Abstract
Background
Immune checkpoint inhibitor-related pneumonitis (CIP) stands out as a particularly severe adverse event caused by immune checkpoint inhibitors, with a substantial real-world incidence ranging from 13 to 19%. While systemic corticosteroids represent the standard treatment for CIP, therapeutic options become limited in cases where patients do not respond to corticosteroid therapy. Such patients are classified as having steroid-resistant CIP, often associated with a poor prognosis. This case study provides insight into the symptoms, diagnostic process, and treatment approach for steroid-resistant CIP. Notably, successful management is demonstrated through the utilization of cyclosporine, highlighting its potential mechanisms of action in effectively treating steroid-resistant CIP.
Case description
We present the case of a 53-year-old male with stage IV. A non-small cell lung cancer (NSCLC), who experienced elevated fever, cough, and dyspnea subsequent to immunotherapy treatment. Based on his medical history, clinical manifestations, and radiological findings, the patient was diagnosed with CIP. Initial administration of led to improvement, but during the subsequent tapering of corticosteroid therapy, a resurgence of CIP occurred, resulting in respiratory failure. Consequently, we arrived at the diagnosis of steroid-resistant CIP, prompting the implementation of a combination therapy with cyclosporine and corticosteroids to establish stable disease control. Upon systematic reduction of corticosteroid dosage, the patient maintained a favorable response with no recurrence.
Conclusions
This marks the first instance of effectively managing steroid-resistant CIP through the combined use of cyclosporine and corticosteroids. Presently, cases of steroid-resistant CIP remain infrequent, necessitating vigilant and meticulous monitoring within clinical settings. Notably, there exists no distinct guideline specifying a singular agent for rescuing patients unresponsive to corticosteroid therapy. Therefore, cyclosporine emerges as a promising and efficacious treatment alternative for individuals unresponsive to corticosteroid intervention in the context of CIP.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-024-03258-5.
Keywords: Cyclosporine, Corticosteroids, Steroid resistance, Checkpoint inhibitor-related pneumonitis, Lung cancer
Backgrounds
Immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment by utilizing the immune system to recognize and attack cancer cells [1, 2]. However, ICI-based treatment accompanied severe immune-related adverse events (irAEs) to which almost all organ systems are susceptible, especially checkpoint inhibitor-related pneumonitis (CIP) [3]. In real-world studies, the incidence of CIP can be as high as 10–19%, and severe cases, especially grade ≥ 3 cases can be fatal [4]. Previous studies have revealed that CIP may occur more frequently and have a faster onset in non-small cell lung cancer (NSCLC) compared to other types of cancers [4]. Further, with the increasing application of ICI treatment, the incidence and mortality of CIP may increase. Major guidelines and expert consensus reports recommend that the first treatment for CIP is still corticosteroids [5, 6]. However, only 70–80% of patients with CIP respond to conventional corticosteroids, and the remaining patients are refractory or become resistant to steroids [7]. Treatment options for these patients are extremely limited, with only limited evidence suggesting the potential efficacy of drugs such as infliximab, and nintedanib, to name a few [8, 9].
Cyclosporine A, a calcineurin inhibitor, is a potent non-cytotoxic immunosuppressive agent that has been widely used in interstitial lung disease associated with autoimmune diseases and rheumatoid arthritis [10]. Given that CIP is an immune-related interstitial lung disease (ILD) that may have overlapping pathophysiological mechanisms with other ILDs, it is possible that such patients can benefit from cyclosporine-based treatment methods [11].
Herein, we report a case of a patient with NSCLC who developed severe CIP after receiving pembrolizumab. The patient was hospitalized four times repeatedly due to steroid-resistant CIP, and the patient’s condition was ultimately controlled with a combination of cyclosporine and low-dose corticosteroid treatment. We further investigated the potential relationship between steroid-resistant CIP in order to provide new treatment options for CIP.
