high-frequency physiologic data in critical care. Physiol Meas. 42,(2021). [DOI] [PubMed] [Google Scholar]
- 19.Vergales BD, et al. Accurate automated apnea analysis in preterm infants. Am J Perinatol. 31,157–162(2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark MT, et al. Stochastic modeling of central apnea events in preterm infants. Physiol Meas. 37,463–484(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finer NN, Higgins R, Kattwinkel J, Martin RJ. Summary proceedings from the apnea-of-prematurity group. Pediatrics. 117,S47–51(2006). [DOI] [PubMed] [Google Scholar]
- 22.Mohr MA, et al. Quantification of periodic breathing in premature infants. Physiol Meas. 36,1415–1427(2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Fiore JM, et al. The relationship between patterns of intermittent hypoxia and retinopathy of prematurity in preterm infants. Pediatr Res. 72,606–612(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrington KJ, Finer NN Periodic Breathing and Apnea in Preterm Infants. Pediatr Res. 27,118–121(1990). [DOI] [PubMed] [Google Scholar]
- 25.Glotzbach SF, Baldwin RB, Lederer NE, Tansey PA, Ariagno RL Periodic breathing in preterm infants: incidence and characteristics. Pediatrics. 84,785–792(1989). [PubMed] [Google Scholar]
- 26.Di Fiore JM, Martin RJ, Gauda EB Apnea of prematurity--perfect storm. RESPNB. 189,213–222(2013). [DOI] [PubMed] [Google Scholar]
- 27.Gauda EB, McLemore GL, Tolosa J, Marston-Nelson J, Kwak D Maturation of peripheral arterial chemoreceptors in relation to neonatal apnoea. Seminars in neonatology : SN. 9,181–194(2004). [DOI] [PubMed] [Google Scholar]
- 28.Erickson G, Dobson NR, Hunt CE. Immature control of breathing and apnea of prematurity: the known and unknown. J Perinatol. 41,2111–2123(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poets CF Apnea of prematurity: What can observational studies tell us about pathophysiology? Sleep Med. 11,701–707(2010). [DOI] [PubMed] [Google Scholar]
- 30.Martin RJ, Wilson CG Apnea of prematurity. Compr Physiol. 2,2923–2931(2012). [DOI] [PubMed] [Google Scholar]
- 31.Eichenwald EC, Aina A, Stark AR Apnea frequently persists beyond term gestation in infants delivered at 24 to 28 weeks. Pediatrics. 100,354–359(1997). [DOI] [PubMed] [Google Scholar]
- 32.Henderson-Smart DJ The effect of gestational age on the incidence and duration of recurrent apnoea in newborn babies. Aust Paediatr J. 17,273–276( 1981). [DOI] [PubMed] [Google Scholar]
- 33.Weintraub Z, et al. The morphology of periodic breathing in infants and adults. Respir Physiol. 127,173–184(2001). [DOI] [PubMed] [Google Scholar]
- 34.Fenner A, Schalk U, Hoenicke H, Wendenburg A, Roehling T Periodic breathing in premature and neonatal babies: incidence, breathing pattern, respiratory gas tensions, response to changes in the composition of ambient air. Pediatr Res. 7,174–183(1973). [DOI] [PubMed] [Google Scholar]
- 35.Seppä-Moilanen M, Andersson S, Kirjavainen T Supplemental Oxygen Treats Periodic Breathing without Effects on Sleep in Late-Preterm Infants. Neonatol. 119,567–574)( 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards BA, Sands SA, Berger PJ Postnatal maturation of breathing stability and loop gain: the role of carotid chemoreceptor development. RESPNB. 185,144–155(2013). [DOI] [PubMed] [Google Scholar]
- 37.McDonald FB, Williams R, Sheehan D, O’Halloran KD Early life exposure to chronic intermittent hypoxia causes upper airway dilator muscle weakness, which persists into young adulthood. Exp Physiol. 100,947–966(2015). [DOI] [PubMed] [Google Scholar]
- 38.Dylag AM, et al. Long-term effects of recurrent intermittent hypoxia and hyperoxia on respiratory system mechanics in neonatal mice. Pediatr Res. 81,565–571(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Fiore JM, MacFarlane PM, Martin RJ Intermittent Hypoxemia in Preterm Infants. Clin Perinatol. 46,553–565(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vagedes J, Poets CF, Dietz K Averaging time, desaturation level, duration and extent. Arch Dis Child Fetal Neonatal. 98,F265–266(2013). [DOI] [PubMed] [Google Scholar]
