Abstract
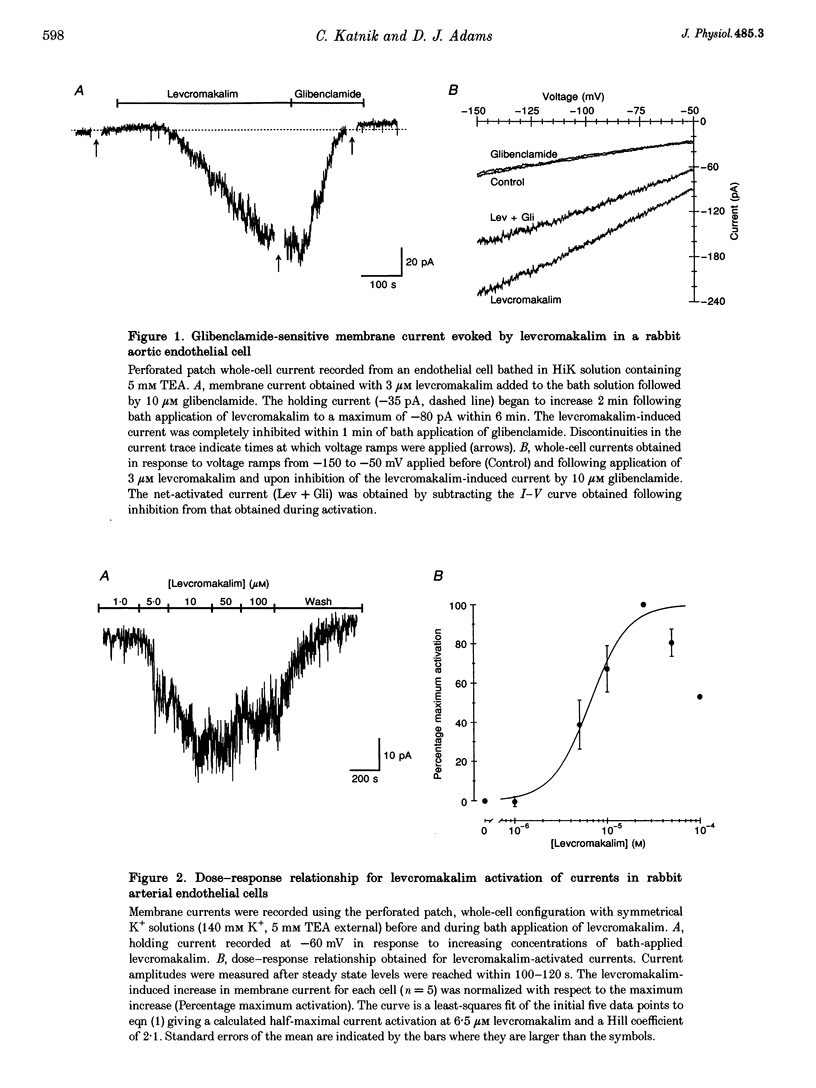
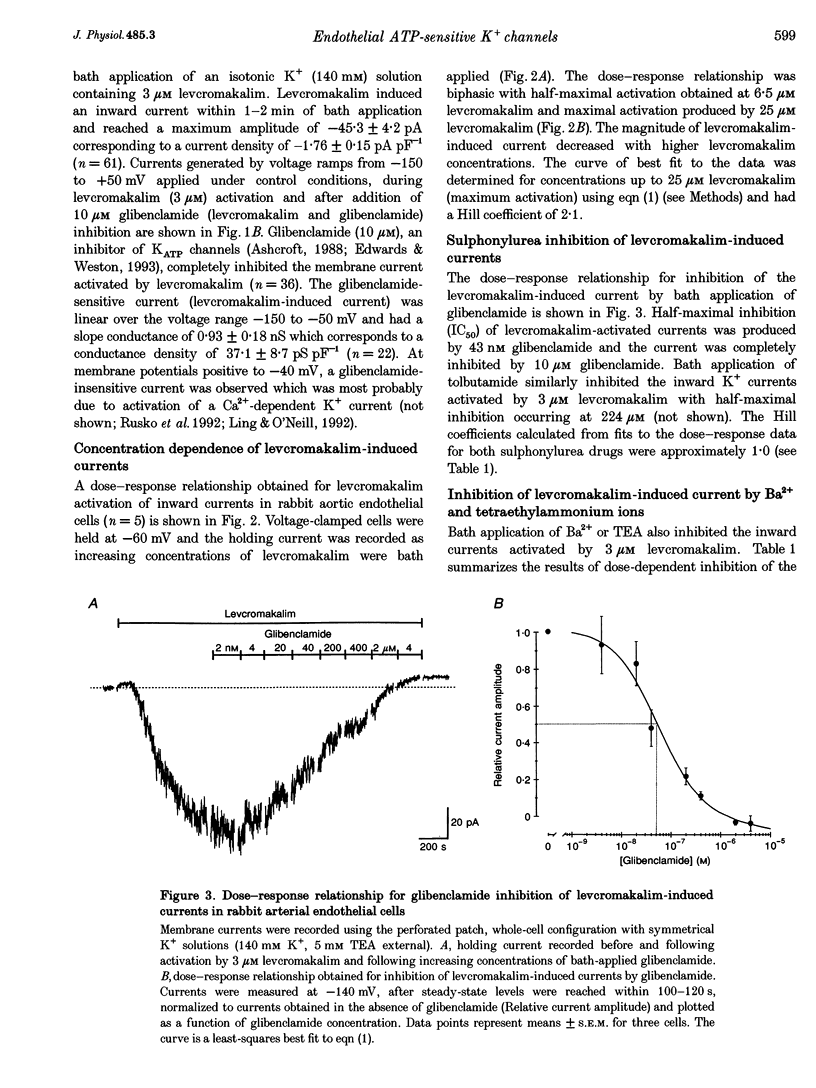
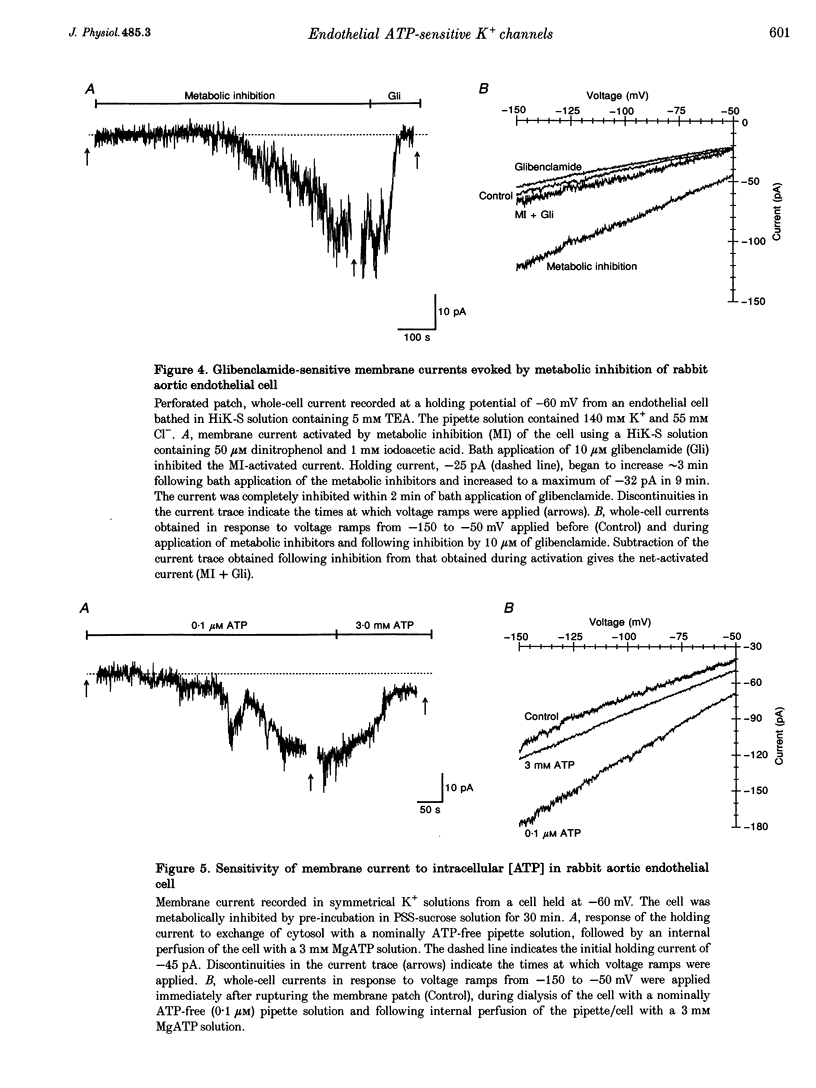
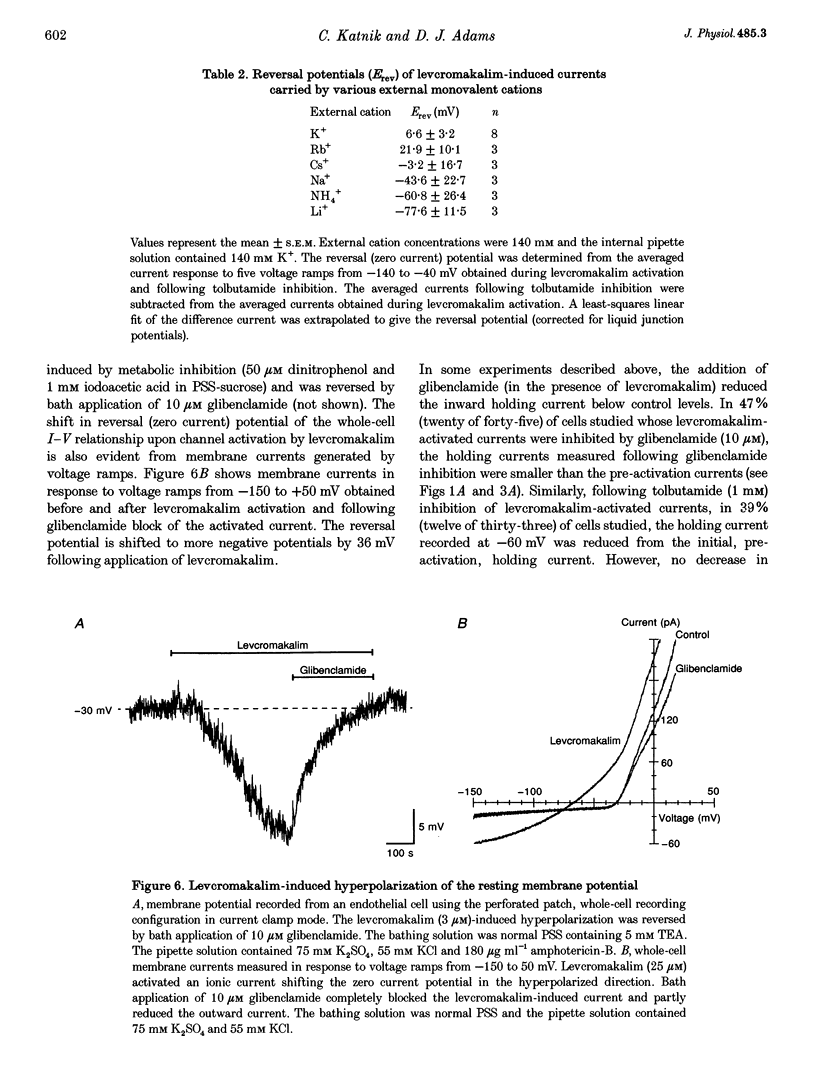
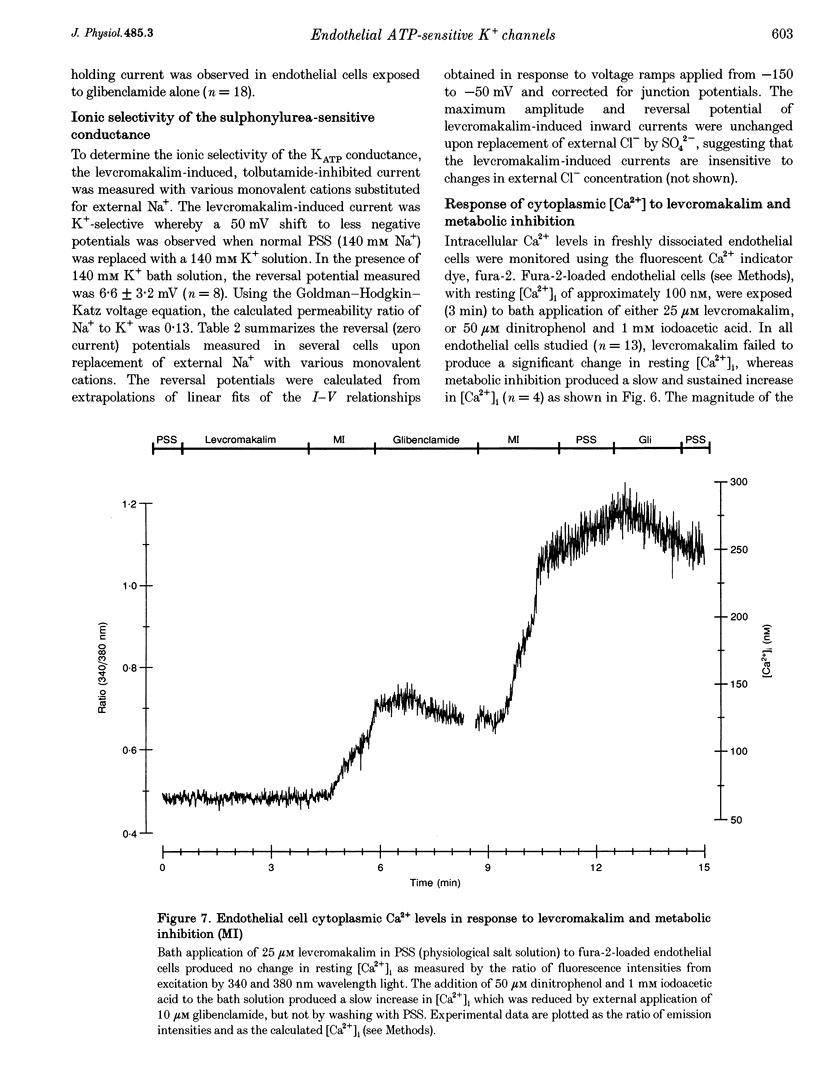
1. Whole-cell patch clamp recording was used to study an ATP-sensitive, sulphonylurea-inhibitable potassium (K+) conductance in freshly dissociated endothelial cells from rabbit arteries. 2. The ATP-sensitive K+ conductance was activated by micromolar concentrations of the K+ channel opener, levcromakalim, and by metabolic inhibition of endothelial cells using dinitrophenol and iodoacetic acid. The current-voltage (I-V) relationship obtained in isotonic K+ solutions was linear between -150 and -50 mV and had a slope conductance of approximately 1 nS. 3. The permeability of the ATP-sensitive K+ conductance determined from reversal potential measurements exhibited the following ionic selectivity sequence: Rb+ > K+ > Cs+ >> Na+ > NH4+ > Li+. 4. Membrane currents activated by either levcromakalim or metabolic inhibition were inhibited by the sulphonylurea drugs, glibenclamide and tolbutamide, with half-maximal inhibitory concentrations of 43 nM and 224 microM and Hill coefficients of 1.1 and 1.2, respectively. Levcromakalim-induced currents were also inhibited by millimolar concentrations of Ba2+ or tetraethylammonium ions in the external solution. 5. Levcromakalim (3 microM) and metabolic inhibition hyperpolarized endothelial cells by approximately 10-15 mV in normal physiological salt solutions. The hyperpolarization induced by levcromakalim or metabolic inhibition was inhibited by bath application of 10 microM glibenclamide. 