Case presentation
Basic clinical information
The patient was a 53-year-old male nonsmoker with a body mass index (BMI) of approximately 22 kg/m² (weight 60 kg, height 165 cm), was diagnosed with right upper lobe adenocarcinoma with pleural metastases (cT2aN2M1a, stage IVA) during routine examination. Treatment with pemetrexed and nedaplatin in combination with pembrolizumab (100 mg q3w) for seven cycles (dose adjustment due to economic distress) was initiated, followed by seven cycles of deplatinated therapy (pemetrexed plus pembrolizumab), resulting in partial response (PR). Subsequently, he underwent monotherapy with pembrolizumab, with the dose adjusted to 200 mg q4w, considering the single treatment modality and the patient’s economic constraints.
Checkpoint inhibitor pneumonitis and grade 3 + respiratory failure
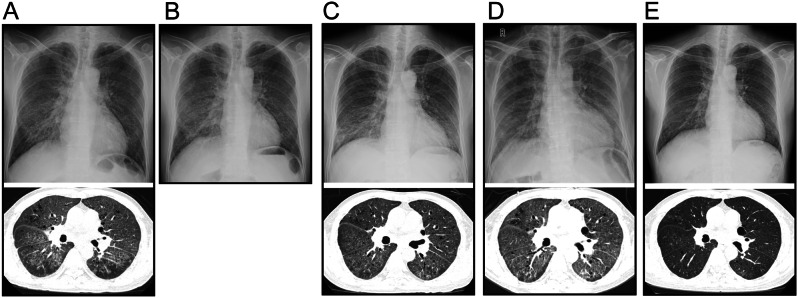
On the 27th day of the fourth cycle of pembrolizumab monotherapy, the patient had a fever of up to 38℃ with cough, and expectoration for three consecutive days, which was not a concern until dyspnea developed. Laboratory tests revealed the white blood cell count and lactate dehydrogenase levels fluctuated within normal limits, except for kreb von den lungen-6 (KL-6) (9345 U/mL) (Fig. 1), which were much higher than normal. Chest computed tomography on admission showed extensive interstitial lesions and ground-glass opacities (GGOs) in both lungs, most of which were grid-like (Fig. 2A). The imaging staging was diagnosed as acute interstitial pneumonia-associated-acute respiratory distress syndrome (AIP-ARDS). To further clarify the diagnosis, a histopathological biopsy of the right upper lung was performed by bronchoscopy, biopsy revealed the tissue changes were consistent with interstitial lung inflammation with fibrinoid exudative inflammation. Finally, he was diagnosed with CIP of grade 3.
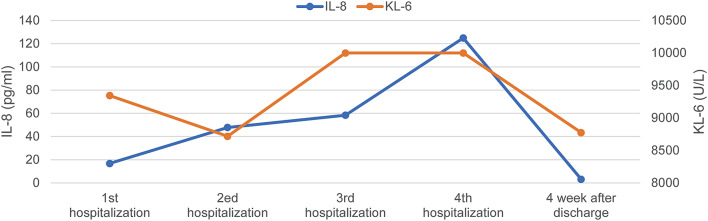
Fig. 1.
The changing trend of IL-8 and KL-6 in the four hospitalization of CIP
Fig. 2.
(A-E) Dynamic changes of chest X-ray and chest computed tomography (CT) in five stages of CIP
Due to his critical condition combined with respiratory failure, the patient was given methylprednisolone (120 mg, QD) on the first day of diagnosis. The aforementioned symptoms were improved (PaO2/FiO2 347.1 mmHg, respiratory rate < 20 per/minute), and chest X-ray showed absorption of interstitial lesions in both lungs indicating that the treatment was effective. He was then instructed to take prednisone acetate tablets (45 mg) and continue to take them orally, and then consider reducing the dosage according to the followup results.
Recurrence of grade 2 checkpoint inhibitor pneumonitis after 10 days
On the tenth day after discharge, the patient returned with shortness of breath after activity and chest tightness. The chest X-ray showed increased interstitial lesions in both lungs than before, and he was hospitalized again with grade 2 CIP. The patient did not discontinue the drug artificially after discharge, and we considered CIP recurrence. Further, the symptoms were also relieved after three days of prednisolone (120 mg QD) treatment (Fig. 2B). After discharge, the patient continued to take a higher dose of oral prednisone acetate tablets (60 mg) than he had taken during the previous hospitalization.
