Abstract
Background
Patients with cirrhosis and portal hypertension may have alterations in intestinal barrier resulting in increased susceptibility for infections. We investigated the effect of propranolol in gastrointestinal motility, permeability and bacterial overgrowth in cirrhosis.
Methods
Patients with cirrhosis and esophageal varices were studied before and after a build-up dose of propranolol according to standard guidelines. Serum TNF-a, IL-6, IL-1b, LPS and bacterial DNA were measured before and during propranolol therapy. Oro-caecal transit time (OCTT) and bacterial overgrowth (BO) have been evaluated with H2 breath testing. Intestinal paracellular (IP), cellular passive non-carrier (ICNC), cellular passive carrier-mediated (ICCM), and gastric permeability (GP) were evaluated by measurement of lactulose, mannitol, D-xylose and sucrose respectively in urine, with high performance liquid chromatography (HPLC).
Results
35 patients with cirrhosis and portal hypertension with median age was 59.6 years (range 42–86) were included in the study. Twenty one had viral hepatitis and 25 were classified as having advanced cirrhosis (Child-Pugh B: 14 or C: 11). Median dose of administrated propranolol was 40 mg/day. After 7 days propranolol treatment BO was resolved in 15 out of 16 patients (93.7%, p = 0.0001) and OCTT was reduced significantly from 180 min to 139 min (SD 58.5, difference − 4 1 min, p = 0.0001). Serum IL-6 levels were reduced in 21/35 (60%) patients from 41.1 to 19 pg/ml (p = 0.01), TNF-a in 10/35 (28.5%) patients from 10.7 to 5.6 pg/ml (p = 0.007) and LPS in 20/35 (57%) from 7.1 to 5.2 mg/L (p = 0.1). No bacterial DNA was detected in serum of all patients either baseline or under propranolol treatment. IP was significantly reduced (0.2 to 0.16, p = 0.04) whereas ICNC (p = 0.9), ICCM (p = 0.4) and GP (p = 0.7) were not affected significantly. Intestinal Permeability (PI) index (Lactulose to Mannitol ratio) was significantly reduced (0.027 to 0.02, p = 0.03).
Conclusion
In patients with cirrhosis and portal hypertension, propranolol use is associated with reduction in BO, increase in intestinal motility and amelioration in intestinal permeability. Moreover IL-6 and LPS levels are being decreased in the majority of patients under propranolol.
Keywords: Cirrhosis, Propranolol, Gastrointestinal motility, Bacterial overgrowth, Gastrointestinal permeability
Background
The development of portal hypertension is the most critical milestone in the natural history of advanced chronic liver disease [1, 2]. Clinically significant portal hypertension (CSPH) [3] becomes evident when the hepatic venous pressure gradient (HVPG) increases to 10 mmHg or greater, promoting the development of gastroesophageal varices. Apart from the risk of variceal hemorrhage and the development of circulatory abdormalities [4, 5], patients with cirrhosis and portal hypertension (PHT), show an increased susceptibility to bacterial infections caused not only by several abnormalities of defence mechanisms but also by decreased bowel motility with bacterial overgrowth, increased intestinal permeability and consequently bacterial translocation [6, 7]. An increased intestinal permeability may facilitate the translocation of bacteria, endotoxin (LPS), or pathogen-associated molecular signatures (PAMPs) to the portal venous system and extraintestinal sites leading to a systemic inflammatory response and progressive hepatic failure [8, 9]. Thus infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis [8, 10–13]. Approximately 30% of these patients will die within one month after an episode of bacterial infection and another 30% by one year [14].
Non-selective betablockers (NSBB) are used for the pharmacological treatment of portal hypertension [3] and are considered effective for the prevention of decompensation in patients with clinically significant portal hypertension. The response to NSBB can be determined by hepatic venous pressure gradient (HVPG) measurement and seems that 30–50% of patients achieve a hemodynamic response to NSBB therapy [15]. Notably, a large proportion of NSBB-treated hemodynamic non-responders also do not bleed [16], probably because NSBB may prevent other triggers of variceal bleeding as infections and spontaneous bacterial peritonitis (SBP) [15, 17, 18]. Data on the correlation between other possible targets like motility, bacterial overgrowth and intestinal permeability in cirrhotic patients with PHT, before and after receiving NSBB therapy, are scarse. A decrease in portal pressure by NSBB may improve the congestion of the intestinal mucous membrane, increase the effective mucosal blood flow, and alleviate ischemia and hypoxia [19]. NSBB may also ameliorate splanchnic perfusion independently of their effect on portal pressure by reducing the sympathetic tone associated with impairment in the defence against bacterial translocation [20]. Potential mechanisms mediated by NSBB with regard to intestinal transit, bacterial overgrowth and translocation have been demonstrated in animal studies [20, 21]. Dynamics of bacterial translocation in patients have been well described by changes in levels of lipopolysaccharide (LPS)-binding protein (LBP) [22–26].
The primary objective of this study was to compare gastrointestinal motility and permeability, both gastric and intestinal, cellular or paracellular, in cirrhotic patients with documented portal hypertension, before and after the initiation of treatment with the NSBB, propranolol. Secondary endpoints were to identify additional changes, from the use of propranolol, in bacterial overgrowth (BO) and bacterial translocation (BT) and their association with staging of liver disease.
Methods
Patients
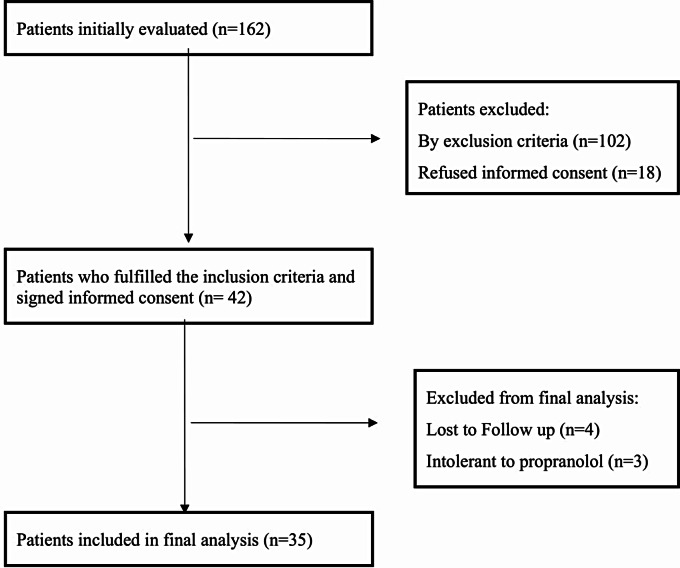
The study was prospective and open label for propranolol. Consecutive patients with cirrhosis who met the following inclusion criteria were selected: Child-Pugh A, B or C cirrhosis, age older than 18 years, esophageal varices during esophagogastroduodenoscopy. Between June 2013 and September 2018, we evaluated 162 patients and 42 patients who were fulfilling all eligibility criteria and had no exclusion criteria were included in the study (Fig. 1). Patients had been informed for the procedures of the study and agreed to sign informed consent. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the local research ethics committee. Exclusion criteria were: presence of systemic inflammatory response syndrome (SIRS) [27], upper gastrointestinal bleeding in the last 2 weeks prior to inclusion, refractory ascites, hepatorenal syndrome, alcohol use disorder (AUD) during the last 6 months, other intestinal disorders including previous gastrointestinal surgery, diseases that can change intestinal motility (diabetes, scleroderma, intestinal pseudo-obstruction), Inflammatory Bowel Diseases, hepatocellular carcinoma, portal vein thrombosis, pregnancy, immobilized patients, not willing to participate in the study. Regarding concomitant medications, patients were excluded if they reported use of lactulose 3 days prior to inclusion, use of β-adrenergic blocking agents or prokinetic drugs or antibiotics or calcium channel blockers or digoxin or anti-arrhythmic agents or vasoactive substances in the previous 2 months. Any use of diuretics has been withdrawn at least 24 h before inclusion in the study protocol.