6. Internal perfusion of the cytosol of whole-cell voltage-clamped endothelial cells with an ATP-free pipette solution activated a membrane current which was reversibly inhibited by internal perfusion with a 3 mM MgATP pipette solution. This current was insensitive to other adenine and guanine nucleotides in the pipette solution. The inward current evoked in a nominally ATP-free internal solution was further increased by bath application of levcromakalim. 7. Levcromakalim (25 microM) did not induce a change in the intracellular Ca2+ concentration of fura-2-loaded endothelial cells, whereas metabolic inhibition caused a slow and sustained increase in intracellular Ca2+ concentration, which was attenuated by 10 microM glibenclamide applied externally.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Barakeh J., Laskey R., Van Breemen C. Ion channels and regulation of intracellular calcium in vascular endothelial cells. FASEB J. 1989 Oct;3(12):2389–2400. doi: 10.1096/fasebj.3.12.2477294. [DOI] [PubMed] [Google Scholar]
- Arnould T., Michiels C., Alexandre I., Remacle J. Effect of hypoxia upon intracellular calcium concentration of human endothelial cells. J Cell Physiol. 1992 Jul;152(1):215–221. doi: 10.1002/jcp.1041520127. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Ashcroft F. M. Properties and functions of ATP-sensitive K-channels. Cell Signal. 