Recurrence of steroid-resistant checkpoint inhibitor pneumonitis grade 3 + respiratory failure
Unfortunately, the patient returned with dyspnea on the 7th day after the second discharge, he was unable to lie down accompanied by cough and fever (up to 38.4℃). He had a PaO2/FiO2 of 197.9 mm Hg and KL-6 > 10,000 U/ml. The recurrence of CIP was evaluated as a grade 3 adverse event (Fig. 2C). Given that a previously reported case of steroid-refractory CIP was successfully treated by pulse steroid therapy [12], we gave methylprednisolone 400 mg high-dose shock on the first day to rule out the recurrence of CIP as a corticosteroid dosage problem. We then gradually reduced the dosage to 80 mg/day by the sixth day according to the improvement of symptoms and imaging results. At discharge, the patient was still taking prednisone acetate tablets 40 mg.
Checkpoint inhibitor pneumonitis stability control: cyclosporine + low dose methylprednisolone
On the fifth day after the third discharge, he presented with an aggravated cough, chest pain, and fatigue, two lungs presented diffuse parenchymal lesions (Fig. 2D). Further, the KL-6 and IL-8 level was significantly higher than normal (Fig. 1). We determined the type of CIP in this patient to be steroid-resistant CIP. Considering the patient’s radiological imaging showed severe diffuse interstitial change in both lungs, and the clinical second-line treatment for CIP has always been based on experience of treating other irAEs and other lung diseases [7], cyclosporine has been reported to be effective in treating interstitial pulmonary fibrosis and extensive pulmonary fibrosis resulting from organ transplantation with no significant adverse effects [13, 14], we tentatively used cyclosporine (25 mg) based on 60 mg/day of prednisolone. Surprisingly, the patient’s symptoms were significantly relieved on the 3rd day, and the corticosteroid dose was rapidly tapered down to 20 mg on the third day. On the 15th day, the patient was discharged asymptomatically and a marked amelioration of pulmonary opacities was observed by chest radiography (Fig. 2E).
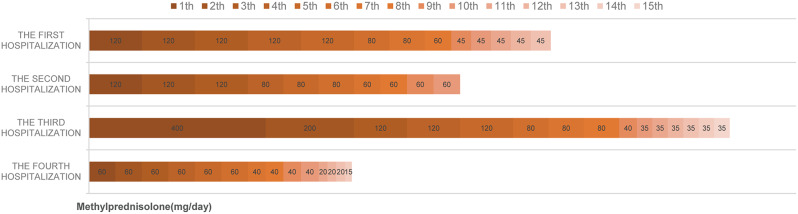
During these four hospitalizations of the patient for CIP, microbiological examination findings were all negative, and immunotherapy was suspended but fortunately, there was no progress in the tumor. In the next course of treatment, we will fully evaluate to immunotherapy rechallenge. Presently, he remains on 15 mg corticosteroids with 25 mg cyclosporine, and there is no recurrent CIP. Further, KL-6 levels also decreased significantly (Fig. 1). His blood pressure, creatinine, and other indicators were in the normal range without any cyclosporine-related side effects. Meanwhile, since we used cyclosporine in conjunction with rapid corticosteroid tapering, we circumvented the adverse consequences of using corticosteroids in large doses for long periods of time (Fig. 3). The patient was also delighted to share his feedback that he was no longer struggling to walk quickly and was breathing much better when he returned to the hospital for follow-up.
Fig. 3.