Fig. 1.
Flow chart of the patient selection
Protocol
Non-invasive gastrointestinal motility, permeability and BO evaluation were scheduled for each patient at baseline (before using propranolol) and one week after propranolol initiation as part of their treatment plan for prevention of variceal bleeding [28] at a dose of 20 mg divided in two doses increasing up to 80 mg if well tolerated (no report of adverse events like postural hypotension, confusion, drowsiness) targeting a heart rate of not less than 55/min and systolic blood pressure of not less than 90mmHg.
Bacterial DNA and levels of lipopolysaccharide (LPS) in blood samples were measured as markers of bacterial translocation whereas cytokines (IL-6 and IL-1b) and tumor necrosis factor (TNF-α) as inflammatory markers. Blood samples were taken twice in two different time points at baseline and one week after propranolol initiation.
Blood samples
All blood samples were collected in a fasting state from a peripheral vein and were transported immediately to the laboratory. Whole blood, serum and plasma aliquots were made under pyrogen-free conditions and all samples were kept frozen at -800C until assayed.
Method of detection of BactDNA
After extraction, specimens were processed in airflow chambers and tubes were never exposed to free air. All laboratory procedures were performed by the same investigator. Detection of bactDNA was performed as previously described [29]. Briefly, a sample of 200 µl of plasma was incubated in a lysozyme-proteinase K buffer for 2 h and placed in ΜΝ Spin Columns (ΜΝ, Germany). Samples were microcentrifuged at full speed, and DNA was finally eluted with 50 µl of 70 °C preheated elution buffer. The yield and purity of DNA were measured by reading A260 and A260/A280 in an Eppendorf biophotometer.
A PCR reaction for the conserved region of the 16S ribosomal RNA prokaryote gene was carried out using the following universal primers: 5’-AGAGTTTGATCATGGCTCAG-3’ and 5’-ACCGCGACTGCTGCTGGCAC-3’ 50ng of template DNA was added into a reaction mix containing 1Xbuffer, 0,2mM of each deoxynucleoside triphosphate, 250nM of primers and 1 U BAC-free Hot Start Taq (Nippon Genetics, Europe GmbH) to complete a final volume of 20 µl. The primers are universal eubacterial primers that will amplify any known bacterial 16 S ribosomal RNA gene. A 35-cycle PCR was run in a T100 Thermal Cycler, Biorad (Biorad, Foster City, CA) using the following profile: 95 °C for 10 min, 95 °C for 5 s, 60 °C for 30 s, 72 °C for 60 s and 72 °C for 5 min. Experiments were repeated twice and verified on 2% agarose gel.
Lipopolysaccharide (LPS) levels
Plasma samples were obtained from blood within 20 min after blood sampling by centrifugation (1500 g at 4oC for 15 min). Plasma LPS was then quantified with a commercially available Human LBP HK315 Elisa kit (HycultBiotech) according to the manufacturer’s protocol [30]. The mean of triplicate determinations was used with appropriate positive and negative samples run simultaneously with the test samples.
Inflammatory markers
We used commercially available sandwich enzyme linked immunosorbent assays (ELISA) to quantify serum levels of TNF-α, IL6 and IL1b cytokines (Legend Max, Biolegend, San Diego, CA).
BO evaluation
Small bowel BO was assessed with the glucose breath hydrogen test. In the evening prior to the test patients were asked to avoid slowly digesting foods like beans and other vegetables. They were instructed to fast for 12 h prior to the test and only water was permitted before the test. Additionally, at least 1/2-hour prior to the test all patients were asked to abstain from smoking, sleep or exercise. Samples were measured by using a hydrogen (H2) breath test analyzer (Gastrolyzer). The hydrogen concentration in the expired air is expressed in parts per million (ppm). A baseline sample was collected to establish baseline value for breath-H2 (typically less than 10 parts per million). A hundred grams of glucose was administered with 240 cc of water after obtaining the baseline sample, and subsequent H2 breath samples were collected every 20 min for 2 h or until a positive increase of 12-20ppm has been recorded.
Orocaecal transit time (OCTT)
OCTΤ was measured using the lactulose hydrogen (H2) breath test as described previously [31, 32]. Although the interpretation of lactulose breath test [33, 34] in the presence of small intestinal bacterial overgrowth (SIBO) may be difficult, we took all necessary precautions and standard criteria to reduce errors [35, 36]. Patients were asked to avoid high fiber cereals (like beans or coarse-grain bread) and complex carbohydrates such as bread or pasta and consume a diet containing rice as the only form of starch on the evening prior to the test. All subjects have been fasting for 12 h before the test, drinking only water, while all medications except propranolol were held for 24 h before the test. Lactulose was held for 72 h. Patients were asked to rinse their mouths with an antiseptic mouthwash at the beginning of the breath test and abstain from smoking or exercise at least one hour before the test. A baseline sample was collected to establish baseline value for breath-H2 (typically less than 10 parts per million). Values over 20 ppm H2 are suspicious of BO and values between 10 and 20 ppm H2 suggest incomplete fasting before the test or the ingestion of slowly digesting foods the day before the test with the colon being the source of the elevated levels. Ten grams (15 cc) of lactulose were administered with 240 cc water after obtaining the baseline sample. Beginning 30 min after lactulose administration H2 breath samples were collected every 10 min intervals until rises to a level at least 3 ppm higher than the immediately previous level for three consecutive measurements have been achieved. After that has been achieved or if not at least 2 h after the lactulose administration samples were taken at 20 min intervals for measurement of H2-breath until a sustained peak is obtained. OCTΤ was defined as the time from baseline when there was either a rise in H2 levels of more than 20 ppm over baseline or more than 10 ppm over baseline sustained over 2 consecutive time points [35]. The breath test was repeated in all patients demonstrating baseline H2 levels 15 ppm to exclude inadequate preparation as a cause of falsely elevated baseline H2 breath levels. In case of small bowel BO defined as a distinct early peak in breath H2 levels seen within 60 min of ingestion of lactulose, or baseline H2 level more than 20 ppm [36], OCTΤ was estimated as the time between the ingestion of lactulose and a second peak of H2 levels.