1990;2(3):197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Sage S. O. Bradykinin-evoked changes in cytosolic calcium and membrane currents in cultured bovine pulmonary artery endothelial cells. J Physiol. 1989 Dec;419:555–568. doi: 10.1113/jphysiol.1989.sp017886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp L. H., Davey R., Gurney A. M. ATP-sensitive K+ channels mediate vasodilation produced by lemakalim in rabbit pulmonary artery. Am J Physiol. 1993 Jun;264(6 Pt 2):H1907–H1915. doi: 10.1152/ajpheart.1993.264.6.H1907. [DOI] [PubMed] [Google Scholar]
- Clapp L. H., Gurney A. M. ATP-sensitive K+ channels regulate resting potential of pulmonary arterial smooth muscle cells. Am J Physiol. 1992 Mar;262(3 Pt 2):H916–H920. doi: 10.1152/ajpheart.1992.262.3.H916. [DOI] [PubMed] [Google Scholar]
- Daut J., Maier-Rudolph W., von Beckerath N., Mehrke G., Günther K., Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990 Mar 16;247(4948):1341–1344. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- Edwards G., Weston A. H. The pharmacology of ATP-sensitive potassium channels. Annu Rev Pharmacol Toxicol. 1993;33:597–637. doi: 10.1146/annurev.pa.33.040193.003121. [DOI] [PubMed] [Google Scholar]
- Findlay I., Dunne M. J. ATP maintains ATP-inhibited K+ channels in an operational state. Pflugers Arch. 1986 Aug;407(2):238–240. doi: 10.1007/BF00580683. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Himmel H. M., Whorton A. R., Strauss H. C. Intracellular calcium, currents, and stimulus-response coupling in endothelial cells. Hypertension. 1993 Jan;21(1):112–127. doi: 10.1161/01.hyp.21.1.112. [DOI] [PubMed] [Google Scholar]