Corticosteroid dosage and its dynamics during four hospitalizations of CIP
Six-month follow-up: the tumor keeps SD with no antitumor therapy
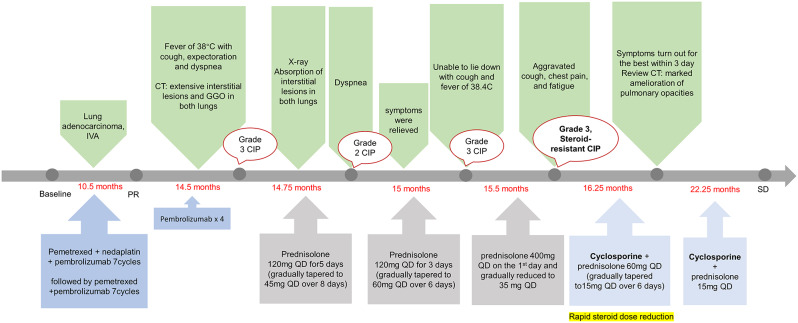
The patient continued to take cyclosporine (25 mg, QD) in combination with a substantially reduced dose of corticosteroids (15 mg, QD) for three weeks after discharge from the hospital and stopped both drugs at week five (Fig. 3). He showed no signs of recurrence, indicating that the previous steroid-resistant CIP was under control. At the same time, the patient stated that he was in good condition, and did not come to the hospital for anti-tumor treatment for half a year. During this period, only routine reexamination was performed, and stable disease (RECIST1.1 criteria) of the primary tumor lesion was evaluated. The patient’s treatment and follow-up were documented in a timeline (Fig. 4). Further, the patient’s weight had increased by 10 kg compared to the last hospitalization.
Fig. 4.
Timeline of events in this case
Discussion
In this work, we present a case of a recurrent steroid-resistant CIP patient treated with pembrolizumab immunotherapy. The patient failed to achieve stable control with corticosteroids and experienced a relapse of CIP during the fourth steroid treatment tapering period. In an exploratory approach, we administered a combination of cyclosporine and corticosteroids, rapidly tapering the corticosteroid dose during the treatment process. There were no signs of CIP relapse, and the tumor remained stable during the six-month follow-up. Cyclosporine is an immunosuppressive agent that specifically targets T lymphocytes, and is frequently utilized in clinical settings to prevent graft rejection in liver, kidney, and heart transplantation. Additionally, it can be combined with steroids to treat immune-related disorders [15]. To our knowledge, cyclosporine has not been previously used to treat pneumonitis associated with ICIs.
Despite our patient showing a moderate response to corticosteroids based on clinical manifestations and imaging, he continued to experience a recurrence of CIP during corticosteroid tapering. Clinicians should be fully aware of the possibility of ICI-induced pulmonary toxicity during ICIs treatment [16]. As a type of interstitial lung disease caused by immunosuppressive medications, it is often associated with irreversible pulmonary fibrosis. Further, without timely and effective control, it can significantly impact the lung function. Indeed, prolonged or high-dose use of corticosteroids may significantly increase the risk of opportunistic infections when used for managing patients with immune-related adverse events (irAEs). Therefore, combining immunosuppressive agents with lower doses of corticosteroids for the treatment of CIP can be effective for stable control and can also reduce the risk of severe infections caused by steroids.
Current guidelines do not clearly specify the use of immunosuppressive agents for steroid-resistant CIP patients. Given that treatment approaches like using infliximab have not shown complete efficacy in some reported cases and are associated with adverse reactions such as infections, we opted for an exploratory approach using the conventional immunosuppressive agent cyclosporine. This treatment regimen led to the stable control of CIP while facilitating a rapid tapering of steroids, thus minimizing the risk of opportunistic infections. Further, we found that the laboratory results of four hospitalizations showed that KL-6 levels in patients with CIP continued to increase, which was well above normal (< 500 U/ml) during four recurrent CIP episodes and even exceeded 10,000 during the third recurrence. There have been studies proving that KL-6 is considered to be one of the best and most reliable serum biomarkers for the diagnosis of Interstitial Lung Disease (ILD) [17]. Serum KL-6/MUC1 is higher in 70–100% of patients with various ILDs, and is often associated with the severity of interstitial lung injury. In our case when cyclosporine was combined with steroid treatment, the KL-6 level was also significantly decreased [18].