Gastrointestinal permeability
Gastrointestinal permeability was assessed using a sugar drink test as previously described in detail [37]. The test is based on the measurement of the urinary excretion of orally administered sugars. Each given sugar is absorbed differently reflecting a different pathway. Intestinal paracellular (IP), cellular passive non carrier mediated (ICNC), cellular passive carrier mediated (ICCM), and gastric permeability (GP) were evaluated by measurement of lactulose, mannitol, D-xylose and sucrose respectively in urine. The lactulose/mannitol ratio (permeability index, PI) served as an additional marker for intestinal permeability to avoid differences in variable gastric emptying, intestinal transit time during the first 5 h, and renal clearance [38, 39]. Patients were asked to refrain from smoking in the morning before and during the test. We performed 2 separate tests within an interval of at least 3 days of each other. During the first test each subject drunk a solution containing 10-g lactulose, 5-g mannitol, and 20-g sucrose dissolved in 100 ml of water. Patients were fasting during the first 5 h and were encouraged to drink water 2 h after the test started. Two urine samples were taken: one before-test sample and another 5-h urine collection sample during the first 5 h after starting the test. Gastroduodenal permeability was analyzed by excretion of sucrose in the 5-h sample, serving as a marker for gastroduodenal permeability [37]. Small intestinal permeability was assessed by the lactulose/ mannitol ratio reflecting passive noncarrier-mediated transport [38, 40]. In the 5-h sample of the second oral sugar test, we further assessed passive carrier-mediated transport in the small intestine by absorption of orally administered D-xylose. Total urine volume was recorded on completion of the test, and a 10-ml aliquot of each urine sample was stored at − 20 °C until analysis.
For urine analysis, protein content was removed using sulfosalicylic acid. Urine was then desalted with amberlite mixed bed-3 resin. Sugars were separated using mesomesoerythritol, 2-deoxy-D-glucose and turanose as internal standards, analyzed and quantified by high-performance liquid chromatography with pulsed electrochemical detection (Dionex, Idstein, Germany; chromatography module: 250 × 40 mm Carbopac PA-1 column [Dionex]; eluent 150 mM NaOH/500 mM NaAC gradient; flow of 1 ml/min). Results were expressed as the percentage recovery of the ingested dose of sugar.
Statistical analysis
All data were analyzed using the Medcalc software (Mariakerke, Belgium). Normality was assessed with the Kolmogorov-Smirnov test. Quantitative variables are expressed as mean values ± one standard deviation (SD) when parametric, or as median values (range) when non-parametric. Categorical data are presented as percentages. Parametric data were compared using t-tests and non-parametric data by the Mann-Whitney’s and Kruskal-Wallis tests. The chi-square test and Fischer’s exact test (when total number of observations were < 20) were used as qualitative tests. In the analyses no cut-off was used to define the increase or decrease of all tests after the use of propranolol. Statistical significance is set to 0.05.
Results
We selected 42 patients who fulfilled eligibility criteria and gave informed consent. In the analysis, we excluded 7 patients of whom 4 were lost of follow up and 3 presented intolerance to propranolol (low systolic pressure and weakness) and did not complete the protocol (Table 1). Motility tests, BO, inflammatory markers, LPS and bacterial DNA were analysed in all included 35 patients. Permeability tests are reported in 32 patients for D-xylose and 25 patients for Lactulose - Sucrose – Mannitol. This was caused by inappropriate execution of the relative tests by the patient himself and revealed during sample analysis when none of the relative sugars was detected in the collected urine samples (Table 2).
Table 1.
Characteristics of 35 patients with cirrhosis and oesophageal varices included in the analysis
| Characteristics | Patients with cirrhosis n = 35 |
|---|---|
| Age (years)** | 59.6 (42–86) |
| Gender (Males), n (%) | 21 (60) |
| Cause of liver disease, n (%) | |
| • Alcohol | 8 (22.8) |
| • Viral (Hepatitis B or C) | 21 (60) |
| • Autoimmune | 1 (2.9) |
| • Cryptogenic or MASLD | 3 (8.5) |
| • PBC/PSC | 1 (2.9) |
| • Others | 1 (2.9) |
| Child Pugh class: A/B/C, n (%) | 10 (28.5) /14 (40) /11 (31.5) |
| MELD score * | 13 (5) |
| Varices: small / medium to large, n (%) | 15 (43) / 20 (57) |
| Variceal Bleeding: No / Yes, n (%) | 25 (71.4) / 10 (28.6) |
| Ascites: No / mild and moderate / large, n (%) | 4 (11.4) / 13 (37.1) / 18 (51.4) |
| Encephalopathy: No / Yes, n (%) | 27 (77) / 8 (23) |
| Hemoglobin (g/dl)* | 11.9 (2.2) |
| White Blood Cells (g/l)* | 5322 (1904) |
| Platelets count (/µL)* | 111,000 (42431) |
| Serum Creatinine (mg/dl)* | 0.86 (0.24) |
| Albumin (g/dl)* | 3.1 (0.49) |
| INR (n.r. 0.8–1.2)* | 1.37 (0.38) |
| Bilirubin (mg/dL)* | 2.9 (3.2) |
| SGOT, IU/L * | 59.2 (32.6) |
| SGPT, IU/L* | 45.1 (30.2) |
| Dose of Propranolol (mg/day)** | 40 (20–100) |
*mean (SD), **median (range)
Table 2.
Results of tests for permeability in 25 patients and motility, bacterial overgrowth, inflammatory markers and lipopolysaccharide levels in 35 patients before (pre-NSBB) and after a build-up dose of propranolol (post-NSBB)
| Test | pre-NSBB | post-NSBB | p-value |
|---|---|---|---|
| Intestinal Permeability Index (Lactulose/Mannitol) | 0.027 (0.01–0.13) | 0.020 (0.01–0.14) | 0,03 |
| Lactulose Permeability (%) | 0.20 (0.04-1) | 0.16 (0.06–0.7) | 0.04 |
| Mannitol Permeability (%) | 7.4 (1.4–16) | 8.4 (0.03-14) | 0.9 |
| Sucrose Permeability (%) | 0,055 (0.01–0.27) | 0,045 (0.01–0.18) | 0.7 |
| D-Xylose Permeability (%) | 12.6 (2.3–33.3) | 12.4 (0.2–34.4) | 0.4 |
| TNF-α (pg/ml) | 3.9 (9.4) | 5.8 (12.8) | 0.2 |
| IL-6 (pg/ml) | 34.8 (39.3) | 33.6 (56.5) | 0,9 |
| IL-1β (pg/ml) | 0.63 (2.3) | 0.22 (0.5) | 0.2 |
| LPS (mg/L) | 7.2 (8.5) | 6.8 (8.7) | 0.7 |
| OCTT (min) | 180 (71.6) | 139 (58.5) | 0.0001 |
| Bacterial Overgrowth | 16 (45.7) | 1 (2.8) | 0.0001 |
Values for permeabilities are expressed as median percentage and range in parenthesis; for TNF-a, IL-6, IL-1β, LPS and OCTT as mean and SD in parenthesis; for bacterial overgrowth as number and percentage in parenthesis
OCTT = Oro Cecal Transit Time
Tests for permeability were performed in 25 patients and motility, bacterial overgrowth, inflammatory markers and lipopolysaccharide levels performed in 35 patients
The etiology of cirrhosis was viral hepatitis in 21 patients (60%), followed by alcohol-related cirrhosis, 8 (22.8%) and MASLD/Cryptogenic, 3 (8.5%). Ten patients (28.5%) had Child-Pugh class A while 14 (40%) and 11(31.5%) had B and C class respectively. The mean MELD score was 13 (SD 5). In addition, medium to large varices have been recorded in 20 (57%) of the 35 patients, 25/35 (71.4%) had no history of previous bleeding, large ascites treated with diuretics in 18/35 (51.4%) and history of encephalopathy in 8/35 (23%). The mean hemoglobin at baseline was 11.9gr/dl, platelets 111,000/µL, albumin 3.1gr/dl, INR 1.37 and bilirubin 2.9 mg/l. Median given dose of propranolol was 40 mg/day (range 20–100). Most of the patients (43%, 15/45) tolerated the dose of 60 mg/day and only 14% (5/35) reached the dose of 80 mg/day.