- Hutcheson I. R., Griffith T. M. Heterogeneous populations of K+ channels mediate EDRF release to flow but not agonists in rabbit aorta. Am J Physiol. 1994 Feb;266(2 Pt 2):H590–H596. doi: 10.1152/ajpheart.1994.266.2.H590. [DOI] [PubMed] [Google Scholar]
- Janigro D., West G. A., Gordon E. L., Winn H. R. ATP-sensitive K+ channels in rat aorta and brain microvascular endothelial cells. Am J Physiol. 1993 Sep;265(3 Pt 1):C812–C821. doi: 10.1152/ajpcell.1993.265.3.C812. [DOI] [PubMed] [Google Scholar]
- Johns A., Lategan T. W., Lodge N. J., Ryan U. S., Van Breemen C., Adams D. J. Calcium entry through receptor-operated channels in bovine pulmonary artery endothelial cells. Tissue Cell. 1987;19(6):733–745. doi: 10.1016/0040-8166(87)90015-2. [DOI] [PubMed] [Google Scholar]
- Laskey R. E., Adams D. J., Johns A., Rubanyi G. M., van Breemen C. Membrane potential and Na(+)-K+ pump activity modulate resting and bradykinin-stimulated changes in cytosolic free calcium in cultured endothelial cells from bovine atria. J Biol Chem. 1990 Feb 15;265(5):2613–2619. [PubMed] [Google Scholar]
- Ling B. N., O'Neill W. C. Ca(2+)-dependent and Ca(2+)-permeable ion channels in aortic endothelial cells. Am J Physiol. 1992 Dec;263(6 Pt 2):H1827–H1838. doi: 10.1152/ajpheart.1992.263.6.H1827. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Activators of potassium channels enhance calcium influx into endothelial cells as a consequence of potassium currents. Naunyn Schmiedebergs Arch Pharmacol. 1990 Jul;342(1):94–99. doi: 10.1007/BF00178979. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Calcium influx into endothelial cells and formation of endothelium-derived relaxing factor is controlled by the membrane potential. Pflugers Arch. 1990 May;416(3):305–311. doi: 10.1007/BF00392067. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Pohl U., Mülsch A., Busse R. Differential role of extra- and intracellular calcium in the release of EDRF and prostacyclin from cultured endothelial cells. Br J Pharmacol. 1988 Sep;95(1):189–196. doi: 10.1111/j.1476-5381.1988.tb16564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrke G., Pohl U., Daut J. Effects of vasoactive agonists on the membrane potential of cultured bovine aortic and guinea-pig coronary endothelium. J Physiol. 1991 Aug;439:277–299. doi: 10.1113/jphysiol.1991.sp018667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. J., Jow B., Jow F. Whole-cell currents in macrophages: I. Human monocyte-derived macrophages. J Membr Biol. 1990 Jul;117(1):29–44. doi: 10.1007/BF01871563. [DOI] [PubMed] [Google Scholar]
- Olesen S. P., Bundgaard M. ATP-dependent closure and reactivation of inward rectifier K+ channels in endothelial cells. Circ Res. 1993 Sep;73(3):492–495. doi: 10.1161/01.res.73.3.492. [DOI] [PubMed] [Google Scholar]
- Rae J., Cooper K., Gates P., Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Methods. 1991 Mar;37(1):15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Richards J. M., Gibson I. F., Martin W. Effects of hypoxia and metabolic inhibitors on production of prostacyclin and endothelium-derived relaxing factor by pig aortic endothelial cells. Br J Pharmacol. 