Based on the existing possible mechanisms of CIP, we explored possible mechanisms of the effectiveness of cyclosporine. For example, Kim et al. found a notable increase of Ki-67 (proliferation markers of PD-1 + CD8 + T cell) in NSCLC patients who received ICIs [19]. Further, Suresh et al. found that the proliferation of lymphocytes in the bronchoalveolar lavage fluid (BALF) of CIP was mainly composed of CD4 + T cells, including a high proportion of central memory CD4 + T cells (Tcms, CD4 + CD45RA − CD62L+). Further, compared with conventional T cells, Tcms were more resistant to steroid-induced apoptosis, which suggests the potential mechanism of steroid-resistant CIP. Additionally, in vitro stimulation showed increased secretion of tumor necrosis factor (TNF-α) and interferon-gamma (IFN-γ), and Th1 cell-mediated immunity with IFN-γ as its signature cytokine. This suggests that during the development of CIP, Th1 may trigger an overactive T cell response and thus mediate T-helper 1 (Th1)-mediated immune responses [20]. Cyclosporine as the first drug identified to have immunosuppressive capabilities, is now newly found to have applications in a variety of diseases such as membranous nephropathy, ulcerative colitis and some autoimmune-related diseases [21–24]. It can selectively regulate the function of peripheral blood lymphocyte subsets, blocks the activation of cytotoxic t cells, and may inhibit the formation or response of memory T cells [25]. In a study exploring the successful treatment of autoimmune uveitis with cyclosporin A by using single-cell sequencing, it was found that cyclosporine may have a superior salvage effect in attenuating the pathogenicity of auto-reactive T-cells compared to glucocorticoids, and focuses on modulating Th1 cells and Th17 cells [26]. Thus in our case combining the existing mechanisms of cyclosporine, the possible mechanism for the effect of cyclosporine on CIP may be the selective inhibition of T lymphocyte subsets in CIP, which effectively inhibits the activity of helper T lymphocytes and B lymphocytes. This is mainly by suppressing the differentiation and proliferation of resting Th cells and the expression of HLA-II antigen [27, 28], thus reducing the synthesis and secretion of cytokines released from Th cells and indirectly affecting B cells.
Conclusions
In conclusion, we successfully treated a case of CIP by using a combination of cyclosporine and corticosteroids. It has been suggested that routine early use of additional immunosuppression may be a strategy to improve prognosis in the future [29]. The same efficacy has been observed in our case and can help reduce the potential risks associated with high corticosteroid use by reducing the dosage of previously used corticosteroids as soon as possible. Corticosteroids along with cyclosporine might be an option for CIP, especially steroid-resistant CIP. Our case not only highlights the significance of cyclosporine as a treatment option for irAEs, but it also underscores the need for further investigation through large-scale clinical studies to validate its safety and efficacy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- CIP
Immune checkpoint inhibitor-related pneumonitis
- ICIs
Immune checkpoint inhibitors
- CT
Computed tomography
- KL-6
Kreb von den lungen-6
- IFN-γ
Knterferon-gamma
- TNF-α
Tumour necrosis factor-α
- irAEs
Immune-related adverse events
- NSCLC
Non-small cell lung cancer
- ILD
Interstitial lung disease
- PR
Partial response
- GGOs
Gound-glass opacities
- AIP-ARDS
Acute interstitial pneumonia-associated-acute respiratory distress syndrome
- PaO2
Pressure of oxygen in arterial blood
- FiO2
Fraction of inspiratory oxygen concentration
- Th1
T-helper 1
- BALF
Bronchoalveolar lavage fluid
Author contributions
J-XD: Case follow-up and manuscript writing. W-HG, H-YD, M-JH: Revision of the article, W-WM, N-S, and R-L Provided advice. C-ZZ and X-QL: Case treatment and response evaluation. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from Guangzhou Science and Technology Major Clinical Project (2023 C-DZ06).
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable: Ethical approval for this case report was not requested. All data in this case were derived from the patient’s routine course of treatment with traceability and no experimental interventions or other manipulations beyond standard medical practice were performed.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chengzhi Zhou, Email: doctorzcz@163.com.
Xinqing Lin, Email: linxinqing81@163.com.