Overall intestinal motility (OCTT) has significantly improved from 180 min at baseline to 139 min after the build up dose of propranolol (difference 41 min, p = 0.0001) (Table 2).
Regarding permeability, the lactulose to mannitol ratio (intestinal permeability-IP index) was significantly improved (p = 0.03). Based on single tests, a significant improvement was seen in paracellular permeability (IP) expressed by the lactulose test from 0.20 to 0.16 (p = 0.04). The other permeability tests (mannitol, sucrose and D-Xylose) did not changed significantly (Table 2).
The overall effect of the above changes on inflammatory markers was not significant. Thus IL-6 and IL-1b decreased but not significantly, whereas TNF-a showed a mild non-significant increase. The levels of LPS binding protein showed also a non-significant decrease (Table 2).
Bacterial DNA on peripheral blood was not detected in any patient both at baseline and on propranolol.
BO was present at baseline in 16 out of 35 patients (45.7%) (Child-Pugh class A: 4/16 25%, B: 5/16 31%, C: 7/16 44%) and it was resolved in a significant number of them (15 out of 16, 93.7%, p = 0.0001) after the use of propranolol. Additionally, we stressed the fact that in the majority (13/15, 86.6%) of patients with BO resolution, OCTT and paracellular lactulose permeability improved significantly from 165 min to 0.24 min at baseline to 124 min and 0.14 min respectively (both p = 0,01).
Subgroup analysis for motility and permeability tests
Sub-group analysis including tests that were significantly improved after the use of propranolol, namely motility (OCTT) and paracellular lactulose permeability (IP), has been performed to identify possible relation with the liver function status (Child-Pugh class) and the dose of propranolol.
OCTT evaluation
OCTT has been decreased significantly from mean 192 min at baseline to 135 min (p = 0.0009) in 25 out of 35 patients (71.4%). The range of percentage change in these patients was between 13% and 56% (median 33%). These patients were compared with the 10 patients who had no improvement in OCTT (149 min at baseline with an increase to 173 min, p = 0.1, while on propranolol). Mean baseline Child-Pugh class was significantly higher, in the subgroup of patients with improved motility compared to the group of no improvement (8.8 and 7, p = 0.01, respectively). However, there was no difference in the mean dose of propranolol given in the two groups (47 and 48 mg/day, p = 0.9, respectively).
IP evaluation
In the subgroup of 25 patients with results from the permeability tests, aetiology of cirrhosis was mainly viral hepatitis, 14 (56%), alcohol-related cirrhosis, 5 (20%), MASLD/Cryptogenic, 3 (12%), autoimmune hepatitis, 1 (4%), PBC/PSC 1, (4%), others 1 (4%). Child-Pugh class was in 7 (28%) patients A, in 9 (36%) B and in 9 (36%) C, while mean MELD score was 13 and median dose of propranolol was 40 mg/day (range 20–80). In the second subgroup analysis lactulose permeability improved significantly in 17 out of 25 patients (68%) from median 0.3–0.14% (p = 0.003), with range of percentage change in these patients been between 11% and 94% (median 49.5%), compared to the 8 patients (32%) who had no change or worsened permeability, resulting in median from 0.11 to 0.25% (p = 0.1). Accordingly, in this subgroup analysis a higher mean Child-Pugh class was seen in the group of patients with improved lactulose permeability compared to unchanged or worsened lactulose permeability, 8.7 vs. 7.7 respectively (p = 0.2).
Subgroup analysis of inflammatory markers and BT
To evaluate the role of propranolol initiation in inflammatory markers and BT, we performed a subgroup analysis between patients who decreased and patients who did not decrease LPS and IL-6 levels as differences between baseline and after propranolol initiation. Moreover, we tried to identify correlations with motility changes, lactulose permeability, Child-Pugh class, presence of BO and dose of propranolol.
LPS evaluation
We compared the characteristics of the 20/35 (57.1%) patients with LPS reduction (from initial mean value 7.1 to 5.2 pg/ml, p = 0.1) with the characteristics of the 15/35 (42.9%) patients who did not present LPS reduction (from initial mean value 7.3 to 9.1 pg/ml, p = 0.08) (Table 3). Median percentage changes of reduction for the first group was 19.4% (range 4-98%) and for the second group which did not present reduction was 12% (range 0-100%). Both groups presented a significant improvement in motility (OCTT) [178 min to 138 min (p = 0.01), 183 min to 140 min (p = 0.004), respectively]. No statistically significant differences among both groups regarding Child-Pugh class, percentage of BO and dose of propranolol have been recorded. However, in the first group lactulose permeability was significantly improved from 0.27 to 0.12% (p = 0.02) whereas in the second group was worsened from 0.20 to 0.25% (p = 0.4). This was an important and expected finding which proved that in patients with cirrhosis under propranolol, LPS levels are decreasing only after paracellular permeability improvement.
Table 3.