1991 Jan;102(1):203–209. doi: 10.1111/j.1476-5381.1991.tb12154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Potassium-induced release of endothelium-derived relaxing factor from canine femoral arteries. Circ Res. 1988 Jun;62(6):1098–1103. doi: 10.1161/01.res.62.6.1098. [DOI] [PubMed] [Google Scholar]
- Rusko J., Tanzi F., van Breemen C., Adams D. J. Calcium-activated potassium channels in native endothelial cells from rabbit aorta: conductance, Ca2+ sensitivity and block. J Physiol. 1992 Sep;455:601–621. doi: 10.1113/jphysiol.1992.sp019318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling W. P. Effect of membrane potential on cytosolic calcium of bovine aortic endothelial cells. Am J Physiol. 1989 Sep;257(3 Pt 2):H778–H784. doi: 10.1152/ajpheart.1989.257.3.H778. [DOI] [PubMed] [Google Scholar]
- Sheppard D. N., Welsh M. J. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J Gen Physiol. 1992 Oct;100(4):573–591. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breemen C., Wuytack F., Casteels R., Martinelli B., Campailla E., Ferrari G. Stimulation of 45Ca efflux from smooth muscle cells by metabolic inhibition and high K depolarization. Pflugers Arch. 1975 Sep 9;359(3):183–196. doi: 10.1007/BF00587378. [DOI] [PubMed] [Google Scholar]
- Xu X., Lee K. S. Characterization of the ATP-inhibited K+ current in canine coronary smooth muscle cells. Pflugers Arch. 1994 May;427(1-2):110–120. doi: 10.1007/BF00585949. [DOI] [PubMed] [Google Scholar]