References
- 1.Remon J, Reguart N, Auclin E, Besse B. Immune-related adverse events and outcomes in patients with Advanced Non-small Cell Lung Cancer: a predictive marker of efficacy? J Thorac Oncology: Official Publication Int Association Study Lung Cancer. 2019;14(6):963–7. [DOI] [PubMed] [Google Scholar]
- 2.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-small-cell Lung Cancer. N Engl J Med. 2018;378(22):2078–92. [DOI] [PubMed] [Google Scholar]
- 3.Wang HA-O, Guo XA-O, Zhou J, Li Y, Duan L, Si XA-O, Zhang L, Liu X, Wang M, Shi J et al. Clinical diagnosis and treatment of immune checkpoint inhibitor-associated pneumonitis. (1759–7714 (Electronic)). [DOI] [PMC free article] [PubMed]
- 4.Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with Advanced Cancer: a systematic review and Meta-analysis. JAMA Oncol. 2016;2(12):1607–16. [DOI] [PubMed] [Google Scholar]
- 5.Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, Budde LE, Costa L, Davies M, Dunnington D, et al. NCCN guidelines insights: management of immunotherapy-related toxicities, Version 1.2020. J Natl Compr Cancer Network: JNCCN. 2020;18(3):230–41. [DOI] [PubMed] [Google Scholar]
- 6.Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, Hamad L, Kim S, Lacouture ME, LeBoeuf NR, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang W, Wang Q, Xu C, Li Z, Song Z, Zhang Y, Cai X, Zhang S, Lian B, Li W, et al. Chinese expert consensus on the multidisciplinary management of pneumonitis associated with immune checkpoint inhibitor. Thorac cancer. 2022;13(23):3420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balaji A, Hsu M, Lin CT, Feliciano J, Marrone K, Brahmer JR, Forde PM, Hann C, Zheng L, Lee V et al. Steroid-refractory PD-(L)1 pneumonitis: incidence, clinical features, treatment, and outcomes. J Immunother Cancer 2021, 9(1). [DOI] [PMC free article] [PubMed]
- 9.Pan L, Meng F, Wang W, Wang XH, Shen H, Bao P, Kang J, Kong D. Nintedanib in an elderly non-small-cell lung cancer patient with severe steroid-refractory checkpoint inhibitor-related pneumonitis: a case report and literature review. (1664–3224 (Electronic)). [DOI] [PMC free article] [PubMed]
- 10.Guada M, Beloqui A, Kumar MN, Préat V, Dios-Viéitez Mdel C, Blanco-Prieto MJ. Reformulating cyclosporine A (CsA): more than just a life cycle management strategy. J Controlled Release: Official J Controlled Release Soc. 2016;225:269–82. [DOI] [PubMed] [Google Scholar]
- 11.Johannson KA, Chaudhuri N, Adegunsoye A, Wolters PJ. Treatment of fibrotic interstitial lung disease: current approaches and future directions. Lancet (London England). 2021;398(10309):1450–60. [DOI] [PubMed] [Google Scholar]
- 12.Lai KC, Hsiao YH, Chen SC. Pulse corticosteroid therapy in the treatment of steroid-refractory immune checkpoint inhibitor-related pneumonitis: case report and review. (1664–3224 (Electronic)). [DOI] [PMC free article] [PubMed]
- 13.Raschko JW, Cottler-Fox M, Abbondanzo SL, Torrisi JR, Spitzer TR, Deeg HJ. Pulmonary fibrosis after bone marrow transplantation responsive to treatment with prednisone and cyclosporine. Bone Marrow Transpl. 1989;4(2):201–5. [PubMed] [Google Scholar]
- 14.Liu K, Zhan Z, Gao W, Feng J, Xie X. Cyclosporine attenuates paraquat-induced mitophagy and pulmonary fibrosis. Immunopharmacol Immunotoxicol. 2020;42(2):138–46. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim SA-O, Abdallah HA-O, El-Halawany AA-O, Mohamed GA-O, Alhaddad AA, Samman WA, Alqarni AA, Rizq AT, Ghazawi KF, El-Dine RS. Natural Reno-Protective Agents against Cyclosporine A-Induced Nephrotoxicity: An Overview. LID – 10.3390/molecules27227771 [doi] LID – 7771. (1420–3049 (Electronic)). [DOI] [PMC free article] [PubMed]
- 16.Zhao B, Zhao H, Zhao J. Fatal adverse events associated with programmed cell death protein 1 or programmed cell death-ligand 1 monotherapy in cancer. Ther Adv Med Oncol. 2020;12:1758835919895753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paloma Millan B, Iván C, Laura Martinez M, Francisco A, Tomás F, Oriol Sibila V, Diego Castillo V. Diagnostic value of serum KL-6 in interstitial lung disease: preliminary results from an European cohort. Eur Respir J. 2018;52(suppl 62):PA2959. [Google Scholar]
- 18.Ishikawa N, Hattori N, Fau - Yokoyama A, Yokoyama A, Fau - Kohno N, Kohno N. Utility of KL-6/MUC1 in the clinical management of interstitial lung diseases. (2212–5353 (Electronic)).