Subgroup analysis between patients that reduced LPS and those who did not reduce LPS, before (pre-NSBB) and after a build-up dose of propranolol (post-NSBB)
| Test | Group with reduced LPS (N = 20) | Group with no reduced LPS (N = 15) | p-value |
|---|---|---|---|
| LPS pre-NSBB (mg/L) | 7.1 (9.1) | 7.3 (7.9) | |
| LPS post-NSBB (mg/L) |
5.2 (6.9) (p = 0.1) |
9.1 (10.4) (p = 0.08) |
|
| Transit time pre-NSBB (min) | 178 (71.8) | 183 (73.8) | |
| Transit time post-NSBB (min) |
138 (58.1) (p = 0.01) |
140 (61.1) (p = 0.004) |
|
| Change in motility | 40 (62) | 43 (49.6) | |
| Child-Pugh class | 8.7 (2.1) | 8 (2.7) | ns |
| BO | 9 (45%) | 7 (47%) | ns |
| Dose of propranolol (mg/day) | 50 (20) | 44 (15.5) | ns |
| Lactulose detected in urine pre-NSBB (mmol/L) | 0.27 (0.24) | 0.20 (0.12) | |
| Lactulose detected in urine post-NSBB(mmol/L) |
0.12 (0.07) (p = 0.02) |
0.25 (0.20) (p = 0.4) |
All values are mean (SD)
Comparisons between pre-NSBB and post-NSBB are having its p-value at post-NSBB box
IL-6 evaluation
In this subgroup analysis, we compared the 21/35 (60%) patients who significantly reduced IL-6 levels (from initial mean value 41.2 to 19.1 pg/ml, p = 0.01) with the 14/35 (40%) patients who did not reduce IL-6 levels (from initial mean value 22.6 to 55.8 pg/ml, p = 0.1) (Table 4). Median percentage changes of reduction was 44.5% (range 11-94%) and for not reduction was 60% (range 12-574%). Motility has been improved in both groups [182 min to 135 min (p = 0.002) and 177 min to 145 min (p = 0.03), respectively]. Lactulose permeability has been, also, improved in both groups [0.25 to 0.18% (p = 0.5) and 0.25 to 0.16% (p = 0.01), respectively]. No statistically significant differences among both groups regarding Child-Pugh class, percentage of BO and dose of propranolol have been recorded. These findings indicated that in patients with liver cirrhosis, changes in IL-6 did not appear to be affected of gastrointestinal motility, lactulose permeability, or BO and were not associated with the Child-Pugh class or the dose of propranolol.
Table 4.
Subgroup analysis between patients that reduced IL-6 and those who did not reduce IL-6, before (pre-NSBB) and after a buildup dose of propranolol (post-NSBB)
| Test | Group with reduced IL-6 (N = 21) | Group with no reduced IL-6 (N = 14) | p-value |
|---|---|---|---|
| IL-6 pre-NSBB (pg/ml) | 41.2 (44.2) | 22.6 (24.7) | |
| IL-6 post-NSBB (pg/ml) |
19.1 (17.4) (p = 0.01) |
55.8 (83.8) (p = 0.1) |
|
| LPS pre-NSBB (mg/L) | 7.3 (9.2) | 7 (7.7) | |
| LPS post-NSBB (mg/L) |
6 (7.6) (p = 0.3) |
8.2 (10.3) (p = 0.4) |
|
| Transit time pre-NSBB (min) | 182 (77) | 177 (65.5) | |
| Transit time post-NSBB (min) |
135 (61.6) (p = 0.002) |
145 (55) (p = 0.03) |
|
| Change in motility | 47 (61) | 32 (49) | |
| Child-Pugh class | 8.5 (2.6) | 8.3 (2.2) | ns |
| BO | 11 (52%) | 5 (35%) | ns |
| Dose of propranolol (mg/day) | 48 (20.6) | 47 (14.9) | ns |
| Lactulose detected in urine pre-NSBB (mmol/L) | 0.25 (0.2) | 0.25 (0.13) | |
| Lactulose detected in urine post-NSBB (mmol/L) |
0.18 (0.15) (p = 0.5) |
0.16 (0.11) (p = 0.01) |
All values are mean (SD)
Comparisons between pre-NSBB and post-NSBB are having its p-value at post-NSBB box
Discussion
The present study demonstrated that in patients with liver cirrhosis and portal hypertension, the use of low-moderate doses of propranolol was associated with significant improvement in intestinal motility and permeability especially the paracellular, accompanying by reduction of BO. This propranolol effect on motility was associated with advanced liver function status according to Child-Pugh class. Moreover, we demonstrated that reduction in LPS levels was associated with paracellular permeability improvement.
A few studies have tried to show that in cirrhotic patients either reduced motility or bacterial overgrowth was correlated with infections such as spontaneous bacterial peritonitis (SBP) [6, 7]. The study of Morencos et al. showed that the prevalence of SBP was significantly higher in cirrhotic patients with BO than without BO [31]. Another study showed that cirrhotic patients with SBP had higher percentage of BO (70% and 20%, respectively) and higher Child-Pugh class compared to a group of cirrhotic patients with no SBP, but this was also accompanied by small intestinal motility impairment [41]. Another study [42] assessed 46 patients with cirrhosis, 60% of them with a Child-Pugh class B and only 8% with C, for the presence of intestinal BO, and changes in OCTT. BO was documented in 50% of the patients. OCTT was significantly reduced in patients with BO compared to non-BO. Additionally, in a subset of patients who received cisapride, an intestinal motor activity stimulator, OCTT improved and BO disappeared. Finally, a randomized study with a 6 months follow-up showed that the effect of cisapride on OCTT and BO, was long term and very similar to antibiotics like norfloxacin and neomycin but significantly better than placebo [43]. In our study the percentage of BO was similar to the above studies, 45.7%. Importantly, our cohort contained patients with advanced cirrhosis and more severe liver failure. We found that propranolol helps significantly the impaired small intestinal motility and resolves the existing BO an event that is associated with baseline Child-Pugh class but not with the dose of propranolol. Additionally, we showed that these significant motility and BO changes were caused by a medium-low dose of propranolol (mean 47 mg/day). In our study we did not observe bacterial DNA in peripheral blood samples; the reason is not clear. Possible explanation may be due to the fact that we excluded patients with active or recent SBP. This could be the reason for no detection of bacterial DNA in peripheral blood.
Significant changes in intestinal permeability under propranolol were also observed in our study. In both active and passive permeability, the enterocytes play a very important role. Substances, microbes and solutes like water have the opportunity to pass through the cells (cellular way) either passively with the presence or not of mediators and no expenditure of energy, actively with the presence of mediators consuming energy or in-between cells through the gap junctions passively (paracellular way). In our study propranolol caused a significant improvement in the intestinal permeability index (PI index) but especially in the lactulose permeability which expresses the paracellular way (IP). Paracellular pathway is regulated by a group of proteins like zonulins and occludins. Many inflammatory, tumoral, autoimmune diseases influence this pathway [44]. In chronic liver diseases and particularly in cirrhosis paracellular pathway seems to be affected by changes in microbiota and bacterial overgrowth. Thus, as we show in our study a positive influence in gut motility by NSBB reduces bacterial overgrowth and possibly has an effect on gut microbiota in general and by that tight junctions regain their function by production or reduced turnover.