- 41.Askie LM, et al. Association Between Oxygen Saturation Targeting and Death or Disability in Extremely Preterm Infants in the Neonatal Oxygenation Prospective Meta-analysis Collaboration. JAMA. 319,2190–2201(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Fiore JM, et al. Prematurity and postnatal alterations in intermittent hypoxaemia. Arch Dis Child Fetal Neonatal. 106,557–559(2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schumacher RE, Farrell PM, Olson EB Jr. Circulating 5-hydroxytryptamine concentrations in preterm newborns. Pediatr Pulmonol. 3,117–122(1987). [DOI] [PubMed] [Google Scholar]
- 44.Cummings KJ, Leiter JC Take a deep breath and wake up: The protean role of serotonin preventing sudden death in infancy. Exp Neurol. 326,113165(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valeeva G, Valiullina F, Khazipov R Excitatory actions of GABA in the intact neonatal rodent hippocampus in vitro. Fron Cell Neurosc. 7,20(2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Myers MM, et al. Developmental profiles of infant EEG: overlap with transient cortical circuits. Clin Neurophysiol. 123,1502–1511(2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanhatalo S, Kaila K Development of neonatal EEG activity: from phenomenology to physiology. Semin Fetal Neonatal Med. 11,471–478(2006). [DOI] [PubMed] [Google Scholar]
- 48.Carroll JL Developmental plasticity in respiratory control. J Applied physiol. 94,375–389(2003). [DOI] [PubMed] [Google Scholar]
- 49.Bavis RW, MacFarlane PM Developmental plasticity in the neural control of breathing. Exp Neurol. 287,176–191(2017). [DOI] [PubMed] [Google Scholar]
- 50.Pawar A, Peng YJ, Jacono FJ, Prabhakar NR Comparative analysis of neonatal and adult rat carotid body responses to chronic intermittent hypoxia. J Appl Physiol. 104,1287–1294(2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bavis RW, Russell KE, Simons JC, Otis JP Hypoxic ventilatory responses in rats after hypercapnic hyperoxia and intermittent hyperoxia. Resp Physiol Neurobiol. 155,193–202(2007). [DOI] [PubMed] [Google Scholar]
- 52.Logan S, et al. Chronic intermittent hyperoxia alters the development of the hypoxic ventilatory response in neonatal rats. Resp Physiol Neurobiol. 220,69–80(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Esquer C, Claure N, D’Ugard C, Wada Y, Bancalari E Role of abdominal muscles activity on duration and severity of hypoxemia episodes in mechanically ventilated preterm infants. Neonatol. 92,182–186(2007). [DOI] [PubMed] [Google Scholar]
- 54.Kato I, et al. Developmental characteristics of apnea in infants who succumb to sudden infant death syndrome. Am J Respir Crit. 164,1464–1469(2001). [DOI] [PubMed] [Google Scholar]
- 55.Bairam A, et al. Sex-based differences in apnoea of prematurity: A retrospective cohort study. Exp Physiol. 103,1403–1411(2018). [DOI] [PubMed] [Google Scholar]
- 56.Nagraj VP, Lake DE, Kuhn L, Moorman JR, Fairchild KD Central Apnea of Prematurity: Does Sex Matter? Am J Perinatol. 38,1428–1434(2021). [DOI] [PubMed] [Google Scholar]
- 57.Dormishian A, Schott A, Aguilar AC, Bancalari E, Claure N Pulse oximetry reliability for detection of hypoxemia under motion in extremely premature infants. Pediatr Res. 93,118–124(2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dimaguila MA, Di Fiore JM, Martin RJ, Miller MJ. Characteristics of hypoxemic episodes in very low birth weight infants on ventilatory support. J Pediatr. 130,577–583(1997). [DOI] [PubMed] [Google Scholar]
- 59.Chavez L, Bancalari E Caffeine: Some of the Evidence behind Its Use and Abuse in the Preterm Infant. Neonatol. 119,428–432(2022). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and analyzed during the current study are not publicly available due to embargo until after publication of the primary outcome manuscripts, at which point they will become available in the NHLBI’s Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC biolincc.nhlbi.nih.gov). The datasets are available from the corresponding author on reasonable request.