- 19.Kim KH, Hur JY, Cho J, Ku BM, Koh J, Koh JY, Sun JM, Lee SH, Ahn JS, Park K, et al. Immune-related adverse events are clustered into distinct subtypes by T-cell profiling before and early after anti-PD-1 treatment. Oncoimmunology. 2020;9(1):1722023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suresh K, Naidoo J, Zhong Q, Xiong Y, Mammen J, de Flores MV, Cappelli L, Balaji A, Palmer T, Forde PM, et al. The alveolar immune cell landscape is dysregulated in checkpoint inhibitor pneumonitis. J Clin Invest. 2019;129(10):4305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponticelli C, Moroni G. Rituximab or Cyclosporine for Membranous Nephropathy. N Engl J Med. 2019;381(17):1688–9. [DOI] [PubMed] [Google Scholar]
- 22.Xu C, Ye Z, Jiang W, Wang S, Zhang H. Cyclosporine a alleviates colitis by inhibiting the formation of neutrophil extracellular traps via the regulating pentose phosphate pathway. Mol Med (Cambridge Mass). 2023;29(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crisafulli S, Boccanegra B, Carollo M, Bottani E, Mantuano P, Trifirò G, De Luca A. Myasthenia Gravis Treatment: from old drugs to innovative therapies with a glimpse into the future. CNS Drugs. 2024;38(1):15–32. [DOI] [PubMed] [Google Scholar]
- 24.Fattizzo B, Cantoni S, Giannotta JA, Bandiera L, Zavaglia R, Bortolotti M, Barcellini W. Efficacy and safety of cyclosporine A treatment in autoimmune cytopenias: the experience of two Italian reference centers. Therapeutic Adv Hematol. 2022;13:20406207221097780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Granelli-Piperno A. In situ hybridization for interleukin 2 and interleukin 2 receptor mRNA in T cells activated in the presence or absence of cyclosporin A. J Exp Med. 1988;168(5):1649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duan R, Xie L, Li H, Wang R, Liu X, Tao T, Yang S, Gao Y, Lin X, Su W. Insights gained from single-cell analysis of immune cells on Cyclosporine A treatment in autoimmune uveitis. (1873–2968 (Electronic)). [DOI] [PubMed]
- 27.Laupacis A, Fau - Keown PA. Keown Pa Fau - Ulan RA, Ulan Ra Fau - McKenzie N, McKenzie N Fau - Stiller CR, Stiller CR: Cyclosporin A: a powerful immunosuppressant. (0008-4409 (Print)). [PMC free article] [PubMed]
- 28.Rodrigues-Diez R, González-Guerrero C, Ocaña-Salceda C, Rodrigues-Diez RR, Egido J, Ortiz A, Ruiz-Ortega M, Ramos AM. Calcineurin inhibitors cyclosporine A and tacrolimus induce vascular inflammation and endothelial activation through TLR4 signaling. (2045–2322 (Electronic)). [DOI] [PMC free article] [PubMed]
- 29.Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K. Management of toxicities from immunotherapy: ESMO Clinical Practice guidelines for diagnosis, treatment and follow-up. Annals Oncology: Official J Eur Soc Med Oncol. 2017;28(suppl4):iv119–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article.