A few years ago, another study reported the status of gastroduodenal and intestinal permeability in 50 cirrhotic patients with portal hypertension [25]. However, a small number of included patients had Child-Pugh class C (4%), and the majority had Child-Pugh class A (74%). At baseline increased gastroduodenal and intestinal permeability was found in 72% and 60% of their patients respectively. Higher Child-Pugh class was not associated with increased prevalence of gastroduodenal or intestinal permeability but significantly correlated with high levels of LPS binding protein and IL-6. A subset of the initial cohort received propranolol and they reported that both gastroduodenal and intestinal permeabilities significantly improved. Also, they addressed significant decrease in LPS binding protein and IL-6 levels after propranolol intake compared to baseline. The last observation was not proved in our study probably due to the higher rate of patients with advanced cirrhosis (Child-Pugh class C) and to the lower median dose of NSBB compared to the Reiberger et al. study [25].
There is a hypothesis “time window” [18] according to which NSBB are beneficial only in decompensated patients with medium-large varices but not in patients with early or end-stage cirrhosis with refractory ascites. This observation concerns mainly the hemodynamic effect of NSBB. In our study as we had more patients with Child-Pugh C class, we showed that NSBB treatment was associated with motility and permeability improvement. This improvement was significantly associated with more advanced cirrhosis, probably patients in this advanced stage had more impaired gut motility and permeability and the improvement after propranolol use was more obvious. Regarding LPS we showed improvement in 57% of patients treated with propranolol. Interestingly, this was the sub-group of patients that apart from a significant motility improvement had also a significant paracellular permeability (IP) improvement. So, even if further studies are needed, we reported that differences in LPS levels in cirrhotic patients before and under propranolol could identify a significant bacterial translocation improvement through restitution of the damaged paracellular way. This may be a possible surrogate marker of improved permeability. We have to admit that the levels of several inflammatory markers are not determined by only one mechanism. The higher levels of several inflammatory markers as TNF-a, IL-6 etc. which are reflecting an increased Th1 reaction or increased monocyte production are subject to different rates of turnover according to liver function at the level of microenvironment and to nitric oxide production. Thus, fluctuations may occur, and studies are not always concordant [45].
At the same time, our study showed that IL-6 levels were improved significantly in 60% of the patients. However, IL-6 based sub-group analysis showed significant changes in motility and permeability in both IL-6 improved and IL-6 not improved subgroups. This may guide to the conclusion that, as a result of the effect of propranolol, IL-6 changes do not depend on motility or permeability but possibly to other factors like decrease of the portal pressure. To strengthen this, if we look at the previous study [18], which measured HVPG before and after propranolol (51% of patients hemodynamically responded and dropped HVPG accordingly), IL-6 levels were decreased more than LPS binding protein levels in hemodynamically responders than non-responders showing that hemodynamic changes (HVPG) may have a larger effect on IL-6. Studies are showing that a better hemodynamic response reflects better nitric oxide levels and consequently, better liver function and clearance of bacterial products by Kupffer macrophages. Thus, a further reduction in IL-6 is expected [25].
Several studies have shown that NSSBs alleviate systemic inflammation [46–48] Mookerjee and coworkers [49] demonstrated that NSBB treatment was associated with lower grades of ACLF, and significantly more patients with ongoing β-blocker treatment experienced improvement. This improvement was associated with a considerably lower white cell count. In comparison, patients who were not treated with β-blocker tended to show worsening of ACLF during their hospital stay. Furthermore, the patients who discontinued NSBB treatment had significantly higher 28-day and 3-month mortality rates due to the development of ACLF episodes as a consequence of severe systemic inflammation. The patients that stopped NSBB were more likely to have circulatory and lung failure [49].
The effect of propranolol on motility as proposed by past studies [50] is through its effect on existing b-adrenergic receptors in the gut. BO may also be resolved by the increased motility. However, recent studies on animal models suggest a possible additional role of propranolol in the production of MUC-2 an important mucin for the intestinal mucus layer, favouring protection of the enterocytes and the existence of a normal intestinal flora [51].
Antibiotics are used in high risk patients and especially in those with recurrent episodes of SBP and hepatic encephalopathy in order to reduce endotoxaemia and bacteriaemia [1]. According to a previous study the prokinetic drug cisapride improved motility and managed to reduce the occurrence of SBP [52]. In this study cisapride along with norfloxacin was more effective than norfloxacin alone in preventing the development of SBP in high-risk patients. As cisapride is no longer used in clinical practice and the addition of propranolol in low-medium doses has a similar effect on motility in cirrhotics, as we proved, it seems a good choice of treatment in patients with or without ascites and recurrent episodes of SBP or hepatic encephalopathy [53].
The strength of our study is the prospective design targeting to explore changes in many parts of gastrointestinal function at the same time. However, there are some limitations. Firstly, the number of included patients is rather small. Secondly, according to last BAVENO VII guidelines [3] for portal hypertension, carvedilol would be a more efective NSSB choice for reducing portal pressure due to its intrinsic anti-alpha adrenergic vasodilator action. In this context, we think that a larger prospective and possibly controlled study comparing antibiotics like rifaximin or norfloxacin to single propranolol or carvedilol at low doses may be necessary. Furthermore, BAVENO VII guidelines introduced non-invasive methods, as liver stiffness and the number of platelets for the definition of cACLD and CSPH, suggesting the use of NSSB for all patients with CSPH. In our study, CSPH was proven by the presence of varices during esophagogastroduodenoscopy. Liver stiffness estimated by transient elastography would be a misleading validation of fibrosis in our patient group as many of them had ascites, a confounding factor for liver stiffness assessment. Thirdly, several tests that assess motility (Tc-99 or Cr-EDTA) and BO (duodenal aspiration which is the gold standard method) were not chosen in our study. However, our study’s design protocol was to use feasible non-invasive tests as our patients were mainly outpatients. Last, we did not study the long-term effects of propranolol in the gut and its stability, something that needs to be studied further in the future.
Conclusions
Our study revealed that propranolol improves motility in patients with cirrhosis, especially in those with more severe stage of liver disease, and reduces small intestinal bacterial overgrowth (SIBO) as well as paracellular permeability. This effect on paracellular permeability causes an improvement on serum LPS levels.
Abbreviations
- AUD
Alcohol use disorder
- BO
Bacterial overgrowth
- BT
Bacterial translocation
- CSPH
Clinically significant portal hypertension
- GP
Gastric permeability
- HPLC
High performance liquid chromatography
- HVPG
Hepatic venous pressure gradient
- ICCM
Cellular passive carrier-mediated
- ICNC
Cellular passive non-carrier
- IP
Intestinal paracellular
- LPS
Lipopolysaccharide
- NSBB
Non-selective betablockers
- OCTT
Oro-caecal transit time
- PHT
Portal hypertension
- SBP
Spontaneous bacterial peritonitis
- SIBO
Small intestinal bacterial overgrowth
- SIRS
Systemic inflammatory response syndrome; *
Author contributions
Conception and Design: E. X.; Manuscript Writing: E.X., S.M., H.K.; Data collection: E.X., S.M, H.K., A.K., M.D., N.P.; Lab Work: E.H., M.V., C.R.; Data Analysis: E.X.; Review: E.X., H.K., M.D., E.H., G.P., S.M.; Supervision: S.M.
Funding
The study was supported by an unrestricted grant from Hellenic Association for the Study of the Liver.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics committee of the participating center (Approval committee of the General Hospital of Athens “Hippocration”) and was conducted according to the principles of the Declaration of Helsinki. Patients had been informed for the procedures of the study and agreed to sign informed consent.
Consent for publication
Not applicable.
Competing interests
M.D: lecturer for Gilead, G.P.: advisor/lecturer for Abbvie, Amgen, Dicerna, Gilead, GlaxoSmithKline, Ipsen, Janssen, Merck Sharp & Dohme, Novo Nordisk, Roche and Takeda; research grants from Abbvie and Gilead, S.M.: advisor/lecturer for Abbvie, Gilead, Ipsen, Merck Sharp & Dohme, Roche, Integris and Genesis; research grants from Abbvie and Gilead. For the remaining authors, there are no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.European Association for the Study of the Liver. Electronic address eee, European Association for the study of the L. EASL Clinical Practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–60. [DOI] [PubMed] [Google Scholar]
- 2.Gines P, Krag A, Abraldes JG, Sola E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359–76. [DOI] [PubMed] [Google Scholar]
- 3.de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C, Baveno VIIF. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwao T, Toyonaga A, Ikegami M, Oho K, Sumino M, Harada H, Sakaki M, et al. Reduced gastric mucosal blood flow in patients with portal-hypertensive gastropathy. Hepatology. 1993;18:36–40. [PubMed] [Google Scholar]
- 5.Iwakiri Y, Groszmann RJ. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology. 2006;43:S121–131. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Tsao G. Bacterial translocation: cause or consequence of decompensation in cirrhosis? J Hepatol. 2001;34:150–5. [DOI] [PubMed] [Google Scholar]
- 7.Pascual S, Such J, Esteban A, Zapater P, Casellas JA, Aparicio JR, Girona E, et al. Intestinal permeability is increased in patients with advanced cirrhosis. Hepatogastroenterology. 2003;50:1482–6. [PubMed] [Google Scholar]
- 8.Goulis J, Armonis A, Patch D, Sabin C, Greenslade L, Burroughs AK. Bacterial infection is independently associated with failure to control bleeding in cirrhotic patients with gastrointestinal hemorrhage. Hepatology. 1998;27:1207–12. [DOI] [PubMed] [Google Scholar]
- 9.Thalheimer U, Triantos CK, Samonakis DN, Patch D, Burroughs AK. Infection, coagulation, and variceal bleeding in cirrhosis. Gut. 2005;54:556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou MC, Lin HC, Liu TT, Kuo BI, Lee FY, Chang FY, Lee SD. Antibiotic prophylaxis after endoscopic therapy prevents rebleeding in acute variceal hemorrhage: a randomized trial. Hepatology. 2004;39:746–53. [DOI] [PubMed] [Google Scholar]
- 11.Montalto P, Vlachogiannakos J, Cox DJ, Pastacaldi S, Patch D, Burroughs AK. Bacterial infection in cirrhosis impairs coagulation by a heparin effect: a prospective study. J Hepatol. 2002;37:463–70. [DOI] [PubMed] [Google Scholar]
- 12.Zambruni A, Thalheimer U, Coppell J, Riddell A, Mancuso A, Leandro G, Perry D, et al. Endogenous heparin-like activity detected by anti-Xa assay in infected cirrhotic and non-cirrhotic patients. Scand J Gastroenterol. 2004;39:830–6. [DOI] [PubMed] [Google Scholar]
- 13.Rasaratnam B, Kaye D, Jennings G, Dudley F, Chin-Dusting J. The effect of selective intestinal decontamination on the hyperdynamic circulatory state in cirrhosis. A randomized trial. Ann Intern Med. 2003;139:186–93. [DOI] [PubMed] [Google Scholar]
- 14.Arvaniti V, D’Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–56. 1256 e1241-1245. [DOI] [PubMed] [Google Scholar]
- 15.Abraldes JG, Tarantino I, Turnes J, Garcia-Pagan JC, Rodes J, Bosch J. Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology. 2003;37:902–8. [DOI] [PubMed] [Google Scholar]
- 16.Turnes J, Garcia-Pagan JC, Abraldes JG, Hernandez-Guerra M, Dell’Era A, Bosch J. Pharmacological reduction of portal pressure and long-term risk of first variceal bleeding in patients with cirrhosis. Am J Gastroenterol. 2006;101:506–12. [DOI] [PubMed] [Google Scholar]
- 17.Thalheimer U, Bosch J, Burroughs AK. How to prevent varices from bleeding: shades of grey–the case for nonselective beta blockers. Gastroenterology. 2007;133:2029–36. [DOI] [PubMed] [Google Scholar]
- 18.Krag A, Wiest R, Albillos A, Gluud LL. The window hypothesis: haemodynamic and non-haemodynamic effects of beta-blockers improve survival of patients with cirrhosis during a window in the disease. Gut. 2012;61:967–9. [DOI] [PubMed] [Google Scholar]
- 19.Yoon KT, Liu H, Lee SS. beta-blockers in advanced cirrhosis: More friend than enemy. Clin Mol Hepatol. 2021;27:425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Worlicek M, Knebel K, Linde HJ, Moleda L, Scholmerich J, Straub RH, Wiest R. Splanchnic sympathectomy prevents translocation and spreading of E coli but not S aureus in liver cirrhosis. Gut. 2010;59:1127–34. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Paramo M, Munoz J, Albillos A, Freile I, Portero F, Santos M, Ortiz-Berrocal J. Effect of propranolol on the factors promoting bacterial translocation in cirrhotic rats with ascites. Hepatology. 2000;31:43–8. [DOI] [PubMed] [Google Scholar]
- 22.Albillos A, de la Hera A, Gonzalez M, Moya JL, Calleja JL, Monserrat J, Ruiz-del-Arbol L, et al. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37:208–17. [DOI] [PubMed] [Google Scholar]
- 23.Hoshino S, Shinoura S, Akamine H. Effect of propranolol for the prevention of spontaneous bacterial peritonitis. Am J Gastroenterol. 2000;95:A349. [Google Scholar]
- 24.Chelarescu O, Chelarescu D, Tircoveanu E, Stratan I. Propranolol administration on post surgical infections in cirrhotic patients. J Hepatol. 2003;38:56–56. [Google Scholar]
- 25.Reiberger T, Ferlitsch A, Payer BA, Mandorfer M, Heinisch BB, Hayden H, Lammert F, et al. Non-selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL-6 in patients with cirrhosis. J Hepatol. 2013;58:911–21. [DOI] [PubMed] [Google Scholar]
- 26.Rickenbacher A, Seiler R, Honegger U, Shaw SG, Balsiger BM. Role of beta1-, beta2-, and beta3-adrenoceptors in contractile hypersensitivity in a model of small bowel transplantation. Surgery. 2008;143:94–102. [DOI] [PubMed] [Google Scholar]
- 27.Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995;273:117–23. [PubMed] [Google Scholar]
- 28.de Franchis R, Baveno VIF. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–52. [DOI] [PubMed] [Google Scholar]
- 29.Such J, Frances R, Munoz C, Zapater P, Casellas JA, Cifuentes A, Rodriguez-Valera F, et al. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology. 2002;36:135–41. [DOI] [PubMed] [Google Scholar]
- 30.Serrano M, Moreno-Navarrete JM, Puig J, Moreno M, Guerra E, Ortega F, Xifra G, et al. Serum lipopolysaccharide-binding protein as a marker of atherosclerosis. Atherosclerosis. 2013;230:223–7. [DOI] [PubMed] [Google Scholar]
- 31.Morencos FC, de las Heras CG, Martin RL, Lopez Arias MJ, Ledesma F, Pons RF. Small bowel bacterial overgrowth in patients with alcoholic cirrhosis. Dig Dis Sci. 1995;40:1252–6. [DOI] [PubMed] [Google Scholar]
- 32.Galati JS, Holdeman KP, Bottjen PL, Quigley EM. Gastric emptying and orocecal transit in portal hypertension and end-stage chronic liver disease. Liver Transpl Surg. 1997;3:34–8. [DOI] [PubMed] [Google Scholar]
- 33.Casellas F, Malagelada J. Influence of the substrate on the reproducibility of the hydrogen breath test to measure the orocecal transit time. Digestion. 1998;59:696–702. [DOI] [PubMed] [Google Scholar]
- 34.Sciarretta G, Furno A, Mazzoni M, Garagnani B, Malaguti P. Lactulose hydrogen breath test in orocecal transit assessment. Critical evaluation by means of scintigraphic method. Dig Dis Sci. 1994;39:1505–10. [DOI] [PubMed] [Google Scholar]
- 35.Romagnuolo J, Schiller D, Bailey RJ. Using breath tests wisely in a gastroenterology practice: an evidence-based review of indications and pitfalls in interpretation. Am J Gastroenterol. 2002;97:1113–26. [DOI] [PubMed] [Google Scholar]
- 36.Riordan SM, McIver CJ, Walker BM, Duncombe VM, Bolin TD, Thomas MC. The lactulose breath hydrogen test and small intestinal bacterial overgrowth. Am J Gastroenterol. 1996;91:1795–803. [PubMed] [Google Scholar]
- 37.Buhner S, Reese I, Kuehl F, Lochs H, Zuberbier T. Pseudoallergic reactions in chronic urticaria are associated with altered gastroduodenal permeability. Allergy. 2004;59:1118–23. [DOI] [PubMed] [Google Scholar]
- 38.Dinmore AJ, Edwards JS, Menzies IS, Travis SP. Intestinal carbohydrate absorption and permeability at high altitude (5,730 m). J Appl Physiol. 1994;76:1903–7. [DOI] [PubMed] [Google Scholar]
- 39.Buhner S, Buning C, Genschel J, Kling K, Herrmann D, Dignass A, Kuechler I, et al. Genetic basis for increased intestinal permeability in families with Crohn’s disease: role of CARD15 3020insC. mutation? Gut. 2006;55:342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson AD, Jain PK, Fleming S, Poon P, Mitchell CJ, MacFie J. Evaluation of a triple sugar test of colonic permeability in humans. Acta Physiol Scand. 2004;182:171–7. [DOI] [PubMed] [Google Scholar]
- 41.Chang CS, Chen GH, Lien HC, Yeh HZ. Small intestine dysmotility and bacterial overgrowth in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 1998;28:1187–90. [DOI] [PubMed] [Google Scholar]
- 42.Pardo A, Bartoli R, Lorenzo-Zuniga V, Planas R, Vinado B, Riba J, Cabre E, et al. Effect of cisapride on intestinal bacterial overgrowth and bacterial translocation in cirrhosis. Hepatology. 2000;31:858–63. [DOI] [PubMed] [Google Scholar]
- 43.Madrid AM, Hurtado C, Venegas M, Cumsille F, Defilippi C. Long-Term treatment with cisapride and antibiotics in liver cirrhosis: effect on small intestinal motility, bacterial overgrowth, and liver function. Am J Gastroenterol. 2001;96:1251–5. [DOI] [PubMed] [Google Scholar]
- 44.Slifer ZM, Blikslager AT. The Integral Role of Tight Junction Proteins in the Repair of Injured Intestinal Epithelium. Int J Mol Sci 2020;21. [DOI] [PMC free article] [PubMed]
- 45.Munoz L, Caparros E, Albillos A, Frances R. The shaping of gut immunity in cirrhosis. Front Immunol. 2023;14:1139554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madsen BS, Havelund T, Krag A. Targeting the gut-liver axis in cirrhosis: antibiotics and non-selective beta-blockers. Adv Ther. 2013;30:659–70. [DOI] [PubMed] [Google Scholar]
- 47.Moctezuma-Velazquez C, Kalainy S, Abraldes JG. Beta-blockers in patients with advanced liver disease: Has the dust settled? Liver Transpl. 2017;23:1058–69. [DOI] [PubMed] [Google Scholar]
- 48.Jachs M, Hartl L, Schaufler D, Desbalmes C, Simbrunner B, Eigenbauer E, Bauer DJM, et al. Amelioration of systemic inflammation in advanced chronic liver disease upon beta-blocker therapy translates into improved clinical outcomes. Gut. 2021;70:1758–67. [DOI] [PubMed] [Google Scholar]
- 49.Mookerjee RP, Pavesi M, Thomsen KL, Mehta G, Macnaughtan J, Bendtsen F, Coenraad M, et al. Treatment with non-selective beta blockers is associated with reduced severity of systemic inflammation and improved survival of patients with acute-on-chronic liver failure. J Hepatol. 2016;64:574–82. [DOI] [PubMed] [Google Scholar]
- 50.Seiler R, Rickenbacher A, Shaw S, Haefliger S, Balsiger BM. Role of Selective alpha and beta Adrenergic Receptor Mechanisms in Rat Jejunal Longitudinal Muscle Contractility. J. Gastrointest; Surg 2007. [DOI] [PubMed]
- 51.Sorribas M, de Gottardi A, Moghadamrad S, Hassan M, Spadoni I, Rescigno M, Wiest R. Isoproterenol Disrupts Intestinal Barriers Activating Gut-Liver-Axis: Effects on Intestinal Mucus and Vascular Barrier as Entry Sites. Digestion 2019:1–13. [DOI] [PubMed]
- 52.Sandhu BS, Gupta R, Sharma J, Singh J, Murthy NS, Sarin SK. Norfloxacin and cisapride combination decreases the incidence of spontaneous bacterial peritonitis in cirrhotic ascites. J Gastroenterol Hepatol. 2005;20:599–605. [DOI] [PubMed] [Google Scholar]
- 53.Facciorusso A, Roy S, Livadas S, Fevrier-Paul A, Wekesa C, Kilic ID, Chaurasia AK, et al. Nonselective Beta-Blockers Do Not Affect Survival in Cirrhotic Patients with Ascites. Dig Dis Sci. 2018;63:1737–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.